Abstract
Background:
Non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) are major public health concerns. Besides the known risk factors, other risk factors, such as vitamin D deficiency, have been suggested for NAFLD.Objectives:
This cross-sectional research aimed to investigate the relationship between serum vitamin D levels and NAFLD in a group of patients with T2DM.Methods:
We investigated various clinical and biochemical parameters, including serum vitamin D level, liver function tests, and liver sonography in 1,110 adult patients with T2DM. The mean difference of numerical variables in NAFLD and non-NAFLD groups was analyzed with an independent sample t-test. Chi-square test was used to evaluate the association between two categorical variables.Results:
Out of 1,110 patients with T2DM, 837 (75.4%) had NAFLD. The mean vitamin D level in diabetic patients with NAFLD was significantly lower than non-NAFLD group (19.71 ng/mL vs. 27.68 ng/mL, respectively; P < 0.001). Furthermore, 410 (49%) patients with NAFLD were found with vitamin D deficiency, while this value was 85 (31.1%) in non-NAFLD group. According to the results of univariate logistic regression analysis, vitamin D deficiency (OR = 3.87) and insufficient vitamin D (OR = 2.83) were the significant variables for NAFLD.Conclusions:
There was a significant association between vitamin D deficiency and NAFLD in patients with T2DM.Keywords
Type 2 Diabetes Mellitus Non-alcoholic Fatty Liver Disease Vitamin D
1. Background
Non-alcoholic fatty liver disease (NAFLD) is defined as the aggregation of triglycerides within hepatocytes exceeding 5% of liver weight. It is not caused by excessive alcohol use or different steatosis sources (1). It encompasses a wide spectrum of liver pathologies, with NAFLD at one end of the spectrum followed by non-alcoholic steatohepatitis (NASH), liver cirrhosis, and hepatocellular carcinoma at the other end (2). In some patients, NAFLD progresses to end-stage liver disease, which has made NAFLD a major reason for morbidity and mortality over the last two decades; it is predicted that NAFLD will be the number one etiology of liver transplantation worldwide (3). Evidence suggests that advanced liver fibrosis can be caused by mild degrees of steatosis and inflammation of the liver (4). Today, many experts believe that a lot of patients diagnosed with cryptogenic cirrhosis have NAFLD/NASH as an underlying disease (5).
NAFLD and type 2 diabetes mellitus (T2DM) have been known as major public health concerns. The prevalence of NAFLD in Western countries is 46.2%, and it is the most common liver disease. Its prevalence in some specific groups, such as obese people and patients with T2DM, reaches 75 to 90%. In recent decades, the prevalence of NAFLD has increased along with obesity worldwide, reaching 46.2% in Europe, 33% in North America, and 31.8% in Asia. It is estimated that about one-fourth of the world’s population suffers from NAFLD. Also, the global prevalence of diabetes in 2019 was 9.3% (463 million people), which is expected to increase to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045. Notably, patients with NAFLD experience T2DM and vice versa. About 25% of patients with NAFLD and 50% of patients with NASH have T2DM, while NAFLD is reported in about 70% of patients with T2DM. These two conditions have mutual effects on each other (6-11). In patients with T2DM, NAFLD increases the risk of mortality. Also, the presence of T2DM causes a three-fold increase in the risk of progressive liver fibrosis and a two-fold increase in the risk of hepatocellular carcinoma. It is also an independent predictor of liver disease mortality and all-cause mortality (12).
Insulin resistance is a common hallmark of NAFLD and T2DM, and NAFLD is a hepatic component of metabolic syndrome (13). Metabolic diseases, such as hypertension, visceral obesity, and dyslipidemia are known risk factors for NAFLD (14). Besides the known risk factors, other risk factors, such as vitamin D deficiency, have been recently suggested for NAFLD. Previous research shows that vitamin D deficiency can enhance the risk of insulin resistance and metabolic syndrome (15). Vitamin D is involved in NAFLD by exerting anti-inflammatory and anti-fibrotic effects on liver cells. It exerts these effects through inflammatory cytokines, like tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β, as well as adipokines, like leptin and adiponectin (16). In addition, it has been shown that vitamin D can decrease the cytokeratin 18 apoptotic fragment M30 concentration as an indicator for liver damage (17). In patients with T2DM, vitamin D deficiency can reduce the expression of glucose transporters on the cell surface, reduce glucose export from the liver, and stimulate intrahepatic lipid synthesis, thereby contributing to the pathogenesis of NAFLD in these patients (18).
Low serum vitamin D levels are associated with NAFLD and are involved in its pathogenesis (19-22). However, this association has not been confirmed in all previous studies, and no relationship has been suggested between the serum vitamin D level and NAFLD in some studies (23, 24).
2. Objectives
This cross-sectional research aimed to investigate the relationship between serum vitamin D levels and NAFLD in a group of patients with T2DM.
3. Methods
The current cross-sectional research was performed on 1,110 patients with T2DM (age range: 31 - 75 years) referring to endocrine clinics of Zahedan, Iran, from March 2018 to August 2020. T2DM was diagnosed based on American Diabetes Association (ADA) criteria (24). A physician completed an information form, including the patient’s age, sex, duration of diabetes, co-morbidities, smoking, alcohol consumption, and drug history.
Participants with evidence of any chronic liver disease, such as autoimmune hepatitis, hemochromatosis, viral hepatitis, primary biliary cirrhosis, Wilson’s disease, and any evidence of liver cirrhosis were excluded from the study. Also, people with other types of diabetes, such as gestational DM, latent autoimmune diabetes of adults, and type 1 DM, were excluded from the study. We also excluded all individuals with acute infection, decreased renal function (eGFR < 60 ml/min/1.73 m2 or plasma creatinine > 2 mg/dL), malignancy, thyroid dysfunction, or alcohol consumption of any volume. Patients with a history of taking any supplements, including vitamin D, as well as pregnant and lactating women were also excluded from the study.
The patients’ height, weight, and blood pressure were evaluated. The weight was measured with minimal clothing by a digital scale, and height was measured while standing without shoes by a stadiometer. Body mass index (BMI) was determined using this formula: Weight (kg) divided by height (m2). Patients’ blood pressure was measured after 15 minutes of rest and before blood sampling using a manual sphygmomanometer.
For all subjects, liver ultrasonography was performed by a sonologist after 12 hours of fasting. Fatty liver was determined based on standard criteria, including liver illumination, variation between the liver and kidneys echogenicity, and the degree of ambiguity of blood vessels. Grading of fatty liver based on the amount of fat deposition in the liver was determined as follows: (1) Grade I – observable periportal and diaphragmatic echogenicity in association with increased liver echogenicity; (2) Grade II – non-observable periportal echogenicity in association with increased liver echogenicity without diaphragmatic ambiguity; and (3) Grade III - non-observable periportal echogenicity in association with increased liver echogenicity with diaphragmatic ambiguity (25). NAFLD was diagnosed according to the American Gastroenterological Association criteria as follows: (1) presence of hepatic steatosis on imaging or histology; (2) no excessive use of alcohol; (3) no other reasons for hepatic steatosis, and (4) no other synchronic reason for chronic liver disease (26).
Fasting venous blood was collected for measurement of the glycemic profile, thyroid function tests, and other biochemical tests. Blood sampling was done between 8, and 9 am following 12 hours of fasting. Plasma glucose was measured with the glucose oxidase method. Measurement of glycated hemoglobin (HbA1c) was carried out using high-performance liquid chromatography (HPLC). Lipids were measured using enzymatic colorimetric tests. Blood urea nitrogen (BUN), creatinine, and liver function tests were assessed by enzymatic colorimetric assays. Serological tests for hepatitis B and C rejection were performed in patients with elevated liver enzymes. The normal AST and ALT were defined as less than 40 u/L. The 25-hydroxyvitamin D (25(OH)D) test was performed using the enzyme immunoassay method. Values less than 20 ng/mL were considered as vitamin D deficiency and values of 20 to 30 ng/mL were regarded as insufficiency (27).
All experiments were performed according to the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments. The study protocol was approved by the Ethics Committee for Human Studies at Zahedan University of Medical Sciences. Informed consent was obtained from all participants.
3.1. Statistical Analysis
Continuous and categorical data are presented as mean ± standard deviation (SD) and frequency (percentage), respectively. Also, we presented the data with histogram and box plot, as appropriate. The normality of variables was assessed with Shapiro–Wilk test and graphical approaches. The mean difference of numerical variable in NAFLD and non-NAFLD groups was analyzed using the independent sample t-test. Chi-square test was used to evaluate the association between two categorical variables. The association between independent factors and NAFLD was assessed using univariate and multivariate logistic regression models. The multivariate logistic regression model was conducted based on the backward stepwise method. A P-value less than 0.05 was considered a significant difference. Data analysis was conducted using Stata software (Release 14. College Station, TX: StataCorp LP).
4. Results
In this study, out of 1,110 patients with T2DM, 837 (75.4%) subjects had NAFLD. Among the patients, 64.9% were females, and gender distribution was not significantly different in patients with and without NAFLD (P = 0.375). The mean age in NAFLD group was significantly higher than non-NAFLD group (54.12 vs. 49.41 years, respectively; P < 0.001). The mean duration of diabetes in patients with NAFLD (10.63 years) was almost twice that of patients without NAFLD (5.84 years), indicating a statistically significant difference (P < 0.001). A comparison of other clinical and laboratory characteristics between the two groups is shown in Table 1.
Clinical and Laboratory Characteristics of Patients with T2DM in NAFLD and Non-NAFLD Groups a, b
| Variables | All (n = 1110) | NAFLD Status | P-Value | |
|---|---|---|---|---|
| NAFLD (n = 837) | Non-NAFLD (n = 273) | |||
| Age (y) | 52.96 ± 10.46 | 54.12 ± 11.22 | 49.41 ± 6.54 | < 0.001 |
| Sex, female | 720 (64.9) | 549 (65.6) | 171 (62.6) | 0.375 |
| Diabetes duration (y) | 9.45 ± 4.24 | 10.63 ± 4.15 | 5.84 ± 1.66 | < 0.001 |
| Positive family history of DM | 780 (70.3) | 636 (76.0) | 144 (52.7) | < 0.001 |
| Use of antihypertensive drug | 601 (54.1) | 492 (58.8) | 109 (39.9) | < 0.001 |
| Use of statin | 939 (84.6) | 711 (84.9) | 228 (83.5) | 0.570 |
| Use of OHA | 644 (58.0) | 449 (53.6) | 195 (71.4) | < 0.001 |
| Use of insulin | 364 (32.8) | 334 (39.9) | 30 (11.0) | < 0.001 |
| BMI (kg/m2) | 27.07 ± 2.93 | 27.39 ± 2.93 | 26.06 ± 2.71 | < 0.001 |
| Systolic blood pressure (mmHg) | 133.26 ± 10.90 | 133.52 ± 11.29 | 132.46 ± 9.57 | 0.131 |
| Diastolic blood pressure (mmHg) | 81.58 ± 9.43 | 81.43 ± 9.68 | 82.05 ± 8.63 | 0.314 |
| Hypertension, BP ≥ 140/90 (%) | 507 (45.7) | 379 (45.3) | 128 (46.9) | 0.644 |
| Fasting plasma glucose (mg/dL) | 163.68 ± 27.50 | 167.72 ± 28.05 | 151.29 ± 21.49 | < 0.001 |
| HbA1c (%) | 8.37 ± 0.94 | 8.63 ± 0.89 | 7.56 ± 0.53 | < 0.001 |
| Total cholesterol (mg/dL) | 185.24 ± 46.96 | 188.48 ± 49.0 | 175.29 ± 38.47 | < 0.001 |
| Triglycerides (mg/dL) | 119.07 ± 62.98 | 119.47 ± 63.99 | 117.84 ± 59.85 | 0.702 |
| LDL cholesterol (mg/dL) | 119.44 ± 44.34 | 122.28 ± 46.41 | 110.75 ± 35.98 | < 0.001 |
| HDL cholesterol (mg/dL) | 45.05 ± 10.08 | 45.67 ± 10.11 | 43.13 ± 9.74 | < 0.001 |
| VLDL (mg/dL) | 23.68 ± 12.69 | 23.81 ± 12.94 | 23.30 ± 11.91 | 0.559 |
| Blood urea nitrogen (mg/dL) | 14.62 ± 3.51 | 14.60 ± 3.55 | 14.70 ± 3.40 | 0.698 |
| Creatinine (mg/dL) | 1.11 ± 0.17 | 1.11 ± 0.17 | 1.10 ± 0.17 | 0.625 |
| ALT (IU/L) | 36.06 ± 13.20 | 38.81 ± 12.99 | 27.63 ± 9.86 | < 0.001 |
| AST (IU/L) | 32.49 ± 12.72 | 35.06 ± 12.55 | 24.59 ± 9.60 | < 0.001 |
| Alk.ph (IU/L) | 108.46 ± 21.59 | 108.91 ± 21.43 | 107.07 ± 22.05 | 0.221 |
| Vit-D (ng/mL) | 21.67 ± 12.78 | 19.71 ± 11.97 | 27.68 ± 13.36 | < 0.001 |
| 25-OH vitamin D status | < 0.001 | |||
| Vit-D < 20 (deficiency) | 495 (44.6) | 410 (49.0) | 85 (31.1) | |
| Vit-D: 20 - 30 (insufficiency) | 377 (34.0) | 294 (35.1) | 83 (30.4) | |
| Vit-D ≥ 30 (normal) | 236 (21.3) | 131 (15.7) | 105 (38.5) | |
| Season of blood sampling | 0.934 | |||
| Spring | 306 (27.6) | 232 (27.7) | 74 (27.1) | |
| Summer | 257 (23.2) | 191 (22.8) | 66 (24.2) | |
| Autumn | 224 (20.2) | 172 (20.5) | 52 (19.0) | |
| Winter | 321 (28.9) | 241 (28.8) | 80 (29.3) | |
| Vit-D by season of blood sampling | ||||
| Spring | 22.99 ± 14.84 | 21.01 ± 13.57 | 29.19 ± 16.89 | < 0.001 |
| Summer | 21.59 ± 11.55 | 19.26 ± 10.62 | 28.33 ± 11.55 | < 0.001 |
| Autumn | 21.57 ± 13.22 | 19.15 ± 12.57 | 29.56 ± 12.23 | < 0.001 |
| Winter | 20.49 ± 11.20 | 19.15 ± 10.83 | 24.51 ± 11.37 | < 0.001 |
The mean vitamin D level in NAFLD group was significantly lower than non-NAFLD group (19.71 ng/mL vs. 27.68 ng/mL, respectively; P < 0.001). According to the 25-OH vitamin D status classification, 410 (49%) patients with NAFLD had vitamin D deficiency, while this value was 85 (31.1%) for patients without NAFLD (Figure 1). There was no statistically significant difference in the blood sampling seasonal distribution between the two groups (P = 0.934).
Vit-D distribution in NAFLD and non-NAFLD patients
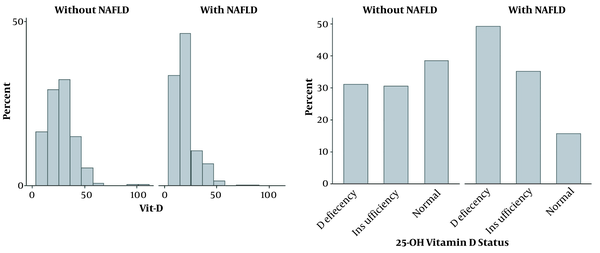
In univariate logistic regression analysis, HbA1c with an odds ratio (OR) of 8.51 and history of insulin use (OR = 5.35) showed the highest OR for NAFLD. Also, duration of diabetes (OR = 2.24), family history of diabetes (OR = 2.84), history of taking the antihypertensive drug (OR = 2.15), vitamin D deficiency compared to normal vitamin D (OR = 3.87), and insufficient vitamin D compared with normal vitamin D (OR = 2.83) were the significant variables with OR > 2 for NAFLD (Table 2).
Univariate Logistic Regression Analysis of NAFLD in Participants
| Variables | OR (95% CI) | P-Value |
|---|---|---|
| Age (y) | 1.05 (1.03 to 1.06) | < 0.001 |
| Sex, female | 1.14 (0.856 to 1.51) | 0.375 |
| Diabetes duration | 2.24 (2.0 to 2.51) | < 0.001 |
| Positive family history of DM | 2.84 (2.13 to 3.77) | < 0.001 |
| Use of antihypertensive drug | 2.15 (1.62 to 2.84) | < 0.001 |
| Use of statin | 1.11 (0.768 to 1.62) | 0.570 |
| Use of OHA | 0.463 (0.344 to 0.622) | < 0.001 |
| Use of insulin | 5.38 (3.59 to 8.05) | < 0.001 |
| BMI | 1.17 (1.12 to 1.23) | < 0.001 |
| Systolic blood pressure | 1.01 (0.996 to 1.02) | 0.164 |
| Diastolic blood pressure | 0.993 (0.979 to 1.01) | 0.342 |
| Hypertension, BP ≥ 140/90 | 0.937 (0.713 to 1.23) | 0.644 |
| Fasting plasma glucose | 1.02 (1.02 to 1.03) | < 0.001 |
| HbA1c | 8.51 (6.40 to 11.33) | < 0.001 |
| Total cholesterol | 1.01 (1.0 to 1.01) | < 0.001 |
| Triglycerides | 1.0 (0.998 to 1.0) | 0.711 |
| LDL cholesterol | 1.01 (1.0 to 1.01) | < 0.001 |
| HDL cholesterol | 1.03 (1.01 to 1.04) | < 0.001 |
| VLDL | 1.0 (0.992 to 1.01) | 0.559 |
| Blood urea nitrogen | 0.992 (0.954 to 1.03) | 0.698 |
| Creatinine | 1.22 (0.553 to 2.68) | 0.625 |
| ALT | 1.11 (1.09 to 1.12) | < 0.001 |
| AST | 1.10 (1.08 to 1.12) | < 0.001 |
| ALK.ph | 1.0 (0.998 to 1.01) | 0.221 |
| Serum vitamin-D level | 0.953 (0.942 to 0.964) | < 0.001 |
| Vitamin D deficiency vs. normal | 3.87 (2.73 to 5.47) | < 0.001 |
| Vitamin D insufficiency vs. normal | 2.83 (1.99 to 4.03) | < 0.001 |
According to the multivariate logistic model, after eliminating the confounding effect of other variables, the chance of developing NAFLD in patients with vitamin D deficiency was 3.15 times higher than patients with normal vitamin D levels. In the multivariate model, the history of insulin consumption (OR = 20.3), HbA1c (OR = 11.76), and the duration of diabetes (OR = 2.92) were the most important variables for NAFLD (Table 3).
The Results of Multivariate Logistic Regression Related to NAFLD in Participants
| Variables | OR (95% CI) | P-Value |
|---|---|---|
| Model 1: Step 1 of Backward Stepwise | ||
| Age (y) | 1.07 (1.03 to 1.11) | < 0.001 |
| Diabetes duration | 2.91 (2.35 to 3.61) | < 0.001 |
| Positive family history of DM | 1.96 (1.04 to 3.69) | 0.038 |
| Use of antihypertensive drug | 9.15 (4.44 to 18.86) | < 0.001 |
| Use of insulin | 20.18 (8.74 to 46.61) | < 0.001 |
| BMI | 1.30 (1.15 to 1.46) | < 0.001 |
| HbA1c | 11.79 (6.64 to 20.92) | < 0.001 |
| Total cholesterol | 1.0 (0.986 to 1.02) | 0.724 |
| LDL cholesterol | 1.01 (0.988 to 1.03) | 0.521 |
| HDL cholesterol | 0.998 (0.996 to 1.03) | 0.911 |
| ALT | 1.14 (1.05 to 1.23) | 0.001 |
| AST | 0.990 (0.916 to 1.07) | 0.792 |
| Vitamin D deficiency vs. normal | 3.12 (1.36 to 7.15) | 0.011 |
| Vitamin D insufficiency vs. normal | 2.82 (1.26 to 6.27) | 0.007 |
| Model 2: Final Step of Backward Stepwise | ||
| Age | 1.07 (1.03 to 1.11) | < 0.001 |
| Diabetes duration | 2.92 (2.36 to 3.61) | < 0.001 |
| Positive family history of DM | 1.96 (1.05 to 3.69) | 0.036 |
| Use of insulin | 20.30 (8.80 to 46.82) | < 0.001 |
| BMI | 1.30 (1.15 to 1.46) | < 0.001 |
| HbA1c | 11.76 (6.64 to 20.83) | < 0.001 |
| LDL cholesterol | 1.01 (1.0 to 1.02) | 0.029 |
| ALT | 1.13 (1.09 to 1.17) | < 0.001 |
| Vitamin D deficiency vs normal | 3.15 (1.38 to 7.19) | 0.011 |
| Vitamin D insufficiency vs normal | 2.82 (1.27 to 6.28) | 0.006 |
5. Discussion
According to our results, the serum vitamin D level was lower in diabetic patients with NAFLD compared to those in non-NAFLD group. Also, vitamin D deficiency was associated with NAFLD in these patients.
These findings are consistent with some previous studies, indicating that the serum vitamin D level was lower in diabetic cases with NAFLD (28, 29). In this regard, a study by Rhee et al. showed that the serum vitamin D levels were lower in NAFLD patients compared with the control group (30). Another study showed that the serum vitamin D levels were significantly lower in patients diagnosed with NAFLD considering liver biopsy than in the control group (31). However, some studies have reported different results regarding the relationship between vitamin D levels and NAFLD. Two different studies carried out in China (32) and Korea (33) reported no significant difference between patients with and without NAFLD regarding the serum vitamin D level.
The discrepancy between the results of different studies can be attributed to factors such as different methods and designs, different criteria for NAFLD diagnosis, different definitions for vitamin D deficiency, lack of matched study groups for interfering factors (such as BMI), and selection bias in cross-sectional studies. Also, genetic factors, such as polymorphisms in vitamin D receptor genes, may be involved. Therefore, vitamin D may affect the evolution and advancement of NAFLD only in subjects with specific genotypes (34).
Previous studies have shown that vitamin D considerably affects immune system regulation, cell differentiation, regulation of cell proliferation, and inflammatory processes. Vitamin D can improve insulin secretion and reduce insulin resistance and liver fibrosis. Through these mechanisms, which are mediated by cytokines and adipokines, vitamin D may contribute to the evolution and advancement of NAFLD (35). Numerous studies have shown that markers of inflammation, such as CRP, TNF-α, and IL-6 are possibly associated with the pathogenesis of NAFLD (36). Elevated serum TNF-α levels have been associated with the increased risk of NAFLD in healthy non-diabetic individuals (36). Moreover, a direct relationship has been found between the increased serum levels of inflammatory markers and NAFLD severity (37). Overall, vitamin D can reduce inflammation in various ways (38). Therefore, it can be proposed that vitamin D reduces the severity of NAFLD, and its deficiency is associated with the exacerbation of NAFLD.
In patients with NAFLD, insulin sensitivity is reduced in the muscles, fat, and liver (39). Vitamin D increases insulin sensitivity by increasing the number of insulin receptors in myocytes, increasing insulin sensitivity in insulin receptors, and affecting peroxisome proliferator-activated receptor delta (PPAR-δ) (40). On the other hand, during oxidative stress, an elevation in reactive oxygen species (ROS) and lipid peroxidation occurs, which ultimately leads to intracellular damage (41). The concentrations of lipid peroxidation biomarkers are correlated with the severity of liver disease (42). According to previous research, vitamin D deficiency increases the concentration of oxidative stress biomarkers, and vitamin D intake reduces the concentration of these biomarkers (43). Also, abnormal lipid metabolism results in fat accumulation in the liver, which in turn increases the production of various adipokines, inflammation, and oxidative stress, all of which play an important role in the NAFLD pathogenesis (44).
This research had some limitations. First, it was a cross-sectional research, in which a cause-and-effect relationship could not be indicated between vitamin D deficiency and NAFLD. Second, no liver biopsy was performed in this study. Generally, liver biopsy is the gold standard technique to diagnose NAFLD and differentiate it from NASH. However, considering the aggressiveness of liver biopsy, ultrasound has been used to diagnose NAFLD in previous studies. Ultrasound sensitivity for the diagnosis of NAFLD ranges from 60 to 94%, depending on the severity of steatosis.
On the other hand, since ultrasound is an operator-dependent method, all liver ultrasounds were performed by an experienced radiologist in this study, which is one of its main strengths. Also, elimination of other reasons for chronic liver disease and relatively acceptable sample size are other strengths of this study.
In summary, vitamin D status is associated with the presence of NAFLD in T2DM patients. However, large-scale prospective studies are needed to demonstrate this association and suggest vitamin D deficiency as a risk factor for NAFLD in diabetic patients. Further investigation is warranted to examine the effect of vitamin D supplementation on liver steatosis status in these cases.
Acknowledgements
References
-
1.
Zhu JZ, Hollis-Hansen K, Wan XY, Fei SJ, Pang XL, Meng FD, et al. Clinical guidelines of non-alcoholic fatty liver disease: A systematic review. World J Gastroenterol. 2016;22(36):8226-33. [PubMed ID: 27688665]. [PubMed Central ID: PMC5037092]. https://doi.org/10.3748/wjg.v22.i36.8226.
-
2.
Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology. 2010;51(5):1820-32. [PubMed ID: 20432259]. https://doi.org/10.1002/hep.23594.
-
3.
Ray K. NAFLD-the next global epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):621. [PubMed ID: 24185985]. https://doi.org/10.1038/nrgastro.2013.197.
-
4.
McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148-55. [PubMed ID: 25477264]. https://doi.org/10.1016/j.jhep.2014.11.034.
-
5.
Clark JM, Diehl AM. Nonalcoholic fatty liver disease: An underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289(22):3000-4. [PubMed ID: 12799409]. https://doi.org/10.1001/jama.289.22.3000.
-
6.
Samji NS, Verma R, Satapathy SK. Magnitude of Nonalcoholic Fatty Liver Disease: Western Perspective. J Clin Exp Hepatol. 2019;9(4):497-505. [PubMed ID: 31516266]. [PubMed Central ID: PMC6728535]. https://doi.org/10.1016/j.jceh.2019.05.001.
-
7.
Andronescu CI, Purcarea MR, Babes PA. Nonalcoholic fatty liver disease: epidemiology, pathogenesis and therapeutic implications. J Med Life. 2018;11(1):20-3. [PubMed ID: 29696060]. [PubMed Central ID: PMC5909941].
-
8.
Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23(47):8263-76. [PubMed ID: 29307986]. [PubMed Central ID: PMC5743497]. https://doi.org/10.3748/wjg.v23.i47.8263.
-
9.
Iqbal U, Perumpail BJ, Akhtar D, Kim D, Ahmed A. The Epidemiology, Risk Profiling and Diagnostic Challenges of Nonalcoholic Fatty Liver Disease. Medicines (Basel). 2019;6(1). [PubMed ID: 30889791]. [PubMed Central ID: PMC6473603]. https://doi.org/10.3390/medicines6010041.
-
10.
Duseja A, Dhiman RK, Premkumar M. Nonalcoholic Fatty Liver Disease: Lessons Learnt in the Last Five Years. J Clin Exp Hepatol. 2021;11(2):159-62. [PubMed ID: 33746439]. [PubMed Central ID: PMC7953007]. https://doi.org/10.1016/j.jceh.2020.07.008.
-
11.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [PubMed ID: 31518657]. https://doi.org/10.1016/j.diabres.2019.107843.
-
12.
Doycheva I, Zhang T, Amjad W, Thuluvath PJ. Diabetes and Hepatocellular Carcinoma: Incidence Trends and Impact of Liver Disease Etiology. J Clin Exp Hepatol. 2020;10(4):296-303. [PubMed ID: 32655232]. [PubMed Central ID: PMC7335702]. https://doi.org/10.1016/j.jceh.2019.11.004.
-
13.
Sayki Arslan M, Turhan S, Dincer I, Mizrak D, Corapcioglu D, Idilman R. A potential link between endothelial function, cardiovascular risk, and metabolic syndrome in patients with Non-alcoholic fatty liver disease. Diabetol Metab Syndr. 2014;6:109. [PubMed ID: 25960770]. [PubMed Central ID: PMC4424578]. https://doi.org/10.1186/1758-5996-6-109.
-
14.
Wang XJ, Malhi H. Nonalcoholic Fatty Liver Disease. Ann Intern Med. 2018;169(9):ITC65-80. [PubMed ID: 30398639]. https://doi.org/10.7326/AITC201811060.
-
15.
Chen LW, Chien CY, Hsieh CW, Chang LC, Huang MH, Huang WY, et al. The Associations Between Helicobacter pylori Infection, Serum Vitamin D, and Metabolic Syndrome: A Community-Based Study. Medicine (Baltimore). 2016;95(18). e3616. [PubMed ID: 27149497]. [PubMed Central ID: PMC4863814]. https://doi.org/10.1097/MD.0000000000003616.
-
16.
Apostolakis M, Armeni E, Bakas P, Lambrinoudaki I. Vitamin D and cardiovascular disease. Maturitas. 2018;115:1-22. [PubMed ID: 30049340]. https://doi.org/10.1016/j.maturitas.2018.05.010.
-
17.
Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: A multicenter validation study. Hepatology. 2009;50(4):1072-8. [PubMed ID: 19585618]. [PubMed Central ID: PMC2757511]. https://doi.org/10.1002/hep.23050.
-
18.
Grammatiki M, Rapti E, Karras S, Ajjan RA, Kotsa K. Vitamin D and diabetes mellitus: Causal or casual association? Rev Endocr Metab Disord. 2017;18(2):227-41. [PubMed ID: 28062940]. https://doi.org/10.1007/s11154-016-9403-y.
-
19.
Chung GE, Kim D, Kwak MS, Yang JI, Yim JY, Lim SH, et al. The serum vitamin D level is inversely correlated with nonalcoholic fatty liver disease. Clin Mol Hepatol. 2016;22(1):146-51. [PubMed ID: 27044765]. [PubMed Central ID: PMC4825160]. https://doi.org/10.3350/cmh.2016.22.1.146.
-
20.
Nelson JE, Roth CL, Wilson LA, Yates KP, Aouizerat B, Morgan-Stevenson V, et al. Vitamin D Deficiency Is Associated With Increased Risk of Non-alcoholic Steatohepatitis in Adults With Non-alcoholic Fatty Liver Disease: Possible Role for MAPK and NF-kappaB? Am J Gastroenterol. 2016;111(6):852-63. [PubMed ID: 27002799]. [PubMed Central ID: PMC5361650]. https://doi.org/10.1038/ajg.2016.51.
-
21.
Dasarathy J, Periyalwar P, Allampati S, Bhinder V, Hawkins C, Brandt P, et al. Hypovitaminosis D is associated with increased whole body fat mass and greater severity of non-alcoholic fatty liver disease. Liver Int. 2014;34(6):e118-27. [PubMed ID: 24118743]. [PubMed Central ID: PMC4012003]. https://doi.org/10.1111/liv.12312.
-
22.
Targher G, Scorletti E, Mantovani A, Byrne CD. Nonalcoholic fatty liver disease and reduced serum vitamin D(3) levels. Metab Syndr Relat Disord. 2013;11(4):217-28. [PubMed ID: 23745619]. https://doi.org/10.1089/met.2013.0044.
-
23.
Patel YA, Henao R, Moylan CA, Guy CD, Piercy DL, Diehl AM, et al. Vitamin D is Not Associated With Severity in NAFLD: Results of a Paired Clinical and Gene Expression Profile Analysis. Am J Gastroenterol. 2016;111(11):1591-8. [PubMed ID: 27644736]. [PubMed Central ID: PMC5331905]. https://doi.org/10.1038/ajg.2016.406.
-
24.
Bril F, Maximos M, Portillo-Sanchez P, Biernacki D, Lomonaco R, Subbarayan S, et al. Relationship of vitamin D with insulin resistance and disease severity in non-alcoholic steatohepatitis. J Hepatol. 2015;62(2):405-11. [PubMed ID: 25195551]. https://doi.org/10.1016/j.jhep.2014.08.040.
-
25.
Tchelepi H, Ralls PW, Radin R, Grant E. Sonography of diffuse liver disease. J Ultrasound Med. 2002;21(9):1023-32. quiz 1033-4. [PubMed ID: 12216750]. https://doi.org/10.7863/jum.2002.21.9.1023.
-
26.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107(6):811-26. [PubMed ID: 22641309]. https://doi.org/10.1038/ajg.2012.128.
-
27.
Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18(2):153-65. [PubMed ID: 28516265]. https://doi.org/10.1007/s11154-017-9424-1.
-
28.
Wang JM, Ye SD, Li SM, Hu W. Correlations of 25(OH)D level with blood lipid, inflammatory factors and vascular endothelial function in diabetic patients. Eur Rev Med Pharmacol Sci. 2018;22(3):731-5. [PubMed ID: 29461603]. https://doi.org/10.26355/eurrev_201802_14303.
-
29.
Hosny SS, Ali HM, Mohammed WA, El Ghannam MH. Study of relationship between total vitamin D level and NAFLD in a sample of Egyptian patients with and without T2DM. Diabetes Metab Syndr. 2019;13(3):1769-71. [PubMed ID: 31235092]. https://doi.org/10.1016/j.dsx.2019.04.002.
-
30.
Rhee EJ, Kim MK, Park SE, Park CY, Baek KH, Lee WY, et al. High serum vitamin D levels reduce the risk for nonalcoholic fatty liver disease in healthy men independent of metabolic syndrome. Endocr J. 2013;60(6):743-52. [PubMed ID: 23411507]. https://doi.org/10.1507/endocrj.ej12-0387.
-
31.
Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17(7):517-24. [PubMed ID: 16928437]. https://doi.org/10.1016/j.numecd.2006.04.002.
-
32.
Li L, Zhang L, Pan S, Wu X, Yin X. No significant association between vitamin D and nonalcoholic fatty liver disease in a Chinese population. Dig Dis Sci. 2013;58(8):2376-82. [PubMed ID: 23589141]. https://doi.org/10.1007/s10620-013-2658-1.
-
33.
Hong HC, Lee JS, Choi HY, Yang SJ, Yoo HJ, Seo JA, et al. Liver enzymes and vitamin D levels in metabolically healthy but obese individuals: Korean National Health and Nutrition Examination Survey. Metabolism. 2013;62(9):1305-12. [PubMed ID: 23643404]. https://doi.org/10.1016/j.metabol.2013.04.002.
-
34.
Grunhage F, Hochrath K, Krawczyk M, Hoblinger A, Obermayer-Pietsch B, Geisel J, et al. Common genetic variation in vitamin D metabolism is associated with liver stiffness. Hepatology. 2012;56(5):1883-91. [PubMed ID: 22576297]. https://doi.org/10.1002/hep.25830.
-
35.
Eliades M, Spyrou E. Vitamin D: A new player in non-alcoholic fatty liver disease? World J Gastroenterol. 2015;21(6):1718-27. [PubMed ID: 25684936]. [PubMed Central ID: PMC4323447]. https://doi.org/10.3748/wjg.v21.i6.1718.
-
36.
Seo YY, Cho YK, Bae JC, Seo MH, Park SE, Rhee EJ, et al. Tumor Necrosis Factor-alpha as a Predictor for the Development of Nonalcoholic Fatty Liver Disease: A 4-Year Follow-Up Study. Endocrinol Metab (Seoul). 2013;28(1):41-5. [PubMed ID: 24396649]. [PubMed Central ID: PMC3811803]. https://doi.org/10.3803/EnM.2013.28.1.41.
-
37.
Manco M, Marcellini M, Giannone G, Nobili V. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;127(6):954-60. [PubMed ID: 17509993]. https://doi.org/10.1309/6VJ4DWGYDU0XYJ8Q.
-
38.
Karkeni E, Bonnet L, Marcotorchino J, Tourniaire F, Astier J, Ye J, et al. Vitamin D limits inflammation-linked microRNA expression in adipocytes in vitro and in vivo: A new mechanism for the regulation of inflammation by vitamin D. Epigenetics. 2018;13(2):156-62. [PubMed ID: 28055298]. [PubMed Central ID: PMC5873365]. https://doi.org/10.1080/15592294.2016.1276681.
-
39.
Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55(5):1389-97. [PubMed ID: 22183689]. https://doi.org/10.1002/hep.25539.
-
40.
Pinelli NR, Jaber LA, Brown MB, Herman WH. Serum 25-hydroxy vitamin d and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care. 2010;33(6):1373-5. [PubMed ID: 20332348]. [PubMed Central ID: PMC2875457]. https://doi.org/10.2337/dc09-2199.
-
41.
Spahis S, Delvin E, Borys JM, Levy E. Oxidative Stress as a Critical Factor in Nonalcoholic Fatty Liver Disease Pathogenesis. Antioxid Redox Signal. 2017;26(10):519-41. [PubMed ID: 27452109]. https://doi.org/10.1089/ars.2016.6776.
-
42.
Liu S, Shi W, Li G, Jin B, Chen Y, Hu H, et al. Plasma reactive carbonyl species levels and risk of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2011;26(6):1010-5. [PubMed ID: 21265881]. https://doi.org/10.1111/j.1440-1746.2011.06672.x.
-
43.
Tamadon MR, Soleimani A, Keneshlou F, Mojarrad MZ, Bahmani F, Naseri A, et al. Clinical Trial on the Effects of Vitamin D Supplementation on Metabolic Profiles in Diabetic Hemodialysis. Horm Metab Res. 2018;50(1):50-5. [PubMed ID: 28958110]. https://doi.org/10.1055/s-0043-119221.
-
44.
Polyzos SA, Kountouras J, Mantzoros CS. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1062-79. [PubMed ID: 26725002]. https://doi.org/10.1016/j.metabol.2015.11.006.