Abstract
Introduction:
Renal arteriovenous fistula is a rare clinical entity which may have a serious effect on the hemodynamics.Case Presentation:
We report a case of renal arteriovenous fistula with undetermined diagnosis at her first presentation. Finally after 9 years her problem was diagnosed and managed successfully with coil embolization.Conclusions:
We should consider renal arteriovenous fistula as a differential diagnosis for hematuria. The gold standard modality for evaluation is DSA. Endovascular management is the best treatment.Keywords
1. Introduction
Renal arteriovenous fistula (AVF) is a rare clinical entity which may have a serious effect on the hemodynamics (1-3). It can be acquired, idiopathic or congenital. The acquired form happens more frequently and its occurrence is increasing because of the increment number of interventional procedures (2-5). Signs and symptoms of renal AVF can mimic other clinical diseases and we usually forget AVF as a differential diagnosis (6). The systemic renal AVF should be usually attempted and treated. There are two kinds of treatments including surgery and endovascular management. Endovascular management is the choice therapy because it is minimally invasive and it preserves renal function (1, 4, 7). We described a complicated case of renal AVF for primary diagnosis that finally was managed successfully.
2. Case Presentation
A 54-year-old woman with a history of cesarean section five months ago was evaluated for one episode of gross hematuria in an outpatient setting. She had no other symptoms. Her vital signs were stable and her hemoglobin was 12.1 g/dL. Urinary tract ultrasonography showed a lobulated cystic mass at the lower pole of left kidney. Intravenous pyelogram showed duplicated collecting system and lower pyelocalyceal dilatation of the left kidney. Abdominopelvic CT angiography demonstrated an enhanced space-occupying mass on the left pyelocalyceal system suggestive of malignant tumor. Given these data right nephrectomy was recommended to the patient but she refused nephrectomy and further evaluation. After nine years she was admitted to the hospital because of a severe left flank pain and an abdominal pain. She had no hematuria and other urinary tract symptoms in the last admission. On review of system she had shortness of breath (NYHA functional class II) and infrequent headaches. On examination, the blood pressure was 150/90 mmHg, the pulse was 110 beats per minute; the temperature was 37.2°C and the respiratory rate 20 breaths per minutes. Cardiac auscultation revealed a crescendo-decrescendo systolic murmur at the right upper sternal border and an S3. A continuous bruit with systolic accentuation was audible over the abdomen particularly left upper quadrant. The rest of the general examinations were normal. Her laboratory data were within normal limit. An electrocardiogram revealed tachycardia and left ventricular hypertrophy. A chest radiograph was normal. Transthoracic echocardiography showed decreased left ventricular ejection fraction (LVEF = 40%), mild left ventricular dilatation (left ventricular end diastolic diameter = 5.7 cm), mild left ventricular hypertrophy (interventricular septal thickness = 1.2 cm and posterior wall thickness = 1.1 cm) and increased left ventricular mass index (LVMI = 162.9 g/m2). According to her past medical history, abdominopelvic CT angiography was performed and it showed early filling of left renal vein, almost at the same time of left renal artery filling most probably due to arteriovenous fistula (Figure 1). To definite the diagnosis and possible interventional management, renal arteries angiography was performed and it demonstrated a large left renal arteriovenous fistula with early opacification of the dilated renal vein and weak left renal parenchymal opacification (Figure 2A). According to acceptable anatomy for endovascular treatment, and patient’s symptoms and signs, coil-embolization of arteriovenous fistula was done (with a 14 diameter Nit-occlude VSD coil). The post embolization angiogram revealed a direct flow via the renal artery to the parenchyma and then normal filling of the renal vein. There was no blood flow via the previous fistula. The procedure did not have any complications such as a partial renal infarction (Figure 2B). The patient was discharged on the second post procedural day with good conditions. After 6 months of follow-up, the patient had complete disappearance of gross hematuria episodes, flank pain, headache and dyspnea. Her blood pressure and heart rate decreased (blood pressure 135/80 mmHg and heart rate 80 beats per minute). On duplex ultrasonography there was no flow via the previous fistula. Color doppler and hemodynamic study of the renal vein were normal. Transthoracic echocardiography was performed for the second time and it showed normalization of the past data (left ventricular ejection fraction; 50%, left ventricular end diastolic diameter of 5.3 cm, interventricular septal thickness of 1 cm, posterior wall thickness=0.9cm and left ventricular mass index about 109.6 g/m2).
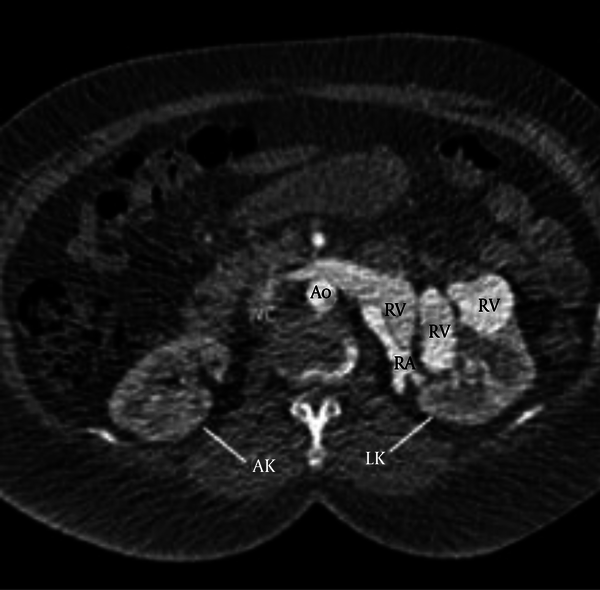
Contrast enhanced axial CT scan of the abdomen within the arterial phase showed dilatation of renal artery and renal vein and early filling of left renal vein almost at the same time of left renal artery filling most probably due to AVF. Ao = Aorta; LK = Left kidney; RA = Renal artery; RK = Right kidney; RV = Renal vein; IVC = Inferior vena cava.
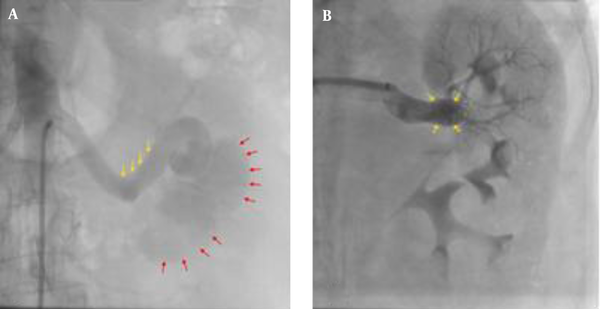
A, Digital subtraction angiography of left renal artery shows a large left renal AVF with early opacification of the dilated renal vein and weak parenchymal opacification; Renal artery (yellow arrows); renal vein (red arrows); B, Digital subtraction angiography after coil embolization shows a VSD coil in the entry of the AVF (yellow arrows); There is no blood flow via the previous fistula; The renal parenchymal opacification is increased.
3. Discussion
Renal AVF is a rare lesion. It might be acquired, idiopathic or congenital. The most common form is the acquired form and is a result of interventional procedures such as renal biopsy, percutaneous nephrostomy, partial nephrectomy and surgery. The acquired form is also due to tumor, trauma and inflammatory disease. Our patient had a history of cesarean section 5 months before starting symptoms so there can be a possibility of those categories. The congenital form is the rarest one and has angiomatous or cricoid formation. The idiopathic form is used to explain those forms in which the etiology is undetermined (2, 4, 5, 8).
Renal AVF is more frequent in right kidney, in women and in ages 30 to 40 years. Our patient was a woman but she was 54 years old and had involvement of left kidney (3).
Hematuria is the most frequent symptom of renal AVF. Other clinical appearances include wide pulse pressure, abdominal bruit, and mild tachycardia, heart failure, left ventricular hypertrophy, hypertension, renal failure and abdominal pain. Our patient was suffering from hematuria at her first presentation and she also had abdominal bruit, tachycardia, signs and symptoms of heart failure, left ventricular hypertrophy, grade 1 hypertension and abdominal pain (2, 3, 9).
Renal AVF can affect heart structure due to volume overload and high-output condition. It’s effect is eccentric left ventricular hypertrophy and heart failure (10). Hypertension creates concentric left ventricular hypertrophy (9). Our patient had left ventricular hypertrophy due to renal AVF. In renal AVF, hypertension arises owing to a renal parenchymal hypoperfusion distal to the fistula with increment renin secretion (2, 6). Treatment of renal AVF eventuates in decrement in LVEDD and LVMI (10). Management of high blood pressure for example with eliminating the underlying cause can lead to regression of LVH (9). In our patient LVEDD, LVMI and left ventricular wall thickness (LVWT) decreased. Decreasing in LVEDD and LVMI was due to treatment of renal AVF and decrease in LVWT might have been due to high blood pressure control. The result of renal AVF closing on left ventricular ejection fraction (LVEF) is controversial (10). In our patient LVEF increased from 40% to 50% about 6 months after treatment.
There are several modalities for diagnosis of renal AVF includes magnetic resonance imaging (MRI), computed tomography (CT), duplex ultra-sonography (DUS) and digital subtraction angiography (DSA). The gold standard modality for assessment of renal AVF is DSA. DUS is very efficient for diagnosis and is very useful in the follow-up after percutaneous intervention or surgery. We used CT scan for primary diagnosis of renal AVF in our patient and then we used DSA for certain diagnosis and management. For follow-up we used DUS for evaluation of patency of the previous renal AVF (1-3).
The treatment is usually performed for symptomatic patients. Some important indications for treatment are heart failure, hypertension, persistent or recurrent hematuria and increment in the extent of renal AVF in follow-up. Our patient had hypertension and symptoms and sings of heart failure (3, 4, 6).
There are two kinds of treatments for renal AVF include surgery and endovascular management. Endovascular management is the choice therapy even for large or high-flow renal AVF Due to the fact that not only it preserves renal function, but also it is minimally invasive and low risk. Large and high-flow renal AVFs have risk of pulmonary or systemic embolization so if there is fear for these problems surgery is preferred over endovascular treatment. Unfavorable anatomy for endovascular management is also an indication for surgery. Among endovascular procedures coil embolization is often used. For small fistulas embolization with methyl cyanoacrylate glue may be useful. In our patient due to large AVF and favorable anatomy for endovascular treatment, coil embolization with VSD coil was done (1, 4, 5, 7, 8).
Although renal artery embolization is a safe intervention, some complications may occur such as dissection or perforation of vessels, groin hematoma, coil migration, pulmonary or systemic embolization, renal failure, post embolization syndrome and infection (1, 4, 7).
3.1. Conclusion
Renal AVF should be considered as a differential diagnosis in patients with urinary complaints (especially hematuria) and indeterminate abnormalities in the urinary system. The gold standard modality for assessment of renal AVF is DSA and when the noninvasive data are non-diagnostic we can have a definite diagnosis with DSA. Endovascular management (coil embolization in most cases) is the best treatment and it is safe but it may have some complications especially in high-flow and large renal AVFs.
References
-
1.
Scheifley CH, Daugherty GW, Greene LF, Priestley JT. Arteriovenous fistula of the kidney; new observations and report of three cases. Circulation. 1959;19(5):662-71. [PubMed ID: 13652358].
-
2.
Paschalis-Purtak K, Januszewicz M, Rokicki A, Pucilowska B, Imiela J, Cybulska I, et al. Arteriovenous fistula of the kidney: a case report of 47-year-old female patient treated by embolisation. J Hum Hypertens. 2003;17(4):293-6. [PubMed ID: 12692574]. https://doi.org/10.1038/sj.jhh.1001544.
-
3.
Donmez FY, Coskun M, Uyusur A, Hunca C, Tutar NU, Basaran C, et al. Noninvasive imaging findings of idiopathic renal arteriovenous fistula. Diagn Interv Radiol. 2008;14(2):103-5. [PubMed ID: 18553286].
-
4.
Sauk S, Zuckerman DA. Renal artery embolization. Semin Intervent Radiol. 2011;28(4):396-406. [PubMed ID: 23204638]. https://doi.org/10.1055/s-0031-1296082.
-
5.
Melo NC, Mundim JS, Costalonga EC, Lucon AM, Santello JL, Praxedes JN. Three cases of hypertension and renal arteriovenous fistula with a de novo fistula. Arq Bras Cardiol. 2009;92(5):36-8. [PubMed ID: 19629281].
-
6.
Thayaparan A, Amer T, Mahdi E, Aboumarzouk O, Hughes O. Complete renal artery embolization in a comorbid patient with an arteriovenous malformation. Case Rep Urol. 2014;2014:856059. [PubMed ID: 24716086]. https://doi.org/10.1155/2014/856059.
-
7.
Ginat DT, Saad WE, Turba UC. Transcatheter renal artery embolization: clinical applications and techniques. Tech Vasc Interv Radiol. 2009;12(4):224-39. [PubMed ID: 20005480]. https://doi.org/10.1053/j.tvir.2009.09.007.
-
8.
Ghoneim TP, Thornton RH, Solomon SB, Adamy A, Favaretto RL, Russo P. Selective arterial embolization for pseudoaneurysms and arteriovenous fistula of renal artery branches following partial nephrectomy. J Urol. 2011;185(6):2061-5. [PubMed ID: 21496835]. https://doi.org/10.1016/j.juro.2011.02.049.
-
9.
Task Force for the management of arterial hypertension of the European Society of H, Task Force for the management of arterial hypertension of the European Society of C. 2013 ESH/ESC Guidelines for the Management of Arterial Hypertension. Blood Press. 2013;22(4):193-278. [PubMed ID: 23777479]. https://doi.org/10.3109/08037051.2013.812549.
-
10.
van Duijnhoven EC, Cheriex EC, Tordoir JH, Kooman JP, van Hooff JP. Effect of closure of the arteriovenous fistula on left ventricular dimensions in renal transplant patients. Nephrol Dial Transplant. 2001;16(2):368-72. [PubMed ID: 11158414].