Abstract
Background:
Anemia is a predictor of no reflow with high rate morbidity and mortality particularly in patients with heart failure.Objectives:
The aim of this study was to investigate the relation between hemoglobin level and no-reflowing in patients with myocardial infarction undergoing primary PCI.Methods:
In this retrospective cross sectional study, all patients with ST elevation myocardial infarction (STEMI) undergoing coronary angiography from 2016 till 2018 were included. According to post procedural TIMI flow, the patients were divided into two groups: patients with TIMI flow III and those with TIMI flow less than III. Patients’ demographic and laboratory data such as hemoglobin, hematocrit, platelets count, white blood cells, serum creatinine level and troponin were collected. To compare the quantitative variables chi square test and to compare qualitative variables t-test were applied by SPSS software version 19.Results:
In this study 1200 patients with acute ST elevation myocardial infarction (STEMI) treated with primary PCI were included. The mean Hemoglobin level in normal TIMI group and low TIMI group were 14.15 ± 1.49 and 13.66 ± 1.69, respectively (P < 0.001). Our results also showed a significant lower RBC count (P < 0.001), lower HCT level (P < 0 .001) and lower RDW (P < 0.001) in those patients with lower TIMI flow. Based on the multivariate regression analysis lower hemoglobin has a significant association with lower TIMI flow and no-reflow (OR = 0.747, CI = 0.618 - 0.888, P < 0.001).Conclusions:
Our study showed that in patients with STEMI undergoing primary PCI, hemoglobin level had a significant association with post procedural low TIMI flow and no-reflow. Our results emphasize the need for randomized control trials to evaluate the importance of pre-simultaneous blood transfusion in patients with anemia undergoing PPCI.Keywords
Hemoglobin Level Myocardial Infarction Coronary Angiography No-Reflow Phenomenon
1. Background
Anemia affects human health adversely. There are different co-morbidities causing anemia (1). The main role of hemoglobin is carrying oxygen to the body tissues. Hemoglobin level varies based on our body mass; hence, women have lower hemoglobin levels than men (2-5). The main factors affecting our hemoglobin level are enough erythropoiesis (Function of bone marrow), erythropoietin (EPO) production for kidney function and red blood cells mass proportionate to plasma volume (dilution of the blood) (5-7).
Anemia is very common in patients with chronic heart failure (8, 9). Existing in up to 50% of selected patients (10, 11), anemia is closely connected to the severity of heart failure and affects its symptoms. Anemia is also common in patients with acute coronary syndrome (12, 13). Although anemia could theoretically reduce oxygen supply to myocardium in acute coronary syndrome, its exact clinical importance is still not clear. Clinical guidelines recommend iron injection and blood infusion for its treatment (14, 15).
Treatment of anemia in acute coronary syndromes can cause undesirable results. It has multiplier adverse impacts on patients, regardless of starting before, during or after hospitalization. In all cases anemia is known as an independent predictor of in-hospital death and long term mortality (3, 16). Outcome of patients admitting with acute myocardial infarction is affected by some factors such as left ventricle performance, arrhythmia and ischemia-reperfusion injury (17, 18). Any decrease in hemoglobin level may harm enough oxygen delivery to myocardium in those coronary arteries occluded by clot and this could increase the chance of arrhythmia and cell death resulting in expansion of the infarcted area (19, 20). No-reflow after revascularization is a multi-factorial phenomenon with unknown etiology. Factors suggested to increase no-reflow are distal emboli, reperfusion injury that can cause oxygen free radical formation, microvascular damage, myocardial necrosis, stunning, released tissue factors from torn plaques, peripheral vasoconstriction, alpha adrenergic receptors and serotonin activity (21).
One study showed that lower levels of hemoglobin was an independent predictor of no-reflow in patients with acute STEMI treated with primary percutaneous intervention (PPCI) (21). Anemia also causes inflammatory responses that can release cytokines causing endothelial dysfunction, instability of atherosclerotic plaques and an increase in coagulation tendency (21). Anemia increases myocardial demand for oxygen by increasing heart rate, and probably by increasing microvascular resistance and microvascular dysfunction (21). Nowadays primary PCI has become the first line treatment in patients with STEMI and no-reflow is common in these patients (22). Hence, no-reflow diagnosis and prevention has become an incremental concern for interventional cardiologist who want to achieve best results with primary PCI (23, 24).
2. Objectives
There are few studies evaluating the role of anemia in no-reflow phenomenon. We decided to evaluate the role of anemia in no-reflow and low TIMI flow in patients undergoing primary PCI to find out its importance in the success rate of PPCI.
3. Methods
In this retrospective cross sectional study, all patients with ST elevation myocardial infarction (STEMI) undergoing coronary angiography from 2016 to 2018 were included. According to post procedural TIMI flow, patients were divided into two groups: patients with TIMI flow III and those with TIMI flow less than III. Patients’ demographic data including age, sex, risk factors for atherosclerosis, history of ischemic heart disease, heart failure and laboratory data including hemoglobin, hematocrit, platelets count, white blood cells, serum creatinine level and troponin were collected. Angiography records of all patients was analyzed by two expert cardiologists. Stenosis more than 70% defined as significant stenosis and post procedural TIMI flow of all patients were calculated and no-reflow during the procedure was determined according to angiography records. TIMI flow grading is defined as: grade 0 (no antegrade flow beyond stenosis), grade 1; (although contrast material passes beyond stenosis it does not fill the entire artery), grade 2; (although the whole vessel is filled by contrast material its flow and clearance is slower than normal vessel) and grade 3; (complete filling of vessel with contrast and its rapid clearance). This study was approved by ethics committee of Tabriz University of Medical Sciences with registration number of IR.TBZMED.REC.1398.490.
To compare the quantitative variables chi square test and to compare qualitative variables t-test were applied by SPSS software version 19. To analyze the independent impact of hemoglobin on no-reflow phenomenon multivariate regression analysis were used.
4. Results
In this study 1200 patients with acute ST elevation myocardial infarction treated with primary PCI were included. The mean Hemoglobin level in normal TIMI group and low TIMI group were 14.15 ± 1.49 and 13.66 ± 1.69, respectively (P < 0.001). Baseline characteristics of patients admitted for primary PCI according to their TIMI flow was shown in Table 1.
Baseline Characteristics of Patients Admitted for Primary PCI According to Their TIMI Flowa
| Parameter | Normal Reflow (N = 608) | No-Reflow (N = 592) | P-Value |
|---|---|---|---|
| Age | 59.80 ± 10.41 | 63.12 ± 10.59 | < 0.001 |
| Sex (male) | 469 (77.1) | 452 (76.4) | 0.75 |
| Left ventricular ejection fraction | 41.59 ± 6.63 | 38.05 ± 7.30 | < 0.001 |
| Systolic blood pressure | 139.02 ± 25.00 | 137.47 ± 26.72 | 0.300 |
| Diastolic blood pressure | 78.68 ± 10.98 | 78.15 ± 11.45 | 0.421 |
| Heart rate | 75.34 ± 15.15 | 80.31 ± 15.91 | < 0.001 |
| Time from symptoms onset to PCI, h | 5.67 ± 1.52 | 6.03 ± 1.53 | < 0.001 |
| Door to balloon time | 19.94 ± 9.27 | 20.29 ± 10.19 | 0.540 |
| Platelets (104) | 25.90 ± 4.92 | 25.95 ± 5.31 | 0.861 |
| Diabetes | 102 (16.8) | 106 (17.9) | 0.61 |
| Hypertension | 230 (37.8) | 305 (51.5) | < 0.001 |
| Hyperlipidemia | 120 (19.7) | 141 (23.8) | 0.09 |
| History of smoking | 269 (44.2) | 248 (41.9) | 0.41 |
| Family history of coronary artery disease | 62 (10.2) | 36 (6.1) | 0.01 |
| Previous PCI | 41 (6.7) | 34 (5.7) | 0.47 |
| Previous CABG | 7 (1.2) | 11 (1.9) | 0.31 |
| Myocardial infarction (anterior) | 300 (49.3) | 396 (66.9) | < 0.001 |
Our results also showed a significant lower red blood cells count (P < 0.001), lower hematocrit level (P < 0 .001) and lower red cell distribution width (P < 0.001) in those patients with lower TIMI flow. Table 2 shows laboratory findings of patients according to their TIMI flow. Table 3 shows angiographic findings of patients admitted for PPCI.
| Parameter | Normal Reflow (N = 608) | No-Reflow (N = 592) | P-Value |
|---|---|---|---|
| White blood cells | 8727.80 ± 1556.07 | 8920.61 ± 1556.41 | 0.032 |
| Hemoglobin | 14.15 ± 1.49 | 13.66 ± 1.69 | < 0.001 |
| Mean corpuscular volume | 87.06 ± 7.17 | 86.50 ± 7.54 | 0.187 |
| Red blood cells | 4.59 ± 0.55 | 4.7 ± 0.60 | 0.001 |
| Hematocrit | 44.73 ± 5.17 | 43.57 ± 4.97 | < 0.001 |
| Red cell distribution width | 11.78 ± 1.90 | 11.31 ± 2.10 | < 0.001 |
| CKMB | 68.46 ± 16.19 | 68.99 ± 16.06 | 0.569 |
| Mean platelet volume | 10.76 ± 1.39 | 10.57 ± 1.47 | 0.020 |
| Parameter | Normal reflow (N = 608) | No-reflow (N = 592) | P-Value |
|---|---|---|---|
| Thrombus burden | 4.25 ± 1.02 | 4.66 ± 0.68 | < 0.001 |
| Length of target lesion | 21.68 ± 6.09 | 22.13 ± 6.18 | 0.203 |
| Reference vessel diameter | 3.07 ± 0.37 | 3.13 ± 0.39 | 0.009 |
| Use of IIb, IIIa inhibitor | 291 (47.9) | 324 (54.7) | 0.02 |
| Thrombus aspiration | 86 (14.1) | 117 (19.8) | 0.01 |
| Outcome | 2 (0.3) | 19 (3.2) | < 0.001 |
| Number of involved vessel | < 0.001 | ||
| Single vessel disease (1VD) | 347 (57.1) | 279 (47.1) | |
| 2VD | 175 (28.8) | 189 (31.9) | |
| 3VD ± left main (LM) disease | 85 (14.0) | 120 (20.3) | |
| Culprit vessel | < 0.001 | ||
| LM | 1 (0.2) | 3 (0.5) | 0.00 |
| Left anterior descending (diagonal) | 291 (47.9) | 394 (66.6) | |
| Left circumflex artery (OM) | 121 (19.9) | 72 (12.2) | |
| Right coronary artery | 188 (30.9) | 119 (20.1) | |
| Reperfusion method | |||
| Balloon dilation | 11 (1.8) | 48 (8.1) | < 0.001 |
| Balloon predilation following Stent implantation | 373 (61.3) | 415 (70.1) | |
| Stent implantation | 222 (36.5) | 124 (20.9) |
Also in univariate analysis higher age, hypertension and anterior myocardial infarction had significant association with lower TIMI flow (Table 4).
Univariate and Multivariable Logistic Regression Analysis for Prediction of No-Reflow
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR | 95% CI | P-Value | Adjusted OR | 95% CI | P-Value | |
| Age | 1.031 | 1.019 - 1.042 | < 0.001 | 1.011 | 0.997 - 1.026 | 0.129 |
| Ejection fraction | 0.930 | 0.914 - 0.946 | < 0.001 | 0.961 | 0.939 - 0.983 | 0.001 |
| Heart rate | 1.021 | 1.013 - 1.028 | < 0.001 | 1.005 | 0.993 - 1.017 | 0.442 |
| Time from symptoms onset to PCI, h | 1.166 | 1.081 - 1.258 | < 0.001 | 1.145 | 1.048 - 1.251 | 0.003 |
| Thrombus burden | 1.756 | 1.519 - 2.029 | < 0.001 | 1.571 | 1.330 - 1.856 | < 0.001 |
| Reference vessel diameter | 1.488 | 1.104 - 2.004 | 0.009 | 1.590 | 1.101 - 2.295 | 0.013 |
| WBC | 1.000 | 1.000 - 1.000 | 0.033 | 1.000 | 1.000 - 1.000 | 0.066 |
| HB | 0.825 | 0.767 - 0.888 | < 0.001 | 0.741 | 0.618 - 0.888 | 0.001 |
| RBC | 0.709 | 0.581 - 0.865 | 0.001 | 1.431 | 0.933 - 2.196 | 0.101 |
| HCT | 0.955 | 0.933 - 0.977 | < 0.001 | 0.969 | 0.925 - 1.015 | 0.189 |
| RDW | 0.891 | 0.841 - 0.943 | < 0.001 | 1.072 | 0.955 - 1.204 | 0.239 |
| MPV | 0.911 | 0.841 - 0.986 | 0.021 | 0.999 | 0.904 - 1.105 | 0.989 |
| HTN | 1.747 | 1.388 - 2.198 | < 0.001 | 0.742 | 0.557 - 0.987 | 0.040 |
| Family history of coronary artery disease | 1.754 | 1.144 - 2.689 | 0.010 | 1.083 | 0.653 - 1.749 | 0.758 |
| MI (anterior) | 2.071 | 1.639 - 2.618 | < 0.001 | 0.083 | 0.009 - 0.743 | 0.023 |
| Use of IIbIIIa inhibitor | 0.764 | 0.609 - 0.959 | 0.020 | 0.784 | 0.591 - 1.039 | 0.090 |
| Thrombus aspiration | 0.674 | 0.497 - 0.914 | 0.011 | 0.723 | 0.504 - 1.039 | 0.080 |
| Outcome | 10.047 | 2.330 - 43.327 | 0.002 | 3.505 | 0.740 - 16.612 | 0.114 |
| No. of involved vessel (3VD ± LM) | 0.001 | 0.181 | ||||
| SVD | 0.570 | 0.414 - 0.784 | 0.001 | 0.655 | 0.408 - 1.050 | 0.079 |
| 2VD | 0.765 | 0.541 - 1.081 | 0.129 | 0.819 | 0.521 - 1.288 | 0.387 |
| Culprit vessel (RCA) | < 0.001 | 0.090 | ||||
| LM | 4.739 | 0.487 - 46.096 | 0.180 | 18.006 | 0.426 - 760.846 | 0.130 |
| LAD (diagonal) | 2.139 | 1.625 - 2.816 | < 0.001 | 16.868 | 1.912 - 148.801 | 0.011 |
| LCX (OM) | 0.940 | 0.649 - 1.363 | 0.744 | 1.096 | 0.703 - 1.708 | 0.687 |
| Reperfusion method (Stent implantation) | < 0.001 | < 0.001 | ||||
| Balloon dilation | 7.812 | 3.914 - 15.592 | < 0.001 | 5.114 | 2.248 - 11.634 | < 0.001 |
| Balloon predilation following stent implantation | 1.992 | 1.535 - 2.585 | < 0.001 | 1.562 | 1.157 - 2.109 | 0.004 |
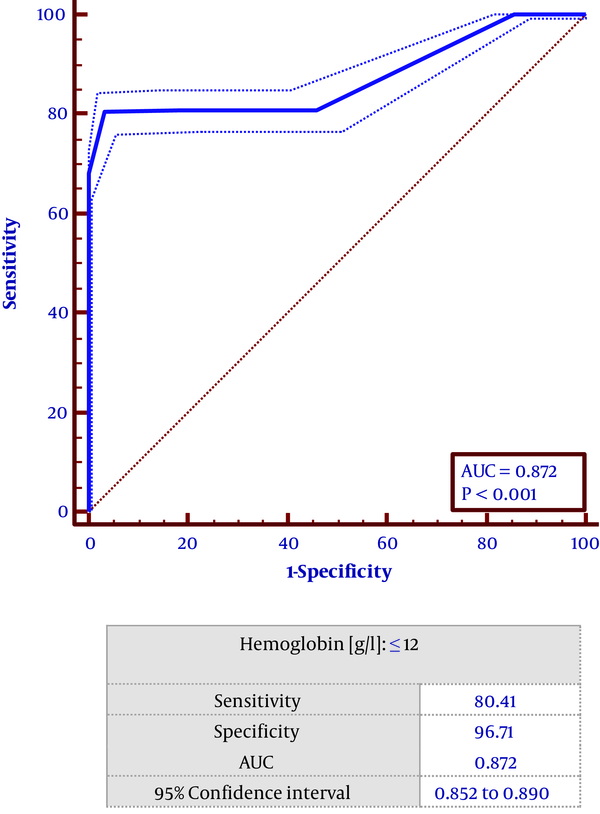
Based on the multivariate regression study left ventricular ejection fraction (OR = 0.94, CI = 0.93 - 0.98, P < 0.001), time from symptoms onset to PCI (OR = 1.14, CI = 1. 48 - 1.9, P < 0 .001), thrombus burden (OR = 1.57, CI = 1.33 - 1.86, P < 0 .001), reference vessel diameter: (OR: 1.59, CI = 1.101 - 2.275, P < 0.05), hemoglobin (OR = 0.747, CI = 0.618 - 0.888 0, P < 0.001), hypertension (HTN) (OR = 5.114, CI = 0.557 - 0.987, P < 0.05) and reperfusion method (OR = 5.114, CF = 2.248 - 11.634, P < 0.001) were found to predict lower TIMI flow in patients undergoing PPCI (Table 2). Receiver operating characteristic (ROC) curve showed that hemoglobin level ≤ 12 had a sensitivity of 80.4% and specificity of 96.7% in predicting low TIMI flow (Figure 1).
Receiver operating characteristic (ROC) curve shows the best cut off point for hemoglobin level in no-reflow phenomenon.
5. Discussion
Our study showed a significant association between lower TIMI flow and anemia. In acute coronary artery syndrome anemia has been shown as a predictor of short term and long term mortality (3, 16). Also in patients with heart failure, anemia is associated with higher mortality. With decreased hemoglobin level and red blood cell count, blood viscosity decreases causing decreased nitric oxide synthesis resulting in adverse effect on coagulation and plaque stability (1).
Similar to our study, Yaylak et al. (1) showed that hemoglobin level with moderate efficacy was an independent predictor of no-reflow in STEMI patients treated with primary PCI. They showed that a hemoglobin level below 11.5 g/dL had a sensitivity of 83.0% in predicting no-reflow phenomenon.
Contrary to our results, Huang et al. showed that hemoglobin level did not have any association with TIMI flow but serum iron concentration had association with TIMI risk score (24).
Lawler et al. (25) in a systematic review showed that in patients admitted with acute coronary syndrome, anemia had significant correlation with all-cause mortality (relative risk 2.08, 95% CI 1.70 - 2.55) and re-infarction (relative risk 1.25, 95% CI 1.02 - 1.53).
Studying patients with acute myocardial infarction undergoing PCI, Nikolsky et al. (26) showed that 12.8% of these patients had anemia. In this study anemia had a significant association with hospital stay, mortality and stroke. In multivariate analysis anemia was an independent predictor of short term and long term mortality.
Dundar et al. (27) also showed that in patients admitted with acute STEMI and treated by primary PCI, hospital mortality, heart failure and major bleeding were significantly higher than those with normal hemoglobin.
Wang et al. (28) showed that in the patients with acute STEMI undergoing primary PCI, a hemoglobin level < 120 g/L on admission had a significant association with major adverse cardiac events including death, coronary artery occlusion and stent restenosis. Our study showed that hemoglobin ≤ 12 g/dL had a sensitivity of 80.4% and specificity of 96.7% in low TIMI flow prediction.
Moghaddam et al. (29) also showed that in patients with STEMI treated with primary PCI, anemia had a great impact on major bleeding but not all cause mortality. They suggested that PCI was safe in anemic patients but preventing major bleeding was very important in these patients. In another study with a large sample size, blood transfusion could decrease cardiovascular death in patients with a hemoglobin level less than 12. About 80% of patients took transfusion when they had bleeding and the influence of blood transfusion when the patient did not have bleeding is not understood well (30). Rousseau et al. showed that recurrent myocardial ischemia had a significant association with anemia. This study shows that not only during PPCI, even after PPCI ischemic anemia continuous to have adverse effects on the perfusion of the heart (31). Because of different results of previous studies we conducted the present study to evaluate the importance of anemia in no-reflow phenomenon and we found that anemia could be an important factor affecting the success rate of PPCI adversely.
5.1. Conclusions
Our study showed that in patients with STEMI undergoing primary PCI, hemoglobin level had a significant association with post procedural low TIMI flow and no-reflow. Our results emphasizes the need for randomized control trials to evaluate the importance of pre-simultaneous blood transfusion in patients with anemia undergoing PPCI.
5.2. Limitation of Study
Our study was a cross sectional study and we had no data about the cause of anemia and the duration of anemia and its impact on no-reflow phenomenon. We did not have long term follow up to evaluate long term effects of anemia on recurrent ischemia and chronic cardiac ischemia.
References
-
1.
Yaylak B, Altintas B, Ozcan KS, Akdeniz E, Avci I, Zeren G, et al. Relation of hemoglobin level to no-reflow in patients with ST-segment elevation myocardial infarction undergoing primary coronary intervention. Postepy Kardiol Interwencyjnej. 2018;14(4):383-90. [PubMed ID: 30603028]. [PubMed Central ID: PMC6309849]. https://doi.org/10.5114/aic.2018.79868.
-
2.
Cornet M, Le Hesran JY, Fievet N, Cot M, Personne P, Gounoue R, et al. Prevalence of and risk factors for anemia in young children in southern Cameroon. Am J Trop Med Hyg. 1998;58(5):606-11. [PubMed ID: 9598449]. https://doi.org/10.4269/ajtmh.1998.58.606.
-
3.
Meneveau N, Schiele F, Seronde MF, Descotes-Genon V, Oettinger J, Chopard R, et al. Anemia for risk assessment of patients with acute coronary syndromes. Am J Cardiol. 2009;103(4):442-7. [PubMed ID: 19195499]. https://doi.org/10.1016/j.amjcard.2008.10.023.
-
4.
Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345(17):1230-6. [PubMed ID: 11680442]. https://doi.org/10.1056/NEJMoa010615.
-
5.
Archbold RA, Balami D, Al-Hajiri A, Suliman A, Liew R, Cooper J, et al. Hemoglobin concentration is an independent determinant of heart failure in acute coronary syndromes: cohort analysis of 2310 patients. Am Heart J. 2006;152(6):1091-5. [PubMed ID: 17161058]. https://doi.org/10.1016/j.ahj.2006.07.020.
-
6.
Sulaiman K, Prashanth P, Al-Zakwani I, Al-Mahmeed W, Al-Motarreb A, Al Suwaidi J, et al. Impact of anemia on in-hospital, one-month and one-year mortality in patients with acute coronary syndrome from the Middle East. Clin Med Res. 2012;10(2):65-71. [PubMed ID: 22593012]. [PubMed Central ID: PMC3355742]. https://doi.org/10.3121/cmr.2011.1032.
-
7.
Kunadian V, Mehran R, Lincoff AM, Feit F, Manoukian SV, Hamon M, et al. Effect of anemia on frequency of short- and long-term clinical events in acute coronary syndromes (from the Acute Catheterization and Urgent Intervention Triage Strategy Trial). Am J Cardiol. 2014;114(12):1823-9. [PubMed ID: 25438908]. https://doi.org/10.1016/j.amjcard.2014.09.023.
-
8.
Yazji K, Abdul F, Elangovan S, Ul Haq MZ, Ossei-Gerning N, Morris K, et al. Baseline anemia in patients undergoing percutaneous coronary intervention after an acute coronary syndrome-A paradox of high bleeding risk, high ischemic risk, and complex coronary disease. J Interv Cardiol. 2017;30(5):491-9. [PubMed ID: 28707308]. https://doi.org/10.1111/joic.12406.
-
9.
Kosiborod M, Curtis JP, Wang Y, Smith GL, Masoudi FA, Foody JM, et al. Anemia and outcomes in patients with heart failure: a study from the National Heart Care Project. Arch Intern Med. 2005;165(19):2237-44. [PubMed ID: 16246989]. https://doi.org/10.1001/archinte.165.19.2237.
-
10.
Maggioni AP, Opasich C, Anand I, Barlera S, Carbonieri E, Gonzini L, et al. Anemia in patients with heart failure: prevalence and prognostic role in a controlled trial and in clinical practice. J Card Fail. 2005;11(2):91-8. [PubMed ID: 15732027]. https://doi.org/10.1016/j.cardfail.2004.05.004.
-
11.
Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113(23):2713-23. [PubMed ID: 16754803]. https://doi.org/10.1161/CIRCULATIONAHA.105.577577.
-
12.
Varadarajan P, Gandhi S, Sharma S, Umakanthan B, Pai RG. Prognostic significance of hemoglobin level in patients with congestive heart failure and normal ejection fraction. Clin Cardiol. 2006;29(10):444-9. [PubMed ID: 17063948]. [PubMed Central ID: PMC6654216]. https://doi.org/10.1002/clc.4960291006.
-
13.
Willis P, Voeltz MD. Anemia, hemorrhage, and transfusion in percutaneous coronary intervention, acute coronary syndromes, and ST-segment elevation myocardial infarction. Am J Cardiol. 2009;104(5 Suppl):34C-8C. [PubMed ID: 19695359]. https://doi.org/10.1016/j.amjcard.2009.06.013.
-
14.
Bassand JP. Impact of anaemia, bleeding, and transfusions in acute coronary syndromes: a shift in the paradigm. Eur Heart J. 2007;28(11):1273-4. [PubMed ID: 17483543]. https://doi.org/10.1093/eurheartj/ehm132.
-
15.
Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, et al. Focused update of the ACC/AHA 2004 guidelines for the management of patients with S''T-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to review new evidence and update the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction, writing on behalf of the 2004 writing committee. Circulation. 2008;117(2):296-329. https://doi.org/10.1161/CIRCULATIONAHA.107.188209.
-
16.
Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey J, et al. ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-579. https://doi.org/10.1161/CIR.0b013e318212bb8b.
-
17.
Hasin T, Sorkin A, Markiewicz W, Hammerman H, Aronson D. Prevalence and prognostic significance of transient, persistent, and new-onset anemia after acute myocardial infarction. Am J Cardiol. 2009;104(4):486-91. [PubMed ID: 19660599]. https://doi.org/10.1016/j.amjcard.2009.03.066.
-
18.
Naccarella F, Lepera G, Rolli A. Arrhythmic risk stratification of post-myocardial infarction patients. Curr Opin Cardiol. 2000;15(1):1-6. [PubMed ID: 10666655]. https://doi.org/10.1097/00001573-200001000-00001.
-
19.
Stone GW, Grines CL, Browne KF, Marco J, Rothbaum D, O'Keefe J, et al. Implications of recurrent ischemia after reperfusion therapy in acute myocardial infarction: a comparison of thrombolytic therapy and primary angioplasty. J Am Coll Cardiol. 1995;26(1):66-72. [PubMed ID: 7797777]. https://doi.org/10.1016/0735-1097(95)00138-p.
-
20.
Levy PS, Kim SJ, Eckel PK, Chavez R, Ismail EF, Gould SA, et al. Limit to cardiac compensation during acute isovolemic hemodilution: influence of coronary stenosis. Am J Physiol. 1993;265(1 Pt 2):H340-9. [PubMed ID: 8342651]. https://doi.org/10.1152/ajpheart.1993.265.1.H340.
-
21.
Levy PS, Quigley RL, Gould SA. Acute dilutional anemia and critical left anterior descending coronary artery stenosis impairs end organ oxygen delivery. J Trauma. 1996;41(3):416-23. [PubMed ID: 8810957]. https://doi.org/10.1097/00005373-199609000-00006.
-
22.
Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54(4):281-92. [PubMed ID: 19608025]. https://doi.org/10.1016/j.jacc.2009.03.054.
-
23.
Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol. 2002;39(1):30-6. [PubMed ID: 11755283]. https://doi.org/10.1016/s0735-1097(01)01711-9.
-
24.
Huang CH, Chang CC, Kuo CL, Huang CS, Chiu TW, Lin CS, et al. Serum iron concentration, but not hemoglobin, correlates with TIMI risk score and 6-month left ventricular performance after primary angioplasty for acute myocardial infarction. PLoS One. 2014;9(8). e104495. [PubMed ID: 25100442]. [PubMed Central ID: PMC4123974]. https://doi.org/10.1371/journal.pone.0104495.
-
25.
Lawler PR, Filion KB, Dourian T, Atallah R, Garfinkle M, Eisenberg MJ. Anemia and mortality in acute coronary syndromes: a systematic review and meta-analysis. Am Heart J. 2013;165(2):143-53 e5. [PubMed ID: 23351816]. https://doi.org/10.1016/j.ahj.2012.10.024.
-
26.
Nikolsky E, Aymong ED, Halkin A, Grines CL, Cox DA, Garcia E, et al. Impact of anemia in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: analysis from the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Trial. J Am Coll Cardiol. 2004;44(3):547-53. [PubMed ID: 15358018]. https://doi.org/10.1016/j.jacc.2004.03.080.
-
27.
Dundar C, Oduncu V, Erkol A, Tanalp AC, Sirma D, Karagoz A, et al. In-hospital prognostic value of hemoglobin levels on admission in patients with acute ST segment elevation myocardial infarction undergoing primary angioplasty. Clin Res Cardiol. 2012;101(1):37-44. [PubMed ID: 21931965]. https://doi.org/10.1007/s00392-011-0361-9.
-
28.
Wang CH, Jin XF, Fang Q, Zhang SY, Shen ZJ, Fan ZJ, et al. [Effects of hemoglobin levels on long-term prognosis in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention]. Zhonghua Yi Xue Za Zhi. 2011;91(42):3003-6. Chinese. [PubMed ID: 22333029].
-
29.
Moghaddam N, Wong GC, Cairns JA, Goodman SG, Perry-Arnesen M, Tocher W, et al. Association of Anemia With Outcomes Among ST-Segment-Elevation Myocardial Infarction Patients Receiving Primary Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2018;11(12). e007175. [PubMed ID: 30562086]. https://doi.org/10.1161/CIRCINTERVENTIONS.118.007175.
-
30.
Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111(16):2042-9. [PubMed ID: 15824203]. https://doi.org/10.1161/01.CIR.0000162477.70955.5F.
-
31.
Rousseau M, Yan RT, Tan M, Lefkowitz CJ, Casanova A, Fitchett D, et al. Relation between hemoglobin level and recurrent myocardial ischemia in acute coronary syndromes detected by continuous electrocardiographic monitoring. Am J Cardiol. 2010;106(10):1417-22. [PubMed ID: 21059430]. https://doi.org/10.1016/j.amjcard.2010.07.009.