Abstract
Background:
Pulmonary hypertension is a rare and fatal disease and several methods are available for its risk stratification. Right heart catheterization is gold standard tool for this target but this method is invasive and expensive. Serum uric acid level is a controversial method for this aim.Objectives:
This paper aims to discover a correlation between serum uric acid level and severity of pulmonary hypertension based on right heart catheterization.Methods:
Uric acid level was measured in 128 pulmonary hypertension patients who had undergone right heart catheterization. Then, the correlations between uric acid level and right heart catheterization findings as well as pulmonary hypertension severity were assessed.Results:
The correlations between serum uric acid level and CI (P = 0.019), DAP (P = 0.032), MAP (P = 0.027), RAP (P = 0.002), SPAP (P = 0.015), MPAP (P = 0.035), PPPA (P = 0.011), SO2S (P = 0.005), MVO2S (P = 0.004) are significant. A positive correlation was found between serum uric acid level and pulmonary hypertension risk based on RAP (P = 0.006) and MVO2S (P = 0.022).Conclusions:
It was found that the serum uric acid level is significantly correlated with some parameters of right heart catheterization including CI, DAP, MAP, SPAP, MPAP, PPPA, especially RAP, SO2S and MVO2S. Also uric acid level is significantly correlated with severity of pulmonary hypertension based on RAP and MVO2S and the level is increased in high risk ranked patients.Keywords
Uric Acid Pulmonary Hypertension Right Heart Catheterization Prognosis
1. Background
Pulmonary hypertension has been known as a rare but fatal disease which is defined by mean pulmonary artery pressure greater than 20 mmHg based on the right heart catheterization (1). Several methods are used for risk stratification of pulmonary hypertension including history, physical examination, BNP (brain natriuretic peptide), pro-BNP, six-minute walk test (6MWT), echocardiography, cardiac MRI (magnetic resonance imaging), cardiopulmonary exercise test and right heart catheterization. Right heart catheterization is known as a gold standard method for diagnosis and evaluation of pulmonary hypertension but this method is invasive and expensive (2). Therefore, there is a need for novel methods to overcome the shortcomings of right heart catheterization.
Serum uric acid, which is the final oxidation product of the purine metabolism, protects cells from the damage induced by reactive oxygen species and reactive nitrogen species (3). Uric acid is known as a prognostic factor in heart failure (4-6), congenial heart disease (7) and obstructive pulmonary disease (8). Allopurinol, a drug for reducing level of uric acid, may be helpful for patients with severe left ventricular systolic dysfunction (9).
Several studies have shown that serum uric acid level rises in adult and pediatric pulmonary hypertension patients (10, 11). Several mechanisms may result in the high uric acid level in pulmonary hypertension, but the exact metabolic mechanism has not been clearly understood. Lung tissue ischemia, impaired renal perfusion and urinary excretion of uric acid may be the main factors for the rise in the level of uric acid level in pulmonary hypertension patients (12).
In spite of specified correlation between serum uric acid level and pulmonary hypertension, there is controversial correlation between uric acid level and prognosis of pulmonary hypertension. Voelkel et al. (10) in a study on 92 cases demonstrated that uric acid was associated with pulmonary artery pressure but it was not correlated to prognosis of pulmonary hypertension. Wensel et al. (13) showed that serum uric acid level was correlated with survival of pulmonary hypertension patients. Bendayan et al. (14) also confirmed the correlation between serum uric acid and the survival of pulmonary hypertension patients.
In previous studies, the relationship between serum uric acid level and severity of pulmonary hypertension has been mainly assessed based on survival, NYHA functional class and 6MWT while the use of right heart catheterization for this assessment has not been comprehensively employed.
2. Objectives
This paper aims to study the correlation between serum uric acid and right heart catheterization findings, as well as, severity of pulmonary hypertension based on right heart catheterization. To this end, the level of uric acid was measured in 128 pulmonary hypertension patients and assessed using the findings of right heart catheterization.
3. Methods
This paper is a retrospective study undertaken on pulmonary hypertension patients from Shahid Rajaei Cardiovascular, Medical and Research Center, Tehran, Iran, for the period of March 2013 to February 2018. Patients with congenital heart disease, reduced ejection fraction (< 45%) and renal failure (Cr > 1.5 mg/dL) were removed from the analysis and 128 cases were used for this study. None of the studied patients had leukemia or myeloproliferative disease. Serum uric acid level was measured by calorimetric method, in the day that right heart catheterization was performed. Right heart catheterization was performed via different accesses involving femoral, brachial, jugular and subclavian vein. In right heart catheterization, right atrial pressure (RAP), systolic pulmonary artery pressure (SPAP), diastolic pulmonary artery pressure (DPAP), wedge pressure (W), mixed venous oxygen saturation (MVO2S) and systemic oxygen saturation (SO2S) were measured. Then, mean pulmonary artery pressure (MPAP), pulse pressure of pulmonary artery (PPPA), cardiac output (CO) using Fick method, cardiac index (CI), systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR) were calculated from these measurements. Systolic aortic pressure (SAP), diastolic aortic pressure (DAP) and mean aortic pressure (MAP) were recorded. The correlation between serum uric acid level and right heart catheterization data was evaluated. Also, the correlation between serum uric acid level and risk of pulmonary hypertension based on right heart catheterization was evaluated according to Table 1.
| Right Heart Catheterization Parameters | Low Risk (1-Year Mortality < 5%) | Moderate Risk (1-Year Mortality 5 - 10%) | High risk (1-Year Mortality > 10%) |
|---|---|---|---|
| CI, L/min/m2 | ≥ 2.5 | 2 - 2.5 | < 2 |
| RAP, mmHg | < 8 | 8 - 14 | > 14 |
| MVO2S, % | > 65 | 60 - 65 | < 60 |
Patients were then divided into three groups based on the severity of pulmonary hypertension risk: (1) high risk patients who had at least two high risk items from three parameters; (2) low risk patients who were evaluated low risk for all three items; (3) moderate risk patients who were not included into the first and second groups. The correlation between serum uric acid level and the categorized severity of pulmonary hypertension risk was evaluated.
3.1. Statistical Analysis
Analyses were carried out using IBM SPSS statistics 19 for Windows (IBM Corp, Armonk, NY, USA) and normality of distributions for each variable was assessed using one sample Kolmogorov - Smirnov test. Continuous variables with and without normal distribution are presented as means ± standard deviation and median (interquartile range; IQR). Categorical data are presented as numbers and percentages. To compare the ordinal variables Kruskal - Wallis test was used. Spearman’s rho correlation coefficient was used to test the correlations between uric acid and right heart catheterization data. All reported probability values were two‐tailed, and a P < 0.05 was considered statistically significant.
4. Results
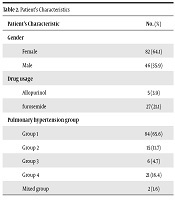
In the surveyed population (128 patients), 35.9% (46 patients) were male and 64.1% (82 patients) were female. Among study subjects 3.9% of cases (5 patients) were using allopurinol and 21.1% of cases (27 patients) were receiving furosemide. Patients were categorized based on WHO classification of pulmonary hypertension where 65.6% (84 patients) fell in group 1, 11.7% (15 patients) in group 2 (include preserved ejection fraction heart failure and restrictive cardiomyopathy), 4.7% (6 patients) in group 3, 16.4% (21 patients) in group 4 and 1.6% (2 patients) were categorized in mixed group. As the patients of group 5 were combined with other groups, it was not considered as a separate group in the analysis. The characteristics of patients are presented in Table 2. Risk of pulmonary hypertension for patients was evaluated based on RAP, CI and MVO2S and the results are summarized in Table 3. Among all, 28.9% (37 patients) based on RAP, 35.9% (46 patients) based on CI and 46.1% (59 patients) based on MVO2S were assessed as high risk. The median of uric acid and right heart catheterization data is listed in Table 4.
Patient’s Characteristics
| Patient’s Characteristic | N (%) |
|---|---|
| Gender | |
| Female | 82 (64.1) |
| Male | 46 (35.9) |
| Drug usage | |
| Allopurinol | 5 (3.9) |
| furosemide | 27 (21.1) |
| Pulmonary hypertension group | |
| Group 1 | 84 (65.6) |
| Group 2 | 15 (11.7) |
| Group 3 | 6 (4.7) |
| Group 4 | 21 (16.4) |
| Mixed group | 2 (1.6) |
Prevalence of Patients Were Categorized Based on Right Heart Catheterization Risk Stratification
| Low Risk, N (%) | Moderate Risk, N (%) | High Risk, N (%) | |
|---|---|---|---|
| Risk base on RAP | 31 (24.2) | 60 (46.9) | 37 (28.9) |
| Risk based on CI | 52 (40.6) | 30 (23.4) | 46 (35.9) |
| Risk based on MVO2S | 42 (32.8) | 27 (21.1) | 59 (46.1) |
Median of Variables
| Variable | Median (IQR) |
|---|---|
| Uric acid | 6.4 (4.9 - 8.3) |
| CO | 4.1 (3.1 - 5.1) |
| CI | 2.26 (1.8 - 2.89) |
| SAP | 120 (110 - 135) |
| DAP | 77 (69 - 85) |
| MAP | 91 (83 - 101.66) |
| RAP | 10 (8 - 15) |
| SPAP | 84 (55 - 110) |
| DPAP | 35 (25 - 40) |
| MPAP | 51.66 (33.3 - 66) |
| PPPA | 44 (30 - 60) |
| W | 12 (11 - 18) |
| PVR | 8.9 (3.5 - 15.3) |
| SVR | 20.5 (15.4 - 26) |
| SO2S | 93 (88 - 95) |
| MVO2S | 62.5 (52 - 67) |
The results of the analyses show that the correlation between serum uric acid level and CI (P = 0.019), DAP (P = 0.032), MAP (P = 0.027), RAP (P = 0.002), SPAP (P = 0.015), MPAP (P = 0.035), PPPA (P = 0.011), SO2S (P = 0.005), MVO2S (P = 0.004) is weak but significant and no correlation was found between other parameters and serum uric acid. As the uric acid level increases, the, RAP, SPAP, MPAP and PPPA increase while it results in a decrease of CI, DAP, MAP, SO2S, and MVO2S. The correlation is sharper for RAP, SO2S, and MVO2S, as shown in Table 5. In addition, the relationship between serum uric acid level and risk of pulmonary hypertension based on RAP (P = 0.006) and MVO2S (P = 0.022) are significant but the relationship between serum uric acid level and risk of pulmonary hypertension based on CI isn’t significant (P = 0.062). It was also found that the level of uric acid for high risk patients’ category is significantly elevated (P = 0.047).
Correlation Between Serum Uric Acid Level and Right Heart Catheterization Variables
| Variable | P Value | Correlation Coefficient |
|---|---|---|
| CO | 0.518 | -0.058 |
| CI | 0.019 | -0.207 |
| SAP | 0.058 | -0.168 |
| DAP | 0.032 | -0.189 |
| MAP | 0.027 | -0.195 |
| RAP | 0.002 | 0.277 |
| SPAP | 0.015 | 0.214 |
| DPAP | 0.094 | 0.149 |
| MPAP | 0.035 | 0.187 |
| PPPA | 0.011 | 0.224 |
| W | 0.458 | 0.066 |
| PVR | 0.116 | 0.140 |
| SVR | 0.992 | -0.001 |
| SO2S | 0.005 | -0.249 |
| MVO2S | 0.004 | -0.253 |
5. Discussion
A number of studies investigated the correlation of serum uric acid level with hemodynamic data and most of them are based on echocardiography while the use of right heart catheterization for assessment of hemodynamic were not fully studied.
Nagaya et al. (15) found a correlation between the serum uric acid level and cardiac output, as well as, pulmonary resistance but the correlation with mean pulmonary arterial pressure was not significant. Voelkel et al. (10) found a positive correlation between serum uric acid level and RAP elevation. Van Albada et al. (11) in a study on the serum uric acid in pulmonary hypertension children demonstrated that serum uric acid level correlates with invasively recorded hemodynamic data. Zhang et al. (12) observed uric acid level positively correlated with MPAP in elevated uric acid level while level of serum uric acid was negatively correlated with RVEF. Boyilla and Madas (16) found an elevated level of uric acid in pulmonary hypertension patients compared to healthy subjects and reported positive correlation between uric acid level and MPAP. Castillo-Martinez et al. (17), found high mean SPAP in hyperuricemia patients after a 6-year follow-up period in a study on systemic lupus erythematosus patients for 7 years. Suteu et al. (18) found that serum uric acid level in pediatric pulmonary arterial hypertension patients was higher than healthy subjects. Seyyedi et al. (19) reported significant correlations between serum uric acid level and SPAP and right ventricular dysfunction.
In this study, it was found that the serum uric acid level is significantly correlated with some parameters of right heart catheterization including CI, DAP, MAP, SPAP, MPAP, PPPA, especially RAP, SO2S and MVO2S. The negative correlation between uric acid level and CI was also observed in Nagaya et al. (15) study. Zhang et al. (12) also detected the correlation between uric acid and MPAP but this correlation was not significant in the Nagaya et al. (15) study. Castillo-Martinez et al. (17) and Seyyedi et al. (19) showed that the uric acid level was associated with SPAP as similar result was found in this study while this correlation was not observed in Njaman et al. (20) study. Voelkel et al. (10) also presented positive correlation between RAP and uric acid level.
Results of this study show that the uric acid level is significantly correlated with severity of pulmonary hypertension based on RAP and MVO2S and the level is increased in high risk ranked patients. In some of the previous studies, the uric acid level has been assessed as prognostic factor in pulmonary hypertension. However, the criterion of severity of pulmonary hypertension has been different in these studies and included survival, NYHA functional class and 6MWT. Wensel et al. (13) followed up the serum uric acid level in idiopathic pulmonary hypertension patients for 6 years and found independent correlation between serum uric acid level and survival. Bendayan et al. (14) observed a strong correlation between serum uric acid level and NYHA functional class and mortality in pulmonary hypertension patients. In their study, six of the eight hyperuricemia patients died and all patients in class IV of NYHA functional class were hyperuricemic. Endothelin receptor antagonists are known as a standard treatment for pulmonary hypertension and they reduce the level of serum uric acid in these patients with both improving survival and longer time to clinical worsening (21). Kang et al. (22) observed 19% increment in the death risk for pulmonary hypertension patients with hyperuricemia by meta-analysis of serum uric acid in pulmonary hypertension patients. However, Voelkel et al. (10) found no significant relationship between serum uric acid level and prognosis in contrast with findings of previous investigations and this study.
5.1. Limitations
In this study, patients who received furosemide and allopurinol were not removed from the analysis and their underlying disease were not considered. It is recommended for future studies to consider larger population in which patients can be differentiated based on the drug usage and underlying disease.
References
-
1.
Galie N, McLaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53(1):1802148. [PubMed ID: 30552088]. [PubMed Central ID: PMC6351332]. https://doi.org/10.1183/13993003.02148-2018.
-
2.
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119. [PubMed ID: 26320113]. https://doi.org/10.1093/eurheartj/ehv317.
-
3.
Pezzuto B, Badagliacca R, Poscia R, Ghio S, D'Alto M, Vitulo P, et al. Circulating biomarkers in pulmonary arterial hypertension: Update and future direction. J Heart Lung Transplant. 2015;34(3):282-305. [PubMed ID: 25682555]. https://doi.org/10.1016/j.healun.2014.12.005.
-
4.
Amin A, Chitsazan M, Shiukhi Ahmad Abad F, Taghavi S, Naderi N. On admission serum sodium and uric acid levels predict 30 day rehospitalization or death in patients with acute decompensated heart failure. ESC Heart Fail. 2017;4(2):162-8. [PubMed ID: 28451453]. [PubMed Central ID: PMC5396033]. https://doi.org/10.1002/ehf2.12135.
-
5.
Amin A, Vakilian F, Maleki M. Serum uric acid levels correlate with filling pressures in systolic heart failure. Congest Heart Fail. 2011;17(2):80-4. [PubMed ID: 21449996]. https://doi.org/10.1111/j.1751-7133.2010.00205.x.
-
6.
Leyva F, Anker S, Swan JW, Godsland IF, Wingrove CS, Chua TP, et al. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur Heart J. 1997;18(5):858-65. [PubMed ID: 9152657]. https://doi.org/10.1093/oxfordjournals.eurheartj.a015352.
-
7.
Hayabuchi Y, Matsuoka S, Akita H, Kuroda Y. Hyperuricaemia in cyanotic congenital heart disease. Eur J Pediatr. 1993;152(11):873-6. [PubMed ID: 8276013]. https://doi.org/10.1007/bf01957519.
-
8.
Braghiroli A, Sacco C, Erbetta M, Ruga V, Donner CF. Overnight urinary uric acid: Creatinine ratio for detection of sleep hypoxemia. Validation study in chronic obstructive pulmonary disease and obstructive sleep apnea before and after treatment with nasal continuous positive airway pressure. Am Rev Respir Dis. 1993;148(1):173-8. [PubMed ID: 8317794]. https://doi.org/10.1164/ajrccm/148.1.173.
-
9.
Ansari-Ramandi MM, Maleki M, Alizadehasl A, Amin A, Taghavi S, Alemzadeh-Ansari MJ, et al. Safety and effect of high dose allopurinol in patients with severe left ventricular systolic dysfunction. J Cardiovasc Thorac Res. 2017;9(2):102-7. [PubMed ID: 28740630]. [PubMed Central ID: PMC5516049]. https://doi.org/10.15171/jcvtr.2017.17.
-
10.
Voelkel MA, Wynne KM, Badesch DB, Groves BM, Voelkel NF. Hyperuricemia in severe pulmonary hypertension. Chest. 2000;117(1):19-24. [PubMed ID: 10631193]. https://doi.org/10.1378/chest.117.1.19.
-
11.
Van Albada ME, Loot FG, Fokkema R, Roofthooft MT, Berger RM. Biological serum markers in the management of pediatric pulmonary arterial hypertension. Pediatr Res. 2008;63(3):321-7. [PubMed ID: 18287971]. https://doi.org/10.1203/PDR.0b013e318163a2e7.
-
12.
Zhang CY, Ma LL, Wang LX. Relationship between serum uric acid levels and ventricular function in patients with idiopathic pulmonary hypertension. Exp Clin Cardiol. 2013;18(1):e37-9. [PubMed ID: 24294046]. [PubMed Central ID: PMC3716500].
-
13.
Wensel R, Opitz CF, Anker SD, Winkler J, Hoffken G, Kleber FX, et al. Assessment of survival in patients with primary pulmonary hypertension: Importance of cardiopulmonary exercise testing. Circulation. 2002;106(3):319-24. [PubMed ID: 12119247]. https://doi.org/10.1161/01.cir.0000022687.18568.2a.
-
14.
Bendayan D, Shitrit D, Ygla M, Huerta M, Fink G, Kramer MR. Hyperuricemia as a prognostic factor in pulmonary arterial hypertension. Respir Med. 2003;97(2):130-3. [PubMed ID: 12587962]. https://doi.org/10.1053/rmed.2003.1440.
-
15.
Nagaya N, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Nakanishi N, et al. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med. 1999;160(2):487-92. [PubMed ID: 10430718]. https://doi.org/10.1164/ajrccm.160.2.9812078.
-
16.
Boyilla N, Madas S. Elevated uric acid levels: A predictor of pulmonary hypertension. Int J Adv Med. 2016;3(3):484-7. https://doi.org/10.18203/2349-3933.ijam20162202.
-
17.
Castillo-Martinez D, Marroquin-Fabian E, Lozada-Navarro AC, Mora-Ramirez M, Juarez M, Sanchez-Munoz F, et al. Levels of uric acid may predict the future development of pulmonary hypertension in systemic lupus erythematosus: A seven-year follow-up study. Lupus. 2016;25(1):61-6. [PubMed ID: 26306740]. https://doi.org/10.1177/0961203315600539.
-
18.
Suteu CC, Benedek T, Toganel R. Significance of serum uric acid in children with pulmonary arterial hypertension. J Interdiscipl Med. 2016;1(1):46-50. https://doi.org/10.1515/jim-2016-0010.
-
19.
Seyyedi SR, Malekmohammad M, Chitsazan M, Behzadnia N, Sadr M, Hashemian SM, et al. Relationship between serum uric acid levels and the severity of pulmonary hypertension. Tanaffos. 2017;16(4):283-8. [PubMed ID: 29849685]. [PubMed Central ID: PMC5971759].
-
20.
Njaman W, Iesaki T, Iwama Y, Takasaki Y, Daida H. Serum uric acid as a prognostic predictor in pulmonary arterial hypertension with connective tissue disease. Int Heart J. 2007;48(4):523-32. [PubMed ID: 17827824].
-
21.
Dhaun N, Vachiery JL, Benza RL, Naeije R, Hwang LJ, Liu X, et al. Endothelin antagonism and uric acid levels in pulmonary arterial hypertension: Clinical associations. J Heart Lung Transplant. 2014;33(5):521-7. [PubMed ID: 24656288]. https://doi.org/10.1016/j.healun.2014.01.853.
-
22.
Kang TU, Park KY, Kim HJ, Ahn HS, Yim SY, Jun JB. Association of hyperuricemia and pulmonary hypertension: A systematic review and meta-analysis. Mod Rheumatol. 2018:1-28. [PubMed ID: 30334638]. https://doi.org/10.1080/14397595.2018.1537555.