Abstract
Background:
Oxidative stress contributes to neuropathic pain. Coenzyme Q10 (CoQ10) is used for the treatment of neurodegenerative diseases. However, there is no report regarding the therapeutic effect of CoQ10 on neuropathic pain.Objectives:
The present study investigated the effects of CoQ10 on pain alleviation in animals with chronic constriction injury (CCI) of the sciatic nerve.Materials and Methods:
Wistar male rats were randomly allocated to four groups. Nerve injury was induced by four loose ligatures around the sciatic nerve. CoQ10 (200 mg/kg) or vehicle were daily injected intraperitoneally for 10 days. Behavioral tests were performed before nerve injury and on fifth and tenth days after injury. Radiant heat, Randall Selitto test, and Von Frey filaments were used to assess the pain threshold. At the end of the experiment, the animals were perfused transcardially. Lumbar spinal cord was removed and prepared for Nissl staining. Analysis of variance (ANOVA) and Mann-Whitney were used for statistical analysis. P-value below 0.05 was considered statistically significant.Results:
Reduction in pain threshold and the number of neurons in dorsal horn of the spinal cord after nerve injury (P ≤ 0.001) were resulted. Injection of CoQ10 and vehicles increased the pain threshold (P ≤ 0.001 and P ≤ 0.01) and prevented cell loss in neuropathic rats compared with injured animals with no treatment (P < 0.05). Significant differences observed between animals that had received coQ10 compared with vehicle treated ones (P < 0.05).Conclusions:
CoQ10 abolished neuropathic pain in CCI rats. Prevention of cell loss might be attributed to the analgesic effect of CoQ10. Hence, it might be used as a therapeutic intervention for neuropathic pain.Keywords
1. Background
Neuropathic pain is a chronic pain, which interferes with daily life activities still remained as one of the most difficult conditions to control or treat (1). Tactile allodynia (pain with non-noxious stimuli) and hyperalgesia (exaggerated pain with noxious stimuli) are the two known symptoms of neuropathic pain (2, 3). Neuropathic pain is opioid resistant, and other drugs are not effective for pain control and might have numerous adverse effects (4). Many efforts have been performed to treat or control the pain to increase the quality of life in affected individuals and to reduce the financial burden imposed on society. To achieve these goals, finding the possible etiology or the involved mechanisms of the pain has received the most attention. The reactive oxygen species (ROS) are toxic agents acting as one of the major causes of neurodegenerative diseases such as Alzheimer disease, Parkinson’s disease, chronic neuropathic, and inflammatory pain (5-7). In pathological conditions, the intracellular level of ROS is more than the level that could be neutralized by antioxidant; therefore, ROS level increase leads to oxidative stress. High levels of ROS accompanied by low levels of antioxidants such as vitamin C and glutathione has been reported in neuropathic pain conditions (8, 9). Mitochondria are the main sources of ROS which are damaged following the oxidative stress and in turn, their damage leads to apoptosis and cell damage (8, 10, 11). Mitochondrial failure is the key event in the pathogenic cascade leading to ischemia-induced cell death from both necrosis and apoptosis (10). Mitochondrial dysfunction followed by cell death has been shown in the dorsal horn of the spinal cord in chronic pain condition (11). In addition, it is shown that cell death caused by peripheral nerve injuries may lead to neuropathic pain. Increased expression of pro-apoptotic genes during the improvement of neuropathic pain in the dorsal horn of spinal cord three days after chronic constriction injury of sciatic nerve has been reported (12). Regarding the critical role of mitochondria in apoptosis cascade, it is expected that the agents protecting mitochondrial function might have beneficial and therapeutic effects in pain relief. Coenzyme Q10 (CoQ10) is an electron transfer agent acting as a cofactor in the mitochondrial electron transport chain and was first used for cancer treatment (13). CoQ10 is also known as a potent antioxidant able to recycle and regenerate other antioxidants such as tocopherol and ascorbate (13, 14). Protective effect of ascorbate against cell death has been confirmed in numerous recent investigations (15-18). It has been reported that exogenous CoQ10 supplementation supports and stabilizes mitochondrial oxidative phosphorylation (19). This might represent one of the important causes of neuropathic pain and explain the probable therapeutic effects for the treatment of neuropathic pain.
2. Objectives
This study was designed to investigate the possible neuroprotective and pain alleviating effect of CoQ10 on chronic constriction injury model of neuropathic pain. In addition, the association between CoQ10 administration and serum level of vitamin C was investigated.
3. Materials and Methods
3.1. Animals
Adult male Wistar rats (200-250 g) were randomly allocated into experimental groups (10 in each) including control, chronic constriction injury (CCI), CCI plus vehicle, and CCI plus CoQ10. The animals were purchased from Pastor Institute, and all the experimental procedures were approved by the Local Research Ethics Committee of Vice Chancellor of the University (according to the Helsinki declaration). The animals were kept in animal house in light-dark cycle of 12:12 for at least two weeks before the experiment. Water and food were provided ad libitum.
3.2. Surgery
For CCI induction, the animals were anesthetized by intraperitoneal (IP) injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). Then, by blunt incision on the dorsal surface of the thigh, the right sciatic nerve was exposed and freed from connective tissue at mid-thigh level just proximal to its trifurcation. According to Bennet and Xie model, by using 4/0 chromic CATGUT, four loosely tied ligatures were applied along 4 mm distance of the exposed nerve (20). Care was taken not to occlude the blood vessels. Blood circulation was checked by a magnifier through epineural vessels.
3.3. Treatment
Animals in CCI plus CoQ10 and CCI plus vehicle groups received 200 mg/kg IP injection of CoQ10 or its vehicle (Tishcon, NY, USA), respectively, at the same time every day for ten days. Behavioral study was performed before the CCI surgery and at fifth and tenth days after the CCI between 10 AM and 2 PM. At the end of the experiment, the animals were deeply anesthetized by IP injection of pentobarbital sodium (45 mg/kg), and blood samples were collected from their heart to determine vitamin C level in serum.
3.4. Behavioral Studies
This experiment was a self-controlled experiment and all animals were behaviorally examined before induction of the nerve injury, which is indicated as control in the graphs, plantar test, Analgesymeter test, and Von Frey test were used for behavioral study. Thermal hyperalgesia was evaluated by using a plantar analgesia meter (Ugo Basile, Varese, Italy). After 30 minutes of habituation period in the apparatus, the plantar surface of the injured hind paw was exposed to a beam of radiant heat through the glass, and the time from onset of radiant heat application to withdrawal of the hind paw was defined as paw withdrawal latency (PWL). A 25-second cut-off time was allocated to prevent tissue damage. The average of three assessments with 5 min intervals were compared between the groups for radiant heat test. To assess mechanical hyperalgesia, increasing pressure was gradually applied to the right hind paw using an Analgesy meter (Ugo Basile, Italy). This method allows the determination of the threshold by vocalization response to mechanical nociceptive stimulation. To study the mechanical allodynia, Von Frey filaments were used. After 15 minutes of habituation in a chamber located over special mesh surface, the mechanical stimuli for the plantar surface of right hind paw was performed by using Von Frey monofilaments (Von Frey numbers: 4.56, 4.74, 4.10, 4.31, 4.93, 5.07, 5.18, 5.46 , 5.88 and 6.00). Von Frey filament was applied perpendicularly to the plantar surface at the base of the third or fourth toes. Sufficient force was applied to bend the filament slightly for two to three sec. Each hair was tested five times. The evaluation was stopped in the case of three positive responses, but if the responses were negative or no response was elicited, the next higher filament was applied.
3.5. Histological Studies
At the end of the experiment, saline was administered transcardially followed by aldehyde solutions containing 4% paraformaldehyde and 0.2% glutaraldehyde in 0.1 M phosphate buffer, PH = 7.4. By a dorsal incision over the thoracic region and then laminectomy of defined segments, the spinal cord was exposed and the lumbar segments from L4 to L6 were removed according to the rat anatomical landmarks. For cryoprotection, the segments were immersed in graded sucrose solution of 30%, 20%, and 10% sequentially. By using freezing microtome, coronal serial sections of 15 μm thickness were prepared. To evaluate the number of cells, Nissl staining was used for certain sections of L4 to L6. Neurons of the equal area (25 µm2) located in laminae I to V of dorsal horn were counted by using Olysia bio report soft imaging system 3.2
3.6. Determination of Plasma Level of Vitamin C
To evaluate the plasma concentration of vitamin C at the end of experiment, blood sample was taken from heart prior to perfusion. The plasma was isolated, stored at -20 ˚C, and the total vitamin C concentration was determined by enzyme-linked immunosorbent assay (ELISA) method.
3.7. Data Analysis
The results were presented as mean ± standard error of means (SEM). Statistical analysis of data was performed using SPSS 16. Data were compared among the groups with two-way analysis of variance (ANOVA) followed by the Tukey post hoc test. Statistical significance was assumed when P < 0.05.
4. Results
4.1. Behavioral Evaluation
4.1.1. Mechanical Hyperalgesia
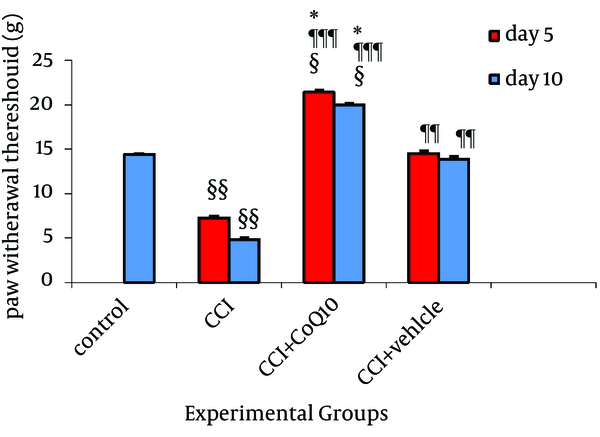
The mean threshold of mechanical hyperalgesia in normal rats before the operation was 14.36 ± 0.08 g which was diminished to 7.25 ± 0.21g after CCI and 5.03 ± 0.22 g on fifth and tenth days, respectively. In other words, chronic constriction injury of sciatic nerve significantly decreased (P < 0.01) the mechanical threshold in Randall-Sellitto test. Following CoQ10 treatment, the mean mechanical threshold was significantly increased to 21.39 ± 0.21g and 20 ± 0.21g on fifth and tenth days, respectively, (P < 0.001, comparing to CCI group). The mechanical threshold of animals with CoQ10 treatment was even higher than the mechanical threshold in control animals. In CCI animals which received vehicle, the mechanical threshold was highly increased to 14.53 ± 0.23 and 13.9 ± 23 g on fifth and tenth days, respectively (P < 0.01, comparing to CCI group). Significant difference existed between the two groups of animal treated with CoQ10 and vehicle as shown in Figure 1.
Effect of IP Administration of CoQ10 on the Development of Mechanical Hyperalgesia
4.1.2. Thermal Hyperalgesia
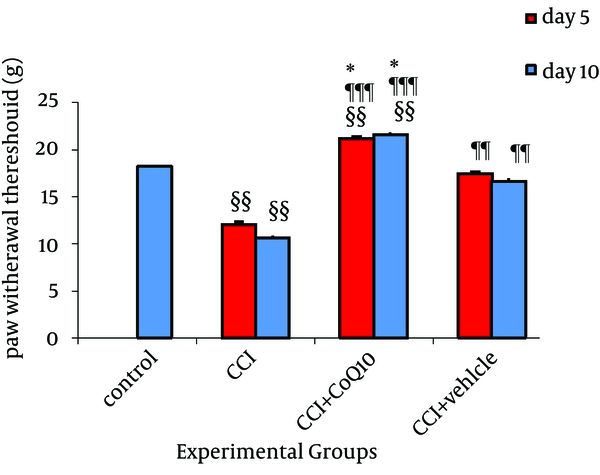
The mean baseline thermal threshold in control group was 18.018 ± 0.65 sec in plantar test. Nerve injured animals showed a significant reduction (P < 0.01 comparing to control) in thermal hyperalgesia on fifth day through tenth day after the operation indicative of hyperalgesic state. The mean thresholds in CCI animals were 12.09 ± 0.25 sec on fifth day and 10.59 ± 0.21sec on tenth day after induction of CCI (Figure 2). In comparison to CCI group, a significant recovery with restoring the thermal sensitivity after treatment with CoQ10 or its vehicle was observed (P < 0.001 and P < 0.01, respectively). This effect lasted up to 10 days after CCI. The significant difference observed between animals treated with vehicle and CoQ10 indicated more effectiveness of treatment with CoQ10 (P < 0.05).
Effect of IP Administration of CoQ10 on the Development of Thermal Hyperalgesia
4.1.3. Mechanical Allodynia
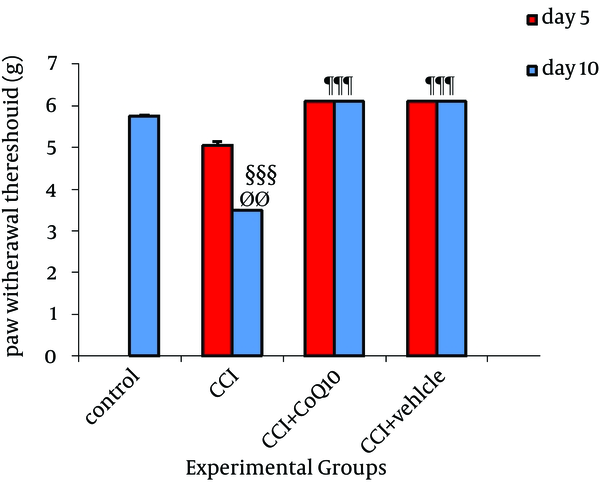
In CCI rats, mechanical allodynia measured with von Frey filament showed a mechanical pressure threshold on the nerve-injured side of 5.15 ± 0.05 g and 3.5 ± 0.09 g on fifth and tenth days, respectively. Although no significant difference was observed on fifth day after the operation with control animals, the mean value on tenth day after the operation was markedly lower (P < 0.001) than the values determined in control non-injured animals (5.8 ± 0.05 g). The mechanical threshold to von Frey hair in animals receiving CoQ10 or its vehicle was markedly higher than the cut-off filament (No. = 6.00) determined before nerve ligation in the same animals. None of the animals treated with vehicle or CoQ10 reacted to the filaments after the treatment. No significant differences were observed between control and CoQ10 or vehicle treated groups (Figure 3).
Effect of IP Administration of CoQ10 on the Development of Mechanical Allodynia
4.2. Histological Evaluation
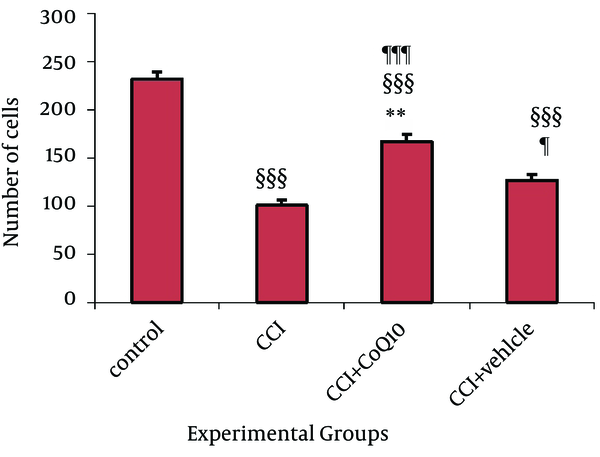
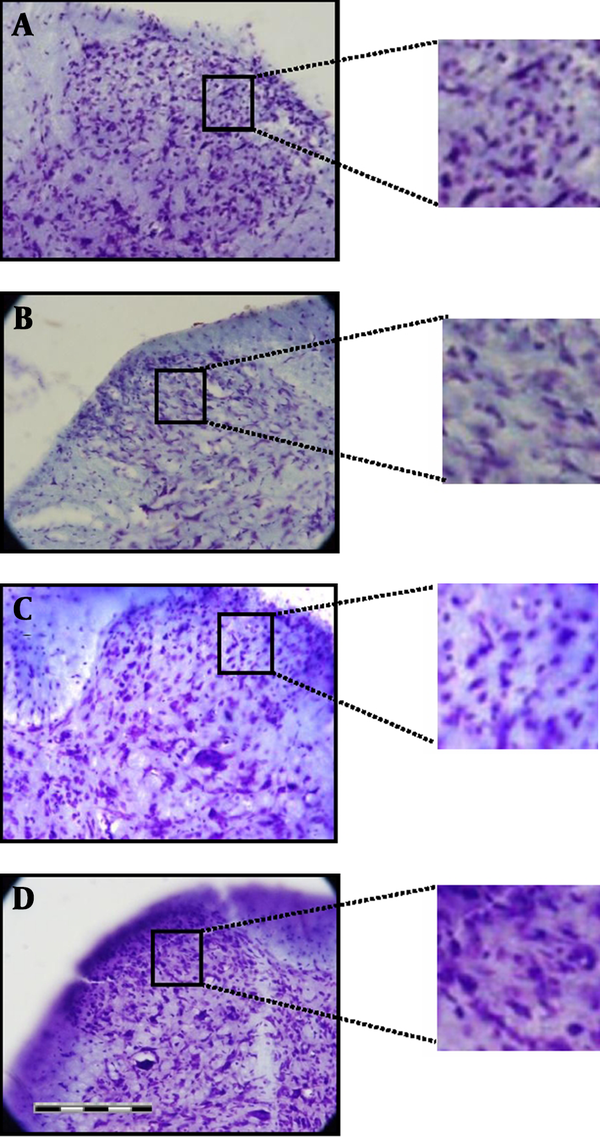
Nissl staining was used to determine certain changes in morphometric parameters of the spinal cord segments related to sciatic nerve. Neurons containing Nissle substance in the cytoplasm, loose chromatin, and prominent nucleoli were considered structurally normal. Main spinal cord segments of sciatic nerve including L4, L5 and L6 were studied. The mean numbers of Nissle-positive neurons in all the three segments were significantly higher in the animals treated with CoQ10 compared to the CCI animals with no treatment (P < 0.001) (Figure 4). Histological study showed that the number of morphological normal neurons was significantly decreased in CCI animals compared to control groups (P < 0.001). In addition, the number of morphologically normal neurons in the vehicle treated group was significantly lower than the number of positive neurons in animals treated with CoQ10 (P < 0.01), (Figure 5).
The Number of Nissl Positive Cells in Dorsal Horn of Lumbar Level of Spinal Cord
Photomicrographs of the Sciatic Nerve Region of the Ipsilateral Dorsal Horn of Spinal Cord in Lumbar Level
4.3. Result of Plasma level of Vitamin C
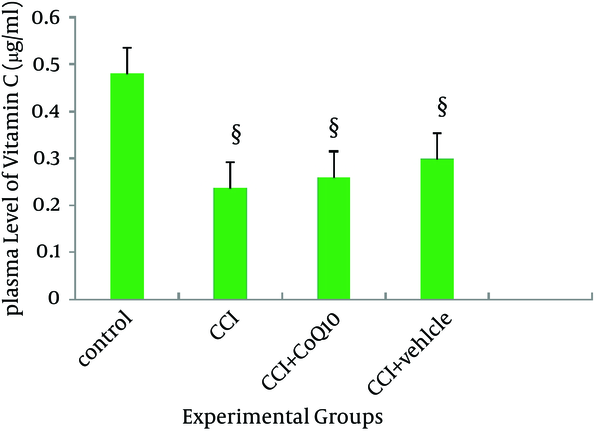
The plasma level of vitamin C was significantly decreased after CCI (P < 0.05). Administration of CoQ10 or its vehicle could not restore the plasma level of vitamin C (Figure 6).
Plasma Level of Vitamin C in Different Experimental Groups
5. Discussion
The present experiment studied the cell loss in the dorsal horn of spinal cord following constriction injury in rats. It confirms that changes in behavioral assessment (increased thermal and mechanical sensitivity) correlates with histological changes in the central nervous system following the induction of nerve injury in a CCI animal model. IP injection of CoQ10 effectively improved the pain threshold and reduced allodynia and hyperalgesia in an animal model with pre-existing nerve injury. Most importantly, histological improvements were accompanied by a significant improvement in behavioral assessment parameters. There are enormous evidences supporting the idea of cell death in the pathogenesis of neuropathic pain. It seems that allodynia and hyperalgesia, which occur after nerve injury, are associated with neuronal apoptosis in the superficial lamina of the rat spinal cord (21). Siniscalco D. et al. emphasized on the role of ROS in neuropathic pain acting mainly throughout spinal mechanisms (22). Excessive ROS production changes pro-apoptotic gene expression of the neurons leading to cell death (23). Hyperalgesia and allodynia are the two main symptoms of neuropathic pain. ROS is also responsible for induction of hyperalgesia (24). khalil, et al. showed that following a nerve injury, production of free radicals will be increased in dorsal horn of the spinal cord (25). They postulated that this phenomenon leads to cell death and might be the cause of hypersensitivity in spinal cord (21, 22). ROS are also involved in NMDA receptor phosphorylation, which leads to activation of NMDA receptors in nerve injury models (22, 26). Activation of NMDA receptors is another mechanism for induction of hyperalgesia seen after nerve injury (24). In addition, activation of NMDA receptors following nerve injury leads to cells death. Glutamate dependent apoptosis following NMDA activation was also reported after one to three days of CCI in substantial gelatinous leading to cell lose. These changes in histological properties of the spinal cord are simultaneous with hyperalgesia, which is seen after nerve injury (27). Activation of NMDA receptors following excessive production of ROS could also lead to decrease in the antioxidant activity (5). Glutathione is an important antioxidant in the nervous system. Reduction in the level of glutathione after excessive NMDA receptor activation has been reported. Imbalance between ROS level and the level of antioxidant agents in the nervous system is another important mechanism of induction of hyperalgesia and allodynia nerve injury (25, 26). ROS are cleared from the cells by the action of antioxidants (28). Vitamin C and E are the two well-known antioxidants able to scavenge the produced ROS. Other antioxidants are introduced recently and among them, the role of CoQ10 is more emphasized. It has been shown that CoQ10 is a potent antioxidant and reduces oxidative stress by reduction of the ROS production (13, 29). Accordingly, the cell protective character of the CoQ10 has also been revealed (30).
Despite the possible well-known role of CoQ10 in apoptotic conditions, the exact mechanisms of its protective action are still unknown. It is believed that the most of the protective effects of CoQ10 is related to the effect of this coenzyme on elevation and reestablishing the serum levels of vitamin C or vitamin E. The ameliorative effect of Vitamin C in neuropathic pain conditions has been reported by Safarpour and Nasirinezhad (31). Although CoQ10 is able to elevate vitamin E and C in serum, our findings did not confirm this regarding the level of vitamin C following CoQ10 administration. We believe that the reduction of vitamin C level after CCI is so critical that CoQ10 could not restore it. It might be due to this issue that the amount of CoQ10 used in this study was not enough to reestablish the level of vitamin C in serum. However, it seems that this amount of CoQ10 was enough to increase the pain threshold and prevent cell loss. Nevertheless, vehicle used in this experiment contained vitamin C, but serum level of vitamin C in the vehicle treated CCI rats was lower than the normal animals, and the amount of vitamin C in vehicle was not enough to restore the reduction in the vitamin C after CCI. We did not measure the level of vitamin E in serum; therefore, we could not exclude the role of CoQ10 on the elevation or changes in the level of this vitamin. Papucci et al. also illustrated a distinct activity for CoQ10. According to their study, antiapoptotic property of CoQ10 was independent of its free radical scavenging ability and may be related to direct inhibition of permeability transition pore (PTP) opening (32). The PTP is a mitochondrial channel which makes the mitochondrial membrane collapse if open. This process finally leads to cell apoptosis. This effect of CoQ10 might be considered as another reason for our result. In addition, we found that the vehicle used in this study had pain-relieving effect and prevented cell lose, but it was not as effective as CoQ10. The vehicle contains vitamin E and C, two effective antioxidants able to protect cells from death during oxidative stress (30, 33). KIM et al. reported that vitamin E is able to reduce responsiveness of dorsal horn neurons to mechanical stimuli in neuropathic rats and reduce NMDA receptors phosphorylation in these cells (34). It is also reported that the analgesic action of vitamin E occurs through desensitization of neurons and inactivation of NMDA receptors in neuropathic rats (31, 34). The same effect has been reported for CoQ10. It is suggested that CoQ10 is able to decrease the activity of NMDA receptors and this effect can introduce it as a good choice for treatment of the neurodegenerative diseases (32).
In conclusion, our study showed for the first time that IP injection of CoQ10 improves the histological features in the dorsal horn following damage of the sciatic nerve, which also correlates with the alleviation of neuropathic pain responses in an animal model of neuropathic pain. Therefore, to increase the efficacy of the treatment of neuropathic pain condition, management may need to include treatment directed at preventing neuronal loss. CoQ10 may facilitate promising therapeutic targets for the treatment of peripheral nerve injury. However, more studies are needed to confirm our findings.
Acknowledgements
References
-
1.
Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-19. [PubMed ID: 19410099]. https://doi.org/10.1016/j.genhosppsych.2008.12.006.
-
2.
Schaeffer V, Meyer L, Patte‐mensah C, Eckert A, Mensah‐nyagan AG. Sciatic nerve injury induces apoptosis of dorsal root ganglion satellite glial cells and selectively modifies neurosteroidogenesis in sensory neurons. Glia. 2010;58(2):169-80.
-
3.
Cui JG, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000;88(3):239-48. https://doi.org/10.1016/S0304-3959(00)00331-6.
-
4.
Mizoguchi H, Watanabe C, Yonezawa A, Sakurada S. New therapy for neuropathic pain. Int Rev Neurobiol. 2009;85:249-60. https://doi.org/10.1016/S0074-7742(09)85019-8.
-
5.
Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689-95. [PubMed ID: 7901908].
-
6.
Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60(1):202-13. [PubMed ID: 19154757]. https://doi.org/10.1016/j.brainresrev.2008.12.010.
-
7.
Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17(10):871-90. [PubMed ID: 11063054].
-
8.
Naik AK, Tandan SK, Dudhgaonkar SP, Jadhav SH, Kataria M, Prakash VR, et al. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur J Pain. 2006;10(7):573-9. [PubMed ID: 16214382]. https://doi.org/10.1016/j.ejpain.2005.08.006.
-
9.
Tan EC, Bahrami S, Kozlov AV, Kurvers HA, Ter Laak HJ, Nohl H, et al. The oxidative response in the chronic constriction injury model of neuropathic pain. J Surg Res. 2009;152(1):84-8. [PubMed ID: 18708193]. https://doi.org/10.1016/j.jss.2008.03.035.
-
10.
Bergmann F, Keller BU. Impact of mitochondrial inhibition on excitability and cytosolic Ca2+ levels in brainstem motoneurones from mouse. J Physiol. 2004;555(Pt 1):45-59. [PubMed ID: 14660707]. https://doi.org/10.1113/jphysiol.2003.053900.
-
11.
Cohen BH, Gold DR. Mitochondrial cytopathy in adults: what we know so far. Cleve Clin J Med. 2001;68(7):625-6.
-
12.
Sekiguchi M, Sekiguchi Y, Konno S, Kobayashi H, Homma Y, Kikuchi S. Comparison of neuropathic pain and neuronal apoptosis following nerve root or spinal nerve compression. Eur Spine J. 2009;18(12):1978-85. [PubMed ID: 19543754]. https://doi.org/10.1007/s00586-009-1064-z.
-
13.
Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271(1):195-204. https://doi.org/10.1016/0925-4439(95)00028-3.
-
14.
Groneberg DA, Kindermann B, Althammer M, Klapper M, Vormann J, Littarru GP, et al. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human CaCo-2 cells. Int J Biochem Cell Biol. 2005;37(6):1208-18. [PubMed ID: 15778085]. https://doi.org/10.1016/j.biocel.2004.11.017.
-
15.
Murata W, Tanaka T, Kubo I, Fujita K. Protective effects of alpha-tocopherol and ascorbic acid against cardol-induced cell death and reactive oxygen species generation in Staphylococcus aureus. Planta Med. 2013;79(9):768-74. [PubMed ID: 23670625]. https://doi.org/10.1055/s-0032-1328555.
-
16.
Gobbo MG, Ribeiro DL, Taboga SR, de Almeida EA, Goes RM. Oxidative stress markers and apoptosis in the prostate of diabetic rats and the influence of vitamin C treatment. J Cell Biochem. 2012;113(7):2223-33. [PubMed ID: 22573545]. https://doi.org/10.1002/jcb.24092.
-
17.
Pavlovic V, Pavlovic D, Kamenov B, Sarac M, Peric Z, Velojic M. Protective role of vitamin C in diazepam-induced apoptosis in rat thymocytes. Bratisl Lek Listy. 2012;113(6):350.
-
18.
Naseer MI, Ullah I, Ullah N, Lee HY, Cheon EW, Chung J, et al. Neuroprotective effect of vitamin C against PTZ induced apoptotic neurodegeneration in adult rat brain. Pak J Pharm Sci. 2011;24(3):263-8. [PubMed ID: 21715258].
-
19.
Yen DH, Chan JY, Huang CI, Lee CH, Chan SH, Chang AY. Coenzyme q10 confers cardiovascular protection against acute mevinphos intoxication by ameliorating bioenergetic failure and hypoxia in the rostral ventrolateral medulla of the rat. Shock. 2005;23(4):353-9. [PubMed ID: 15803059].
-
20.
Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87-107. https://doi.org/10.1016/0304-3959(88)90209-6.
-
21.
Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A. 2010;107(33):14851-6. [PubMed ID: 20679217]. https://doi.org/10.1073/pnas.1009926107.
-
22.
Siniscalco D, Fuccio C, Giordano C, Ferraraccio F, Palazzo E, Luongo L, et al. Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res. 2007;55(2):158-66. [PubMed ID: 17207636]. https://doi.org/10.1016/j.phrs.2006.11.009.
-
23.
Reichling DB, Levine JD. Pain and death: neurodegenerative disease mechanisms in the nociceptor. Ann Neurol. 2011;69(1):13-21.
-
24.
Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309(3):869-78. [PubMed ID: 14988418]. https://doi.org/10.1124/jpet.103.064154.
-
25.
Khalil Z, Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic Biol Med. 2001;31(4):430-9. https://doi.org/10.1016/S0891-5849(01)00597-4.
-
26.
Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, et al. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111(1-2):96-103. [PubMed ID: 15327813]. https://doi.org/10.1016/j.pain.2004.06.004.
-
27.
de Novellis V, Siniscalco D, Galderisi U, Fuccio C, Nolano M, Santoro L, et al. Blockade of glutamate mGlu5 receptors in a rat model of neuropathic pain prevents early over-expression of pro-apoptotic genes and morphological changes in dorsal horn lamina II. Neuropharmacology. 2004;46(4):468-79. [PubMed ID: 14975670]. https://doi.org/10.1016/j.neuropharm.2003.10.014.
-
28.
Calabrese V, Cornelius C, Trovato A, Cavallaro M, Mancuso C, Di Rienzo L, et al. The hormetic role of dietary antioxidants in free radical-related diseases. Curr Pharm Des. 2010;16(7):877-83. [PubMed ID: 20388101].
-
29.
Ferrante RJ, Andreassen OA, Dedeoglu A, Ferrante KL, Jenkins BG, Hersch SM, et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington's disease. J Neurosci. 2002;22(5):1592-9. [PubMed ID: 11880489].
-
30.
Sharma SK, El Refaey H, Ebadi M. Complex-1 activity and 18F-DOPA uptake in genetically engineered mouse model of Parkinson's disease and the neuroprotective role of coenzyme Q10. Brain Res Bull. 2006;70(1):22-32. [PubMed ID: 16750479]. https://doi.org/10.1016/j.brainresbull.2005.11.019.
-
31.
Safapoor S, Nasirnejad F. NMDA Receptors in the analgesic effect of vitamin C in a neuropathic pain model. Razi J Med sci. 2008;15:1-2.
-
32.
Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, et al. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278(30):28220-8. [PubMed ID: 12736273]. https://doi.org/10.1074/jbc.M302297200.
-
33.
Aabdallah DM, Eid NI. Possible neuroprotective effects of lecithin and alpha-tocopherol alone or in combination against ischemia/reperfusion insult in rat brain. J Biochem Mol Toxicol. 2004;18(5):273-8. [PubMed ID: 15549708]. https://doi.org/10.1002/jbt.20037.
-
34.
Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, et al. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. 2006;122(1-2):53-62. [PubMed ID: 16524661]. https://doi.org/10.1016/j.pain.2006.01.013.