Abstract
Background:
Minimizing ischemic-reperfusion injury following valvular heart surgeries is very important with the goal of providing appropriate cardiac systolic function, preventing arrhythmic events as well as inhibiting ischemic related processes. Due to the protective effects of adenosine, this chemical has been used as an additive to a cardioplegic solution for achieving this purpose.Objectives:
The present study aimed to assess the effects of cold blood cardioplegia with adenosine on hemodynamic status of patients undergoing mitral valve surgery.Methods:
This randomized single-blinded clinical trial was performed on 40 consecutive patients who were candidates for mitral valve surgery. The patients were randomly assigned to receive cold blood cardioplegia in combination with or without adenosine or hyperkalemic cardioplegia as the control. Primary endpoints were returning sinus rhythm, requiring inotropes after cardiopulmonary bypass and secondary endpoints were the change in hemodynamic parameters and postoperative complications.Results:
Except for mean time required for induction of cardiac arrest that was significantly shorter in intervention group, no differences were found between the two groups regarding cardiopulmonary bypass time, cross clamp duration, and rhythm return time. In assessment of postoperative consequences, there were no differences in post-procedural events with respect to returning sinus rhythm, requiring inotropes, requiring DC shock, mean intubation time, length of ICU stay and also left ventricular systolic function.Conclusions:
Except for reducing the time for inducing cardiac arrest within mitral valve surgery, adding adenosine to cold blood cardioplegia may not be beneficial regarding the improvement of postoperative outcome.Keywords
Cardiopulmonary Bypass Mitral Valve Surgery Adenosine Cardioplegia Solution
1. Background
Patients after heart valve surgery are at risk of multiple problems, such as atrial fibrillation, bleeding, thrombosis, respiratory complications and digestive complications, leading to death and potential complications. One of the most important cardiovascular disorders leading to death can be referred to the phenomenon of ischemic-reperfusion event (1). Cardiac ischemia leads to complex complications such as myocardial arrhythmias and consequently congestive heart failure. The ischemia-reperfusion phenomenon in myocardium will irreparably lead to ischemic injury by activating pathological inflammatory processes and apoptosis resulting in tissue death through inducing excessive production of free radicals and oxidative stress (2, 3). In fact, due to damage caused by free radical production and active oxygen species during ischemic rejection of perfusion and non-compliance with the antioxidant system of the muscular tissue of the heart, myocardium will always be exposed to necrosis and apoptosis.
Ischemia-reperfusion injury is known as one of the most important clinical problems. For these reasons, researchers have been focusing on protecting and preventing damage from ischemic-reperfusion injury over recent years (4). On the other hand, for cardiac surgery, stopping heart movement is needed to provide a relaxed and bloodless environment for the action required by a solution called cardioplegia (5). During the surgery, oxygenating the bloodstream in the rest of the body is supplied by cardiopulmonary bypass. The heart muscle itself is exhausted through the bypass of the circulatory system and lacks blood flow. Therefore, there is a possibility of serious and sometimes irreversible damage in this situation. It may even be accompanied by metabolic acidosis in the heart’s ischemia, lowering blood pressure and reducing cardiac output (6). But despite the inactivity of the heart by injecting a cardioplegic solution, energy consumption and heart oxygenation never go down to zero. Therefore, as a result of ischemia and accumulation of metabolic waste, there is considerably lower possibility of cellular damage. Since the protective effect of the cardiopulmonary solution on the heart is limited, it is necessary to repeatedly inject it at specific intervals. The most basic principle during heart surgery is to maintain cardiac function by preventing cardiac ischemia as no cardiopulmonary approach to protecting the entire heart from myocardial damage against ischemic damage has been demonstrated. The results of the studies show that cardioplegic solutions containing high potassium concentrations may cause damage to myocardial cells (7). However, the results of some studies indicate that the use of solutions containing normal potassium concentration such as adenosine, magnesium and lidocaine can play an important role in the better protection of the cardiac myocardium and, consequently, improve cardiac function during ischemia and reperfusion of blood circulation (5, 8). Among the cases mentioned, adenosine by induction of vascular dilatation plays an important role in reducing the inflammatory response caused by reperfusion-injury with intermediate receptor mechanisms (9). Adenosine regulates response to an imbalance between supply and demand for myocardial oxygen during induction of ischemia (10). In other words, adenosine increases energy supply to the heart’s myocardial tissue. Several studies in animals have shown the effectiveness of protecting the heart through adenosine as part of the cardioplegia method (11). Using a cold blood cardioplegia can increase the level of cardiac protection during surgery (12). Due to the protective effects of adenosine, this chemical has been used as an additive to a cardioplegic solution for more than two decades. Although there are several studies that report improving cardiac function with adenosine supplementation during the course of ischemic heart disease, some others could not demonstrate such significant effects.
2. Objectives
The present study aimed to assess the effects of cold blood cardioplegia with adenosine on hemodynamic status of patients undergoing mitral valve surgery.
3. Methods
This randomized single-blinded clinical trial was performed on 40 consecutive patients who were candidates for mitral valve surgeries at Rajaie Cardiovascular Medical and Research Center in Tehran in 2018. All eligible patients aged 30 to 65 years, had willingness for participation in the study and filled the written informed consent to take part in the study. The exclusion criteria were patients with aortic valve and/or pulmonic valve problems, any evidence of coronary artery disease, liver or renal insufficiencies, pulmonary disorders, asthma, history of cardiac surgery, left ventricular ejection fraction less than 30%, or history of adenosine hypersensitivity. Before transferring to surgery room and using a random table number, the patients were randomly assigned into two groups as the intervention group and the control group. Before beginning the operation and after inducing anesthesia and medial sternotomy, heparin was used to increase coagulation time. After connecting to cardiopulmonary pump, cold blood cardioplegia consisted of 1 liter of plasmalyte solution (containing 140 mEq/L sodium, 3 mEq/L magnesium, 5 mEq/L potassium, 27 mEq/L acetate, 98 mEq/L chloride and 23 mEq/L gluconate with a pH of 7.4) in addition to adenosine (2 mmol) were infused in the intervention group and hyperkalemic cardioplegia group was infused for the control and case group. During operation, moderate hypothermia status (temperature of 28ºC) was induced and blood pressure was maintained at the range of 50 to 80 mmHg. Hemodynamic parameters were assessed before, as well as 15 min, 6 hours and 24 hours after cardiopulmonary bypass. In addition, the time for reversing cardiac rhythm, the length of stay in hospital and amount of inotrope use was also assessed in both groups. The levels of serum potassium, calcium, cardiac troponin T, high-sensitive CRP, lactate, and bicarbonate were also measured in both groups 24 and 48 hours after surgery.
The results were demonstrated as mean ± standard deviation (SD) for quantitative variables and were summed up by percentages and absolute frequencies for categorical variables. The Kolmogorov-Smirnoff test was used to analyze data normality. In case over 20% of cells with expected count of less than 5 were observed, chi-square test or Fisher’s exact test were used to compare categorical variables. T-test, or Mann-Whitney U test were also used to compare quantitative variables. SPSS version 16.0 for windows (SPSS Inc., Chicago, IL) was used for statistical analysis. P values of < 0.05 were considered statistically significant.
4. Results
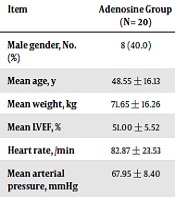
Totally, 20 patients were scheduled for infusion of cold blood cardioplegia combined with adenosine and 20 for infusing only hyperkalemic cardioplegia as the control. The two groups were similar in terms of baseline variables including average age, gender, mean weight, baseline systolic function status, as well as hemodynamic parameters (Table 1). Assessing the trend of the change in hemodynamic parameters (heart rate and mean blood pressure) as well as serum biomarkers (electrolytes, and blood gas analysis) showed no significant difference within 48 hours after operation (Table 2). Intra-operatively, except for mean time required for induction of cardiac arrest that was significantly shorter in intervention group, no difference was found between the two groups regarding cardiopulmonary bypass time, cross clamp duration, and rhythm return time (Table 3). Also, in assessment of postoperative consequences, there was no difference in post-procedural events with respect to returning sinus rhythm, requiring balloon pump insertion, requiring DC shock, mean intubation time, length of ICU stay and also left ventricular systolic function (Table 3). No cases of myocardial infarction, cardiac arrhythmias such as atrial fibrillation, pace maker insertion, abnormal ECG changes, or death were occurred.
Comparing Baseline and Intraoperative Characteristics Between Intervention and Control Groups
| Item | Adenosine Group (N = 20) | Control Group (N = 20) | P Value |
|---|---|---|---|
| Male gender, No. (%) | 8 (40.0) | 8 (40.0) | 1.000 |
| Mean age, y | 48.55 ± 16.13 | 48.65 ± 13.21 | 0.925 |
| Mean weight, kg | 71.65 ± 16.26 | 74.30 ± 13.04 | 0.678 |
| Mean LVEF, % | 51.00 ± 5.52 | 48.75 ± 9.16 | 0.583 |
| Heart rate, /min | 82.87 ± 23.53 | 81.85 ± 17.87 | 0.231 |
| Mean arterial pressure, mmHg | 67.95 ± 8.40 | 71.35 ± 14.62 | 0.583 |
| HS-CRP | 2.72 ± 1.40 | 2.76 ± 1.55 | 0.968 |
| CTNI | 3.27 ± 6.13 | 3.16 ± 6.10 | 0.968 |
| Serum potassium, mmol/L | 3.68 ± 0.57 | 3.69 ± 0.55 | 0.999 |
| Serum calcium, mg/dL | 8.89 ± 1.30 | 8.42 ± 1.23 | 0.242 |
| Serum bicarbonate, mEq/L | 24.21 ± 4.92 | 22.90 ± 4.36 | 0.398 |
| Serum lactate, mg/dl | 1.23 ± 0.67 | 1.12 ± 0.56 | 0.640 |
Comparing the Trend of the Change in Hemodynamic Parameters Between Intervention and Control Groups
| Item | Adenosine Group (N = 20) | Control Group (N = 20) | P Value |
|---|---|---|---|
| Heart rate, /min | |||
| Baseline | 82.87 ± 23.53 | 81.85 ± 17.87 | 0.231 |
| 6 hours later | 86.40 ± 19.29 | 88.45 ± 27.01 | 0.134 |
| 24 hours later | 80.00 ± 9.34 | 82.55 ± 11.95 | 0.602 |
| Mean arterial pressure, mmHg | |||
| Baseline | 67.95 ± 8.40 | 71.35 ± 14.62 | 0.583 |
| 15 min later | 63.55 ± 7.93 | 67.10 ± 12.28 | 0.211 |
| 6 hours later | 66.35 ± 6.74 | 70.00 ± 11.78 | 0.529 |
| 24 hours later | 65.70 ± 5.16 | 68.40 ± 8.16 | 0.718 |
| HS-CRP | |||
| Baseline | 2.72 ± 1.40 | 2.76 ± 1.55 | 0.968 |
| 6 hours later | 12.21 ± 6.09 | 10.31 ± 5.41 | 0.369 |
| 24 hours later | 11.37 ± 5.57 | 10.14 ± 6.54 | 0.461 |
| 48 hours later | 8.46 ± 5.10 | 8.32 ± 5.61 | 0.883 |
| CTNI | |||
| Baseline | 3.27 ± 6.13 | 3.16 ± 6.10 | 0.968 |
| 6 hours later | 8.41 ± 4.84 | 10.16 ± 6.48 | 0.327 |
| 24 hours later | 8.41 ± 5.44 | 8.98 ± 6.49 | 0.758 |
| 48 hours later | 5.03 ± 4.51 | 7.15 ± 5.67 | 0.242 |
| Serum potassium, mmol/L | |||
| Baseline | 3.68 ± 0.57 | 3.69 ± 0.55 | 0.999 |
| 6 hours later | 4.25 ± 0.34 | 4.27 ± 0.47 | 0.925 |
| 24 hours later | 4.03 ± 0.45 | 4.11 ± 0.39 | 0.529 |
| 48 hours later | 4.06 ± 0.38 | 4.17 ± 0.44 | 0.383 |
| Serum calcium, mg/dL | |||
| Baseline | 8.89 ± 1.30 | 8.42 ± 1.23 | 0.242 |
| 6 hours later | 8.59 ± 1.11 | 8.22 ± 1.00 | 0.301 |
| 24 hours later | 8.38 ± 1.03 | 7.99 ± 0.84 | 0.157 |
| 48 hours later | 7.62 ± 0.51 | 7.32 ± 1.44 | 0.461 |
| Serum bicarbonate, mEq/L | |||
| Baseline | 24.21 ± 4.92 | 22.90 ± 4.36 | 0.398 |
| 6 hours later | 19.71 ± 5.52 | 20.55 ± 3.68 | 0.989 |
| 24 hours later | 22.26 ± 3.43 | 21.75 ± 3.21 | 0.565 |
| 48 hours later | 22.97 ± 4.54 | 20.85 ± 5.26 | 0.414 |
| Serum lactate, mg/dL | |||
| Baseline | 1.23 ± 0.67 | 1.12 ± 0.56 | 0.640 |
| 6 hours later | 2.05 ± 1.01 | 1.85 ± 1.25 | 0.289 |
| 24 hours later | 1.61 ± 0.61 | 1.66 ± 1.64 | 0.127 |
| 48 hours later | 1.05 ± 0.30 | 1.09 ± 0.71 | 0.314 |
Comparing Intraoperative and Postoperative Outcome Between Intervention and Control Groups
| Item | Adenosine Group (N = 20) | Control Group (N = 20) | P Value |
|---|---|---|---|
| CPB time, min | 76.75 ± 23.84 | 73.35 ± 26.47 | 0.495 |
| Cross clamp time, min | 47.95 ± 17.50 | 42.50 ± 15.67 | 0.383 |
| Arrest induction time, Sec | 11.65 ± 3.77 | 25.80 ± 23.46 | 0.001 |
| Rhythm return time, Sec | 143.50 ± 189.75 | 94.40 ± 63.49 | 0.529 |
| Need for mitral valve repair, % | 7 (30.0) | 8 (40.0) | 0.744 |
| Post declamping rhythm, % | 0.545 | ||
| VF | 4 (20.0) | 2 (10.0) | |
| VT | 2 (10.0) | 1 (5.0) | |
| Sinus rhythm | 14 (70.0) | 17 (85.0) | |
| Antiarrhythmic drug use, % | 4 (20.0) | 2 (10.0) | 0.661 |
| DC shock, % | 6 (30.0) | 2 (10.0) | 0.235 |
| Balloon pump insertion, % | 0 (0.0) | 1 (5.0) | 0.999 |
| Intubation time, min | 504.50 ± 157.56 | 483.00 ± 210.99 | 0.678 |
| Length of ICU stay, d | 2.25 ± 0.85 | 2.55 ± 0.60 | 0.157 |
| Postoperative EF, % | 49.75 ± 5.95 | 48.50 ± 9.04 | 0.820 |
5. Discussion
Minimizing ischemic-reperfusion injury following valvular heart surgeries is very important with the goal of providing appropriate cardiac systolic function, preventing arrhythmic events as well as inhibiting ischemic related processes. In this regard, the use of adenosine has multi-potential role in the prevention of cardiac adverse events by induction of vascular dilatation, reduction of the inflammatory responses and thus protection of cardiac tissue during surgical intervention. However, such effects have not been demonstrated in some studies that might be due to the drug dosages, type of surgical intervention, study power and employing small sample sizes, inclusion and exclusion criteria, and also simultaneous medications considered. As shown in our study, we could not demonstrate beneficial effects of intraoperative adenosine use combined with cold blood cardioplegia as compared to hyperkalemic cardioplegia alone on postoperative status. In other words, the use of cardioplegia solutions with and without adenosine may lead to similar postoperative outcome regarding mortality and morbidity. However, we found that only the time required for inducing cardiac arrest during cardioplegia infusion was few seconds shorter when using adenosine that seems not to clinically affect postoperative outcome. Thus, despite demonstrating clinical benefits of adenosine to prevent cardiac ischemic injuries, due to the probability of drug-related adverse events, adenosine may not be used in patients candidate for valvular procedures, however our low study power because of small sample size and considering minimum dose of drug as a supportive, emphasizes further studies to prove or reject of our initial hypotheses.
Similar to our study, Ahlsson et al. in 2012 demonstrated that a combination of cold blood cardioplegia and adenosine 400 μmol/L showed no cardioprotective effects with regard to oxygen myocardial metabolism, lactate and adenine nucleotides, hemodynamic performance or postoperative enzyme release (13). No differences were found in two other large studies of pre-ischemic delivery of adenosine in either clinical performance or cardiac enzymatic release (14, 15). Contrarily, Jin et al. in the study of assessing post-ischaemic effects of adenosine showed that 0.3 - 0.5 mM of adenosine intraarterial injection at cross clamp removal was associated with decreased need for postoperative inotropic support and a lower troponin-I release (16). Similarly, Liu et al. could show that in comparison with simple cold blood cardioplegia in patients who need heart valve replacement, ADO pretreatment as a supplement to 1 mmol/L cold blood cardioplegia could reduce troponin I as well as inflammatory biomarkers, resulting in reduced myocardial injury in ultrastructure after surgery (17). Jin et al. also revealed that an adenosine (1.5-mg/kg bolus) post-conditioning in addition to high potassium cold blood myocardial protection resulted in lower troponin-I release, shorter ICU stay, and less inotropic drug use (16). The dose-dependence of adenosine beneficial cardiac effects has been also indicated in a study by Mentzer et al. so that in comparison with those receiving intermediate or high dose treatments, 100 microM adenosine groups had a lower ejection fraction (18). In other words, to achieve benefits of adenosine, considering its optimal doses is necessary to attenuate myocardial stunning in valvular heart disease patients.
5.1. Conclusions
Adding adenosine to cold blood cardioplegia seems to have no significant role in the improvement of postoperative outcome of mitral valve replacement; however it could reduce the time required for inducing cardiac arrest for a few seconds which does not seem to have any clinical significance.
5.2. Limitations
Regarding the short time of aortic cross clamp times (48 min in adenosine and 43 min in control groups) in our study we fund (an expected) low levels of postoperative HS-CRP and CTNI in both study groups and unable to show statistically different results. However, favorite effects of adding adenosine to cardioplegia solution could be more detectible in multi-valve operations and prolonged CPB time; so more investigation in complex cardiac surgery in suggested.
References
-
1.
Casos K, Ferrer-Curriu G, Soler-Ferrer P, Perez ML, Permanyer E, Blasco-Lucas A, et al. Response of the human myocardium to ischemic injury and preconditioning: The role of cardiac and comorbid conditions, medical treatment, and basal redox status. PLoS One. 2017;12(4). e0174588. [PubMed ID: 28380047]. [PubMed Central ID: PMC5381881]. https://doi.org/10.1371/journal.pone.0174588.
-
2.
Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, et al. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid Med Cell Longev. 2019;2019:7092151. [PubMed ID: 31341533]. [PubMed Central ID: PMC6612399]. https://doi.org/10.1155/2019/7092151.
-
3.
Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: From basic science to clinical bedside. Semin Cardiothorac Vasc Anesth. 2012;16(3):123-32. [PubMed ID: 22368166]. [PubMed Central ID: PMC3457795]. https://doi.org/10.1177/1089253211436350.
-
4.
Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106(3):360-8. [PubMed ID: 20643246]. [PubMed Central ID: PMC2957093]. https://doi.org/10.1016/j.amjcard.2010.03.032.
-
5.
Habertheuer A, Kocher A, Laufer G, Andreas M, Szeto WY, Petzelbauer P, et al. Cardioprotection: A review of current practice in global ischemia and future translational perspective. Biomed Res Int. 2014;2014:325725. [PubMed ID: 25276778]. [PubMed Central ID: PMC4172998]. https://doi.org/10.1155/2014/325725.
-
6.
Brown AH, Morritt GN, Hammo M. Avoidance of reperfusion injury after cardioplegia. Thorax. 1982;37(4):275-9. [PubMed ID: 7112456]. [PubMed Central ID: PMC459297]. https://doi.org/10.1136/thx.37.4.275.
-
7.
Boning A, Hagmuller S, Heep M, Rohrbach S, Niemann B, Muhlfeld C. Is warm or cold Calafiore blood cardioplegia better? Hemodynamic, metabolic, and electron microscopic differences. Thorac Cardiovasc Surg. 2014;62(8):683-9. [PubMed ID: 25068771]. https://doi.org/10.1055/s-0034-1383722.
-
8.
Sacli H, Kara I, Diler MS, Percin B, Turan AI, Kirali K. The relationship between the use of cold and isothermic blood cardioplegia solution for myocardial protection during cardiopulmonary bypass and the ischemia-reperfusion injury. Ann Thorac Cardiovasc Surg. 2019. [PubMed ID: 31308305]. https://doi.org/10.5761/atcs.oa.18-00293.
-
9.
Zeng J, He W, Qu Z, Tang Y, Zhou Q, Zhang B. Cold blood versus crystalloid cardioplegia for myocardial protection in adult cardiac surgery: A meta-analysis of randomized controlled studies. J Cardiothorac Vasc Anesth. 2014;28(3):674-81. [PubMed ID: 24721161]. https://doi.org/10.1053/j.jvca.2013.06.005.
-
10.
Vinten-Johansen J, Thourani VH, Ronson RS, Jordan JE, Zhao ZQ, Nakamura M, et al. Broad-spectrum cardioprotection with adenosine. Ann Thorac Surg. 1999;68(5):1942-8. [PubMed ID: 10585108]. https://doi.org/10.1016/s0003-4975(99)01018-8.
-
11.
Boros D, Thompson J, Larson DF. Adenosine regulation of the immune response initiated by ischemia reperfusion injury. Perfusion. 2016;31(2):103-10. [PubMed ID: 25987550]. https://doi.org/10.1177/0267659115586579.
-
12.
Boehm DH, Human PA, von Oppell U, Owen P, Reichenspurner H, Opie LH, et al. Adenosine cardioplegia: Reducing reperfusion injury of the ischaemic myocardium? Eur J Cardiothorac Surg. 1991;5(10):542-5. [PubMed ID: 1756047]. https://doi.org/10.1016/1010-7940(91)90108-v.
-
13.
Ahlsson A, Sobrosa C, Kaijser L, Jansson E, Bomfim V. Adenosine in cold blood cardioplegia--a placebo-controlled study. Interact Cardiovasc Thorac Surg. 2012;14(1):48-55. [PubMed ID: 22108937]. [PubMed Central ID: PMC3420270]. https://doi.org/10.1093/icvts/ivr027.
-
14.
Belhomme D, Peynet J, Florens E, Tibourtine O, Kitakaze M, Menasche P. Is adenosine preconditioning truly cardioprotective in coronary artery bypass surgery? Ann Thorac Surg. 2000;70(2):590-4. [PubMed ID: 10969685]. https://doi.org/10.1016/s0003-4975(00)01502-2.
-
15.
Rinne T, Laurikka J, Penttila I, Kaukinen S. Adenosine with cold blood cardioplegia during coronary revascularization. J Cardiothorac Vasc Anesth. 2000;14(1):18-20. [PubMed ID: 10698386]. https://doi.org/10.1016/s1053-0770(00)90049-1.
-
16.
Jin ZX, Zhou JJ, Xin M, Peng DR, Wang XM, Bi SH, et al. Postconditioning the human heart with adenosine in heart valve replacement surgery. Ann Thorac Surg. 2007;83(6):2066-72. [PubMed ID: 17532398]. https://doi.org/10.1016/j.athoracsur.2006.12.031.
-
17.
Liu R, Xing J, Miao N, Li W, Liu W, Lai YQ, et al. The myocardial protective effect of adenosine as an adjunct to intermittent blood cardioplegia during open heart surgery. Eur J Cardiothorac Surg. 2009;36(6):1018-23. [PubMed ID: 19683936]. https://doi.org/10.1016/j.ejcts.2009.06.033.
-
18.
Mentzer RJ, Rahko PS, Molina-Viamonte V, Canver CC, Chopra PS, Love RB, et al. Safety, tolerance, and efficacy of adenosine as an additive to blood cardioplegia in humans during coronary artery bypass surgery. Am J Cardiol. 1997;79(12A):38-43. [PubMed ID: 9223362]. https://doi.org/10.1016/s0002-9149(97)00262-2.