Abstract
Keywords
Treatment OAB (Overactive Blader) OABSS (Overactive Bladder Symptoms Scale) TTNS (Transcutaneous Posterior Tibial Nerve Stimulation)
1. Introduction
The Overactive Bladder (OAB) is a common disorder (14%-16%) (1), in which there is an urgent repetitive need to urinate with or without incontinency and probable nocturia. This condition can not only cause psychological problems but also impose economic costs and reduce the quality of life (2) The treatment frontline of OAB involves antimuscarinics medications and behavioral therapy. There are limitations to using drugs for the treatment of OAB due to the side effects, contraindications, and inadequate response (3-5). The second line of treatment currently involves the Sacral Nerve Stimulation (SNS) neuromodulation techniques and percutaneous or transcutaneous posterior tibial nerve stimulation (PTNS or TTNS) (6). In SNS neuromodulation, a stimulator is placed in the sacral plexus. However, this method is more successful than PTNS (61%-90% versus 54%-79%) (7), it has more side effects than PTNS and is more expensive. Besides, there might be a need to repeat the surgery. However, PTNS is available with minimum invasion and at a lower cost. Besides, it is more effective than anticholinergic drugs (8). However, the use of the TTNS method has also had positive effects and used noninvasively comparing with PTNS (9, 10). The effectiveness of these methods in the short run has been confirmed (9-11). The following different names are used to describe the types of electrical stimulation of the posterior tibial nerve: Percutaneous Tibial Nerve Stimulation (PTNS) - Percutaneous Posterior Tibial Nerve Stimulation (PPTNS) - peripheral Tibial Nerve Stimulation (PTNS) - Transcutaneous Tibial Nerve Stimulation (TTNS) – Transcutaneous Posterior Tibial Nerve Stimulation (TPTNS). The authors recommend the use of the word Transcutaneous Tibial Nerve Stimulation (TTNS) to stimulate the posterior tibial nerve using superficial skin electrodes electrically, and the word Percutaneous Tibial Nerve Stimulation (PTNS) to use electrical stimulation of the posterior tibial nerve using needles, which will save the reader from confusion. In this study, the word TTNS is used for posterior tibial nerve stimulation by superficial patch electrodes.
2. Case Presentation
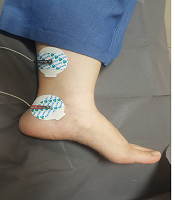
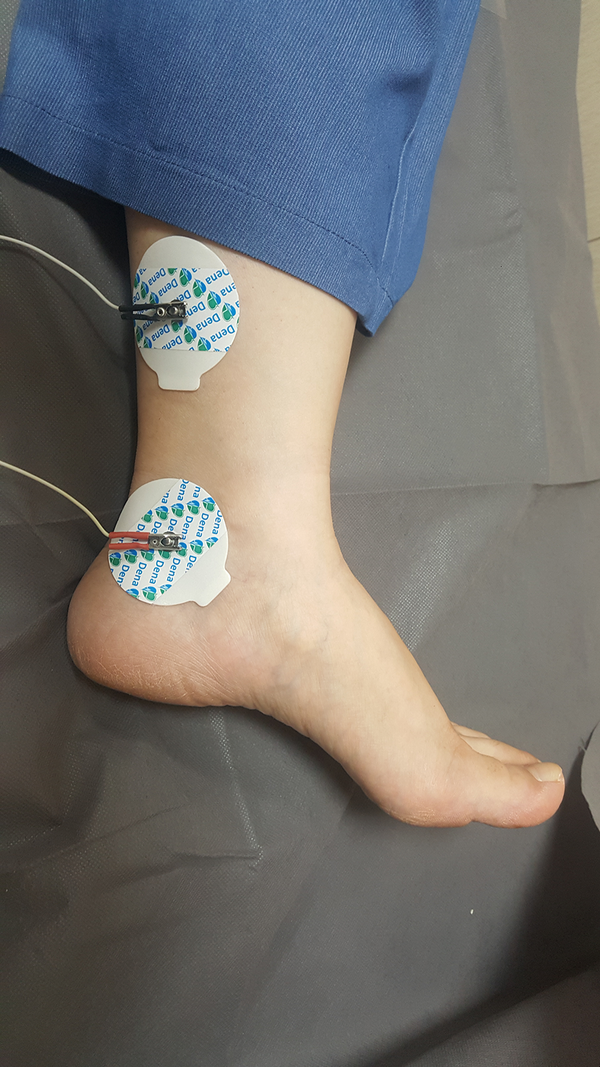
The patient is a 7-year-old girl who visited in Mobasher Physiotherapy Clinic at the Rehabilitation Sciences Faculty of Hamedan University of Medical Sciences in date 31 August 2019 with complaints about frequent urination and nocturnal enuresis. According to the reports by the patient's mother, the patient was unable to attend school as she needed to go to the bathroom repeatedly and was under mental pressure. Urodynamic tests are usually used to evaluate OAB patients, which are invasive, time-consuming, require prior preparation, and the patient should not have an infection. Using these tests in children is much more complicated as it is associated with the non-acceptance of the child. In the previous assessments of this child, the relevant doctor used an urodynamic assessment once a year ago, causing the child to be upset and have terrible psychological effects. The second time the doctor requested the test, the child's parents did not consent. The present study has used the Persian version of the overactive bladder symptoms scale (OABSS) for the first time to assess the symptoms following the use of electrical stimulation. This questionnaire is practical and quite easy to complete for patients and interpreted easily by the therapist (12). The patient completed OABSS and 24 Voiding Diary questionnaires before, immediately after, and one month after the TTNS treatment. The Persian version of the OABS questionnaire is a valid and repeatable questionnaire (13), consisting of four questions. Each question may have a score between 0 and 3-5. The total score is calculated to obtain a total score. A total score greater than or equal to 12 represents the severe stage, while a score smaller than or equal to 11 shows the moderate stage. A score smaller than or equal to five also refers to the mild stage. The degree of the OABS questionnaire before the treatment equaled 15, which was in the severe range. Regarding the Voiding Diary, the patient felt an urgent need seven times, went to the bathroom 26 times, and needed to change her underwear nine times within 24 hours. The patient was exposed to the TTNS treatment for 12 sessions on alternate days. In treatment with TTNS, two patch electrodes were used instead of needle electrodes for patient comfort. Transcutaneous stimulation was carried out at the 20Hz frequency, 200 microseconds duration by stimulation device (610p model, Novin Co, Iran), and a treatment period of 30 minutes (14). The current intensity was increased as much as the patient satisfaction was obtained, and we increased the current intensity after the adaptation phenomenon. One of the electrodes was beneath the inner malleoli, and the other was placed 4cm higher in proximal (Figure 1). The OABS and Voiding Diary questionnaires were completed immediately after twelve sessions of treatment. The score of the OABS questionnaire of the patient was eleven after the treatment, which was in the average range. Regarding the Voiding Diary, the patient felt an urgent need to urinate three times, twelve times went to the bathroom and did not need to change underwear within 24 hours. In the re-analysis carried out one month after the end of the treatment, the patient’s score on the OABS questionnaire was five, falling into the mild range. Regarding the Voiding Diary, the urgent need to urinate was felt five times, the patient went to the bathroom seventeen times, and the patient needed to change underwear four times within 24 hours. Using these electrodes can be recommended for the TTNS treatment.
Electrode placement
3. Discussion
Previous studies revealed the positive effect of PTNS or TTNS on overactive bladder patients with neurogenic or non-neurogenic origins due to the resulting neuromodulation (14-21). Neuromodulation is an approved method for treating patients with pelvic floor disorders. The exact mechanism of action of PTNS or TTNS in neuromodulation is not clear. However, the output of the efferent pathways to the lower urinary tract is modulated by the stimulation of the afferent pathways of the sacral roots (22). The present study also confirms the positive effects of treatment in line with the previous studies. In the PTNS method, at least one needle electrode is used to stimulate the posterior tibial nerve, which increases the invasiveness of the treatment. This needle can be a cause of anxiety and distress in these patients, especially children. A recent study showed that there was no difference between the advantages of TTNS and needle using in PTNS for adults with OAB (23). In the present study, the patient was treated using patch electrodes, fixated easily and noninvasively on the patient's skin, and was connected to the device via alligator connectors. The advantages of the patch electrode include easier stabilization and localization of stimulation compared to the plate electrode. Hence, using these electrodes can be recommended for TTNS treatment based on the positive results of this study.
Another finding was the increasing improvement within one month following the treatment. Seemingly, the effects of neuromodulation resulting from the transcutaneous tibial nerve stimulation through 12 sessions within a month are not limited by the treatment duration and treatment finish time. In other words, the effects show a positive trend within a month following the treatment. Hence, TTNS is recommended for a child of OAB and in other randomized clinical trials. Finally, the OABSS questionnaire is highly recommended for the evaluation of children with OABS.
References
-
1.
Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306-14. discussion 1314-5. [PubMed ID: 17049716]. https://doi.org/10.1016/j.eururo.2006.09.019.
-
2.
de Wall LL, Heesakkers JP. Effectiveness of percutaneous tibial nerve stimulation in the treatment of overactive bladder syndrome. Res Rep Urol. 2017;9:145-57. [PubMed ID: 28861404]. [PubMed Central ID: PMC5565382]. https://doi.org/10.2147/RRU.S124981.
-
3.
Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326(7394):841-4. [PubMed ID: 12702614]. [PubMed Central ID: PMC153465]. https://doi.org/10.1136/bmj.326.7394.841.
-
4.
Scheife R, Takeda M. Central nervous system safety of anticholinergic drugs for the treatment of overactive bladder in the elderly. Clin Ther. 2005;27(2):144-53. [PubMed ID: 15811477]. https://doi.org/10.1016/j.clinthera.2005.02.014.
-
5.
Andersson K. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004;3(1):46-53. https://doi.org/10.1016/s1474-4422(03)00622-7.
-
6.
Ellsworth P. Treatment of overactive bladder symptoms beyond antimuscarinics: current and future therapies. Postgrad Med. 2012;124(3):16-27. [PubMed ID: 22691895]. https://doi.org/10.3810/pgm.2012.05.2544.
-
7.
Tutolo M, Ammirati E, Heesakkers J, Kessler TM, Peters KM, Rashid T, et al. Efficacy and Safety of Sacral and Percutaneous Tibial Neuromodulation in Non-neurogenic Lower Urinary Tract Dysfunction and Chronic Pelvic Pain: A Systematic Review of the Literature. Eur Urol. 2018;73(3):406-18. [PubMed ID: 29336927]. https://doi.org/10.1016/j.eururo.2017.11.002.
-
8.
Abello A, Das AK. Electrical neuromodulation in the management of lower urinary tract dysfunction: evidence, experience and future prospects. Ther Adv Urol. 2018;10(5):165-73. [PubMed ID: 29623108]. [PubMed Central ID: PMC5881994]. https://doi.org/10.1177/1756287218756082.
-
9.
de Seze M, Raibaut P, Gallien P, Even-Schneider A, Denys P, Bonniaud V, et al. Transcutaneous posterior tibial nerve stimulation for treatment of the overactive bladder syndrome in multiple sclerosis: results of a multicenter prospective study. Neurourol Urodyn. 2011;30(3):306-11. [PubMed ID: 21305588]. https://doi.org/10.1002/nau.20958.
-
10.
Amarenco G, Ismael SS, Even-Schneider A, Raibaut P, Demaille-Wlodyka S, Parratte B, et al. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J Urol. 2003;169(6):2210-5. [PubMed ID: 12771752]. https://doi.org/10.1097/01.ju.0000067446.17576.bd.
-
11.
Wibisono E, Rahardjo HE. Effectiveness of short term percutaneous tibial nerve stimulation for non-neurogenic overactive bladder syndrome in adults: a meta-analysis. Acta Med Indones. 2015;47(3).
-
12.
Homma Y, Yoshida M, Seki N, Yokoyama O, Kakizaki H, Gotoh M, et al. Symptom assessment tool for overactive bladder syndrome--overactive bladder symptom score. Urology. 2006;68(2):318-23. [PubMed ID: 16904444]. https://doi.org/10.1016/j.urology.2006.02.042.
-
13.
Hakimi S, Dargahi R, Mobaraki Asl N, Ranjbar M, Mohammadi M, Nikan F, et al. Iranian Version of Overactive Bladder Symptom Scale: A Methodological Study. Cres J Med Biol Sci. 2018;5(4):366-70.
-
14.
Valles-Antuna C, Perez-Haro ML, Gonzalez-Ruiz de LC, Quintas-Blanco A, Tamargo-Diaz EM, Garcia-Rodriguez J, et al. Transcutaneous stimulation of the posterior tibial nerve for treating refractory urge incontinence of idiopathic and neurogenic origin. Actas Urol Esp. 2017;41(7):465-70. [PubMed ID: 28325529]. https://doi.org/10.1016/j.acuro.2017.01.009.
-
15.
Canbaz Kabay S, Kabay S, Mestan E, Cetiner M, Ayas S, Sevim M, et al. Long term sustained therapeutic effects of percutaneous posterior tibial nerve stimulation treatment of neurogenic overactive bladder in multiple sclerosis patients: 12-months results. Neurourol Urodyn. 2017;36(1):104-10. [PubMed ID: 26352904]. https://doi.org/10.1002/nau.22868.
-
16.
Gobbi C, Digesu GA, Khullar V, El Neil S, Caccia G, Zecca C. Percutaneous posterior tibial nerve stimulation as an effective treatment of refractory lower urinary tract symptoms in patients with multiple sclerosis: preliminary data from a multicentre, prospective, open label trial. Mult Scler. 2011;17(12):1514-9. [PubMed ID: 21757534]. https://doi.org/10.1177/1352458511414040.
-
17.
Kabay S, Kabay SC, Yucel M, Ozden H, Yilmaz Z, Aras O, et al. The clinical and urodynamic results of a 3-month percutaneous posterior tibial nerve stimulation treatment in patients with multiple sclerosis-related neurogenic bladder dysfunction. Neurourol Urodyn. 2009;28(8):964-8. [PubMed ID: 19373898]. https://doi.org/10.1002/nau.20733.
-
18.
Propst Katie, Butler Haylie, O'sullivan DM, Tunitsky-Bitton Elena. Peripheral Tibial Nerve Stimulation for Overactive Bladder Syndrome: Treatment Success and Patient Satisfaction. Conn Med. 2017;81(4).
-
19.
Sanagapalli S, Neilan L, Lo JYT, Anandan L, Liwanag J, Raeburn A, et al. Efficacy of Percutaneous Posterior Tibial Nerve Stimulation for the Management of Fecal Incontinence in Multiple Sclerosis: A Pilot Study. Neuromodulation. 2018;21(7):682-7. [PubMed ID: 29575432]. https://doi.org/10.1111/ner.12764.
-
20.
Scaldazza CV, Morosetti C, Giampieretti R, Lorenzetti R, Baroni M. Percutaneous tibial nerve stimulation versus electrical stimulation with pelvic floor muscle training for overactive bladder syndrome in women: results of a randomized controlled study. Int Braz J Urol. 2017;43(1):121-6. [PubMed ID: 28124534]. [PubMed Central ID: PMC5293392]. https://doi.org/10.1590/S1677-5538.IBJU.2015.0719.
-
21.
Vecchioli-Scaldazza C, Morosetti C, Berouz A, Giannubilo W, Ferrara V. Solifenacin succinate versus percutaneous tibial nerve stimulation in women with overactive bladder syndrome: results of a randomized controlled crossover study. Gynecol Obstet Invest. 2013;75(4):230-4. [PubMed ID: 23548260]. https://doi.org/10.1159/000350216.
-
22.
Gupta P, Ehlert MJ, Sirls LT, Peters KM. Percutaneous tibial nerve stimulation and sacral neuromodulation: an update. Curr Urol Rep. 2015;16(2):4. [PubMed ID: 25630918]. https://doi.org/10.1007/s11934-014-0479-1.
-
23.
Ramirez-Garcia I, Blanco-Ratto L, Kauffmann S, Carralero-Martinez A, Sanchez E. Efficacy of transcutaneous stimulation of the posterior tibial nerve compared to percutaneous stimulation in idiopathic overactive bladder syndrome: Randomized control trial. Neurourol Urodyn. 2019;38(1):261-8. [PubMed ID: 30311692]. https://doi.org/10.1002/nau.23843.