Abstract
Background:
Diabetes type 1 has a variety of destructive effects on different parts of the body including kidney tissue. Diabetic nephropathy is among the serious complications of diabetes. Oxidative stress plays a central role in the development of diabetic nephropathy with glomeruli dilation.Objectives:
Due to the known anti-oxidant and anti-diabetic effects of Origanum majorana (OM), this study was conducted to survey its effects on oxidative stress and histopathology of renal tissue among diabetic rats.Materials and Methods:
In this experimental investigation, 32 male rats, weighing 200 - 250 g, were divided into four groups: 1: control, 2: control receiving treatment with OM, 3: diabetic, and 4: diabetic receiving treatment with OM. Diabetes was induced by intraperitoneal injection of streptozotocin (60 mg/kg). Groups under treatment received food containing 6.25% of OM. Tissue levels of malondialdehyde (MDA) were measured to evaluate the oxidative stress. In addition, renal histopathology was evaluated using hematoxylin and eosin (H & E) staining. Data were analyzed using ANOVA.Results:
A significant increase in the renal tissue level of MDA was observed in diabetic and OM-treated diabetic groups versus control group (P = 0.01 and P = 0.04, respectively). OM treatment non-significantly reduced the tissue level of MDA (P = 0.11). Also, diabetes caused a significant increase in glomerular size compared to the control group (P = 0.03), and treatment of diabetic rats with OM caused a non-significant decrease in glomerular size compared to the diabetic group (P = 0.17).Conclusion:
Taken together, long term treatment of diabetic rats with OM can partially protect the renal tissue via attenuation of oxidative stress and glomerular expansion.Keywords
Origanum Majorana Diabetes Mellitus Kidney Oxidative Stress Rat
1. Background
Diabetes mellitus is a metabolic disorder with chronic hyperglycemia and defects in carbohydrates, lipids and proteins metabolism (1). Chronic diabetes can lead to retinopathy, nephropathy and cardiovascular disorders. According to some reports, diabetes prevalence is increasing worldwide (2-4). Presumably, one third to half of diabetic patients will finally develop diabetic nephropathy which is the most important cause of morbidity and mortality among diabetics. Mortality rate among patients with diabetic nephropathy is 30 times more than diabetic patients without nephropathy (5). Diabetes mellitus also has a close relation with oxidative stress due to the increased formation of oxygen free radicals. Different studies have shown that hyperglycemia is related to increased oxidative stress. Increased oxidative stress has a pivotal role in the renal damage of patients with diabetes (6-8). Increased tissue level of malondialdehyde (MDA) is one of the important markers of oxidative stress (8, 9). Moreover, one of the complications of diabetic nephropathy is distention and destruction of glomeruli in the renal tissue (10).
Because of the low accessibility and high expenses of most of the new therapies, finding new agents with less side effects such as herbal derivatives is further highlighted (11). One of these herbal plants is Organium vulgarea ssp. viridie (Origanum majorana). Investigations have shown that Origanum majorana (OM) can decrease blood glucose level in diabetic rats (12). Also its long term prescription hampered streptozotocin-induced diabetes tissue injuries (13). OM supplement is a modulator of tissue lipid peroxidation and is considered as an anti-oxidant in colon cancer with probable anti-cancer effects (14). With an increase in predisposing factors like obesity, sedentary life style, low body movements and inappropriate diets, a precise programming seems essential to manage the disease (15, 16).
2. Objectives
Due to the known anti-oxidant and anti-diabetic properties of this herb, we conducted this study to investigate the effects of Origanum majorana on renal histopathology and oxidative stress in diabetic rats.
3. Materials and Methods
3.1. Animals
This experimental investigation was performed on 32 three-month-old male albino Wistar rats weighing 200 to 250 g (Razi Institute). Animals were kept at a standard temperature of 21 - 23°C on a 12/12 light dark cycle with an appropriate air conditioning. All 32 rats were in 4 groups including: 1, control; 2, control given food containing OM powder; 3, diabetic; 4, diabetic given food containing OM powder. Four rats were kept in each cage. At the first stage, rats were fed for one week for accommodation with the lab environment. Animals were provided with food and water without any limitation. This study was performed according to the American National Health Institute (NIH) guidelines and also available local manuals for keeping and working with animals. Rats with serum glucose levels below 130 mg/dL were selected. Blood sampling was performed using a capillary tube from the retro-orbital plexus after overnight fasting in normal conditions. Sample volume was about 1 ml. For induction of diabetes, streptozotocin (STZ) (Sigma, Germany) dissolved in cold physiologic saline was injected intraperitoneally (60 mg/kg). After one week post-injection, diabetes verification was done using dipstick (Glucoyab co., Tehran). Only animals with urine glucose levels of more than 50 which equals to the glucose level of 250 in plasma were qualified for the next step. Moreover, some symptoms like polyphagia, polydipsia, diuresis and gradual weight loss were indicators of diabetes induction in rats. After three weeks, all rats were weighed and blood sampling was performed. Six weeks after initiation of the investigation, final weighing and blood sampling were performed. Serum glucose level was measured with glucose oxidase enzyme method (Zistchemi, Tehran) using spectrophotometer with the following formula:
Standard absorption × 100/test absorption = glucose (mg/dL)
3.2. Preparation of OM Powder
The plant leaves were prepared and the identification was approved by Shahid Beheshti University of Medical Sciences Biology Department. Leaves were grinded. Then the powder was mixed with the standard rats’ food with a proportion of 6.25%. Adequate water was added to the mixture and the pasta was formed in a shape available for consumption. Then it was dried for 24 hours with sunlight, and was provided in cages with water without any limitation.
3.3. Determination of Renal Malondialdehyde (MDA) Concentration
Rats were anesthetized with diethyl ether (100 mg/kg) (Merck, Germany), decapitated, kidneys were removed, blotted dry, weighed, then made into 5% tissue homogenate in ice-cold 0.9% saline solution, centrifuged (1000 × g, 4ºC, 10 min), and obtained supernatant was liquated and stored at -80°C until assayed. In order to measure the MDA concentration (thiobarbituric acid reactive substances, TBARS) in the supernatant, trichloroacetic acid and TBARS reagent were added to the supernatant, then mixed and incubated at 100ºC for 80 min. After cooling on ice, samples were centrifuged at 1000 × g for 10 min and the absorbance of the supernatant was read at 532 nm. TBARS results were expressed as MDA equivalents using tetraethoxypropane as standard (17).
3.4. Protein Assay
The protein content of the supernatant was measured with Bradford method using bovine serum albumin (Sigma Chemical, St. Louis, MO) as the standard (Bradford, 1976) (17).
3.5. Histological Assessment
Kidneys were resected and put into 10% formalin (10, 18). After 24 hours, the fixator solution was changed. After processing, 5 µm sections were prepared using rotary microtome and sections were transferred into gelatin slides. For evaluation of sections, hematoxylin and eosin (H & E) staining was done (Ziess, Germany). In each group, at least 100 glomeruli (20 glomeruli from each rat) were observed and evaluated (10) by a pathologist who was blinded to the treatments.
3.6. Statistical Analysis
Results were reported as averages and standard deviations. After determining data distribution, one way ANOVA was used for analysis of the histological data. Other parameters were assessed using repeated measure ANOVA and Tukey’s post-test. P value less than 0.05 was considered significant for all analyses.
4. Results
4.1. Weight of Rats
Thirty two male Wistar rats were used for the study. Animal weights were measured one week before the evaluation as well as three and six weeks after evaluation. Results showed that weight does not show any significant difference one week before evaluations in all groups, while there was a significant difference among groups after three and six weeks. After six weeks, diabetic group showed a significant reduction in weight compared to the control group. Although this decrease was also observed after three weeks, it was not significant. Moreover, difference was not significant after three and six weeks between the two groups of diabetic and diabetic under treatment with OM. Although body weight was higher in diabetic group receiving treatment compared to diabetic group without treatment after three and six weeks, the observed difference was not significant. In addition, treatment of control group with OM resulted in a significant difference compared to control group without treatment and weight was less than control group (Figure 1).
Weights of Rats in Different Groups During the Course of Experiment

4.2. Serum Glucose Level
Assessing the serum level of glucose, no significant differences were found among the groups at the beginning of the study. Moreover, the serum level of glucose of the diabetic and OM-treated diabetic group were considerably higher compared to the primary measures at the beginning of the study. Also, the control group with OM did not show a significant difference in comparison with the control group. In addition, an insignificant drop of serum level of glucose was documented in the diabetic rats receiving treatment compared to the diabetic group (Figure 2)
Data of Glucose Measurement in Serum From Different Groups in Different Weeks
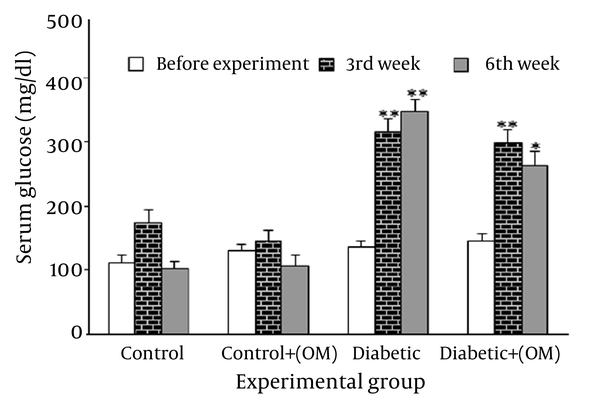
4.3. Tissue Level of MDA
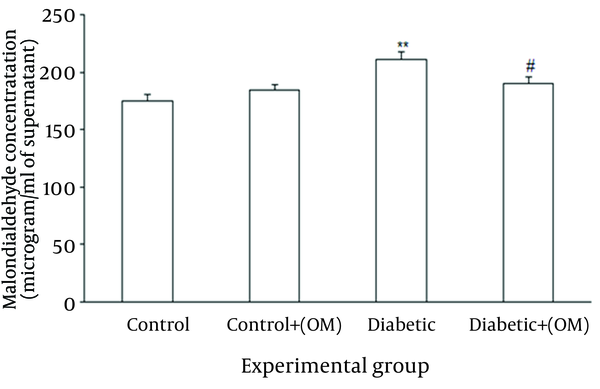
Regarding renal lipid peroxidation marker, treatment with OM in control group did not produce any significant change versus untreated control group. In contrast, STZ-induced diabetes resulted in significant elevation of MDA content compared to the control group (P = 0.009), and treatment with OM in diabetic group caused a significant reduction of MDA level (P = 0.03) versus untreated diabetics. Meanwhile, OM-treated diabetics did not show a significant elevation of MDA content in comparison with control group (Figure 3).
MDA Measurement in Renal Homogenates Among Different Groups
4.4. Kidney Histopathology
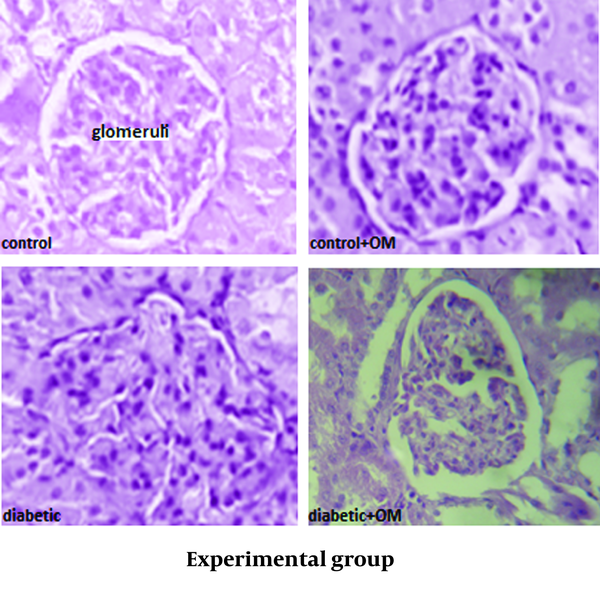
Staining of the renal tissue showed that the glomeruli of the control group were morphologically normal and that the treatment of the control group with OM did not have a clear effect on the structure of the glomeruli. Meanwhile, the glomeruli from the diabetic rats had expansion and dispersion after six weeks, even though such alterations were yet statistically non-significant. Also, the treatment of the diabetic group with OM non-significantly reduced glomerular size with a more regular pattern (Figure 4) (Table 1).
Light Microscopic Photomicrograph From Cortex of Kidney Tissue Using H & E Method (400×)
Glomerular Size in Different Groups
5. Discussion
The results of this experiment showed that treatment of the diabetic group with OM does not increase the weight of the diabetic rats, nor does it reduce the serum level of glucose in any significant way. Diabetic rats showed a significant rise in the levels of malondialdehyde in the renal tissue which was significantly reduced after treatment with OM. Furthermore, undergoing treatment with OM for the diabetic rats showed a non-significant reduction of the glomeruli size and their irregularity in shape and pathological expansion.
Reports of the previous experimentations show that in both diabetes insipidus and mellitus (respectively type 1 and 2), the occurrence of oxidative stress rises due to increased levels of oxygen free radicals and that the body’s anti-oxidative capabilities are somewhat diminished which is the main cause of tissue damage in diabetic patients (7, 8). The activities of anti-oxidative agents in the body play a major role in the upkeep of cells against internal and external toxic free radicals (8, 19). The STZ-induced diabetes can result in lowered levels of activities of endogenous anti-oxidative enzymes and subsequent tissue degradations (8). In time, the occurrence of oxidative stress and lipid peroxidation is imminent in the diabetic patient, for which the best marker would be the tissue levels of MDA (20). Moreover, chronic hyperglycemia affects the renal tissue, either by direct effect or indirect induction of the damage through hemodynamic alterations, while in such situations, hyperfilteration and microalbuminuria play the most important roles. Such alterations will affect the adjacent resident cells into an abnormal production of cytokines and growth factors which will subsequently facilitate the synthesis of extracellular matrix proteins and the depositions in the glomerular level. Such events will eventually lead to mesangial expansion and glomerular basement thickening (10).
It has been previously reported that OM can act in many ways to stack up the free radicals of oxygen (i.e. superoxide), protect the cell against harmful chemical substances (i.e. environmental toxins), reduce lipid peroxidation and protect the liver against a variety of chemically-induced stresses, which can be attributed to its high values of anti-oxidants like flavonoids (21, 22). To this, consumption of this herb can lead to protection of the body’s tissues against the aforementioned harms (22) in the healthy person and the diabetic patient who experience the occurrence of oxidative stress more (20) and show the consequent biochemical changes in the serum. In addition, flavonoids of this herb may balance the enzymatic activities of the liver such ways as to help balance the levels of hormones in the metabolism pathway of carbohydrates like reduction of hepatic phosphorylase and increasing glucokinase and glycogen synthetase, which will in turn, lower the serum level of glucose and maintain a healthy body weight (23). Trials regarding OM have shown that administering the extract of this herb in healthy and diabetic rats will cause a drop in serum glucose levels within six hours of administration, and repeated daily administration will result in a normal serum glucose level (12); though such allegations are inconsistent with our findings in this trial. This contrast might be partially explained by the fact that diabetes has been chemically induced with a cytotoxic substance in our rats, which will cause a more severe set of biochemical effects. Accordingly, the serum level of insulin and the number of insulin-producing cells would reduce to an absolute minimum. Furthermore, in another study, the anti-diabetic properties of OM had been confirmed. The results of this study showed that long-term administration of OM will withhold the organ and tissue damage and this treatment does not affect the serum levels of glucose among animals (13); again, such allegations are inconsistent with our findings.
In our study, the increase in renal tissue levels of MDA was documented and oral administration of OM resulted in decreasing the levels of oxidative stress markers in the renal tissue. Correspondingly, some positive attributes of OM in this study may be the ability to lower the oxidative stress. On this matter, it was shown that some flavonoids can boost the activity of some free-radical-fighting mechanisms of anti-oxidants and enzymes (24). Accordingly, flavonoids can lower the occurrence of lipid peroxidation as well (25), which can lead to lower levels of MDA in the renal tissue.
Some inconsistencies with other studies can be attributed to the varying dosages of administration of OM, considering that our dosages have been closer to the minimum effective dosage in the diet of the subjects. Also, another cause may be the route of OM administration which was oral in this study. Lower consumed amounts mean lower levels of entry in the blood. We recommend re-assessing the ability of OM in lowering the serum glucose levels and its antioxidant properties with different administration routes or higher dosages. One of our limitations was that we did not measure the Glomerular Filtration Rate (GFR), and we recommend similar studies with GFR measurement to assess the effects of OM on physiological functions of the glomeruli.
Acknowledgements
References
-
1.
Wandell PE. Quality of life of patients with diabetes mellitus. An overview of research in primary health care in the Nordic countries. Scand J Prim Health Care. 2005;23(2):68-74. [PubMed ID: 16036544]. https://doi.org/10.1080/02813430510015296.
-
2.
Tripathi BK, Srivastava AK. Diabetes mellitus: complications and therapeutics. Med Sci Monit. 2006;12(7):RA130-47. [PubMed ID: 16810145].
-
3.
Suji G, Sivakami S. Approaches to the treatement of diabetes mellitus: an overview. Cell Mol Biol (Noisy-le-grand). 2003;49(4):635-9. [PubMed ID: 12899455].
-
4.
Shapiro K, Gong WC. Natural products used for diabetes. J Am Pharm Assn. 2002;42(2):217-226.
-
5.
Yokozawa T, Yamabe N, Cho EJ, Nakagawa T, Oowada S. A study on the effects to diabetic nephropathy of Hachimi-jio-gan in rats. Nephron Exp Nephrol. 2004;97(2):e38-48. [PubMed ID: 15218322]. https://doi.org/10.1159/000078405.
-
6.
Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C, et al. Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud. 2010;7(1):15-25. [PubMed ID: 20703435]. https://doi.org/10.1900/RDS.2010.7.15.
-
7.
Descorbeth M, Anand-Srivastava MB. Role of oxidative stress in high-glucose- and diabetes-induced increased expression of Gq/11alpha proteins and associated signaling in vascular smooth muscle cells. Free Radic Biol Med. 2010;49(9):1395-405. [PubMed ID: 20691780]. https://doi.org/10.1016/j.freeradbiomed.2010.07.023.
-
8.
Chang YC, Chuang LM. The role of oxidative stress in the pathogenesis of type 2 diabetes: from molecular mechanism to clinical implication. Am J Transl Res. 2010;2(3):316-31. [PubMed ID: 20589170].
-
9.
Pazdro R, Burgess JR. The role of vitamin E and oxidative stress in diabetes complications. Mech Ageing Dev. 2010;131(4):276-86. [PubMed ID: 20307566]. https://doi.org/10.1016/j.mad.2010.03.005.
-
10.
Baig MA, Gawali VB, Patil RR, Naik SR. Protective effect of herbomineral formulation (Dolabi) on early diabetic nephropathy in streptozotocin-induced diabetic rats. J Nat Med. 2012;66(3):500-9. [PubMed ID: 22116744]. https://doi.org/10.1007/s11418-011-0614-y.
-
11.
Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr. 2007;85(3):662-77. [PubMed ID: 17344486].
-
12.
Lemhadri A, Zeggwagh NA, Maghrani M, Jouad H, Eddouks M. Anti-hyperglycaemic activity of the aqueous extract of Origanum vulgare growing wild in Tafilalet region. J Ethnopharmacol. 2004;92(2-3):251-6. [PubMed ID: 15138008]. https://doi.org/10.1016/j.jep.2004.02.026.
-
13.
Lermioglu F, Bagci S, Onderoglu S, Ortac R, Tugrul L. Evaluation of the long-term effects of oleum origani on the toxicity induced by administration of streptozotocin in rats. J Pharm Pharmacol. 1997;49(11):1157-61. [PubMed ID: 9401956].
-
14.
Srihari T, Sengottuvelan M, Nalini N. Dose-dependent effect of oregano (Origanum vulgare L.) on lipid peroxidation and antioxidant status in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. J Pharm Pharmacol. 2008;60(6):787-94. [PubMed ID: 18498716]. https://doi.org/10.1211/jpp.60.6.0015.
-
15.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27(5):1047-1053.
-
16.
Bergeron R, Yao J, Woods JW, Zycband EI, Liu C, Li Z, et al. Peroxisome proliferator-activated receptor (PPAR)-α agonism prevents the onset of type 2 diabetes in Zucker diabetic fatty rats: a comparison with PPARγ agonism. Endocrinology. 2006;147(9):4252-4262.
-
17.
Bagheri M, Joghataei MT, Mohseni S, Roghani M. Genistein ameliorates learning and memory deficits in amyloid beta(1-40) rat model of Alzheimer's disease. Neurobiol Learn Mem. 2011;95(3):270-6. [PubMed ID: 21144907]. https://doi.org/10.1016/j.nlm.2010.12.001.
-
18.
Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton). 2007;12(3):261-6. [PubMed ID: 17498121]. https://doi.org/10.1111/j.1440-1797.2007.00796.x.
-
19.
Chtourou Y, Fetoui H, Sefi M, Trabelsi K, Barkallah M, Boudawara T, et al. Silymarin, a natural antioxidant, protects cerebral cortex against manganese-induced neurotoxicity in adult rats. Biometals. 2010;23(6):985-96. [PubMed ID: 20503066]. https://doi.org/10.1007/s10534-010-9345-x.
-
20.
Akiyama S, Katsumata S, Suzuki K, Nakaya Y, Ishimi Y, Uehara M. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki rats with type 2 diabetes. Biosci Biotechnol Biochem. 2009;73(12):2779-82. [PubMed ID: 19966469].
-
21.
Folli F, Corradi D, Fanti P, Davalli A, Paez A, Giaccari A, et al. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev. 2011;7(5):313-24. [PubMed ID: 21838680].
-
22.
Nwosu BU, Stavre ZG, Maranda L, Cullen K, Lee MM. Hepatic dysfunction is associated with vitamin D deficiency and poor glycemic control in diabetes mellitus. J Pediatr Endocrinol Metab. 2012;25(1-2):181-6. [PubMed ID: 22570973].
-
23.
Abascal K, Yarnell E. The many faces of silybum marianum (Milk Thistle): Part 1-Treating cancer and hyperlipidemia and restoring kidney function. Alternative Complementary Therapies. 2003;9(4):170-175.
-
24.
Soto C, Recoba R, Barrón H, Alvarez C, Favari L. Silymarin increases antioxidant enzymes in alloxan-induced diabetes in rat pancreas. Comp Biochem Physiol. 2003;136(3):205-212.
-
25.
Toklu HZ, Tunali-Akbay T, Erkanli G, Yuksel M, Ercan F, Sener G. Silymarin, the antioxidant component of Silybum marianum, protects against burn-induced oxidative skin injury. Burns. 2007;33(7):908-16. [PubMed ID: 17521818]. https://doi.org/10.1016/j.burns.2006.10.407.