Abstract
Context:
Different factors such as the site of infection, the etiological agent, and the immune system can modify the antifungal response of the host. Differences in Sporothrix schenckii strains’ virulence and the host’s immune competency may be involved in the development of sporotrichosis. Nevertheless, the mechanisms related to the disease’s development and progression remain not fully elucidated. Nowadays, no model outweighs the usefulness of mice in biological studies. In these models, transient controlled immunity is created by depressed inflammatory cells during the acute phase of the disease. This is also related to nitric oxide-induced T-cell apoptosis and the lack of a mitogen response.Evidence Acquisition:
The recognition of the lipid components of S. schenckii can induce and prolong inflammation. This recognition occurs mainly through the Toll-like receptor (TLR)-4 or the inflammasome. At the same time, TLR-2-mediated identification of fungal exoantigen can serve as an immune evasion process, continuing and worsening the infection. Cell-mediated immune mechanisms have a predominant influence on modulating the clinical expression of sporotrichosis, which is mainly related to Th1/Th17 immunity.Conclusions:
In this study, we aimed to explore the innate and acquired immune mechanisms involved in sporotrichosis, as well as the most commonly used animal models for experimental studies.Keywords
Sporothrix schenckii Innate Immunity Acquired Immunity Inflammasome Experimental Sporotrichosis
1. Context
Sporotrichosis is a common subcutaneous mycosis in Latin America, acquired through traumatic inoculation of the fungus via contaminated organic material. The host’s immune system plays a crucial role in responding against the disease and reducing the progression of the infection. Immune cells, including neutrophils, macrophages, and numerous innate and acquired defense mechanisms interact with each other for recognizing and orchestrating coordinated immune responses against the pathogen (1).
The innate immune system consists of several specific cells representing the first line of defense against many fungal infections including sporotrichosis. These innate immune mechanisms include a variety of cells and pattern recognition receptors that are important to control systemic infections (2). Different factors such as the site of the infection, the causal agent, and the immune system can modulate the antifungal reaction of the host. Infectious agents such as Sporothrix sp. benefit from the resources provided to them by the host as long as the damage suffered from the immune system is not strong enough to affect the viability of the microbe (3). Here, we investigated the main immune mechanisms against sporotrichosis.
2. Evidence Acquisition
2.1. The Importance of the Innate Immune System in Sporotrichosis
The innate immune system allows distinguishing the host’s cells (self) from foreign structures (non-self). The system is the first line of defense against external, mostly pathogenic, agents (3). A properly functioning immune system is necessary for decreasing predisposition to numerous infections (4). The main mechanisms of the innate immunity against sporotrichosis, determining susceptibility to the infection, will be reviewed.
Several studies have been conducted in congenitally athymic mice (nu/nu) to assess the role of T-cell mediated immune responses in sporotrichosis, showing greater susceptibility and death rates in the infections caused by S. schenckii (5). In animal models, S. schenckii infection was shown to be promptly limited by effectively and persistently controlling the fungal load.
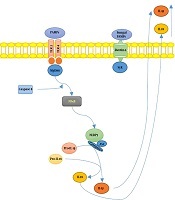
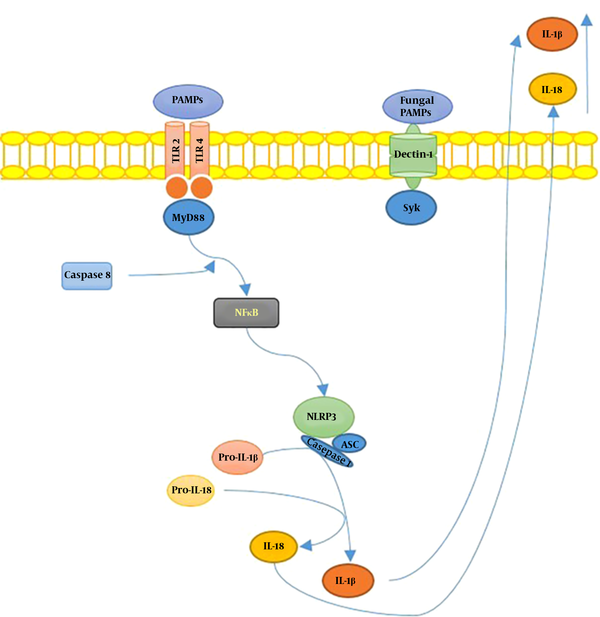
S. schenckiisensu stricto induced the release of several proinflammatory cytokines in mice experimental models (6), including TNFα and IL-6, at the infection site 24h after inoculation. However, in the case of S. brasiliensis, cytokines’ levels remained similar to uninfected mice. In Figure 1, we illustrated the hypothetical mechanisms of the interplay between Sporothrix sp. and the host’s immune system.
Summary of Sporotrix sp. and the host’s immune system interplay. Toll-like receptors (TLR) 2 and 4 recognize S. schenckii-derived mannans. The membrane-bound C-type lectins dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), macrophage-inducible C-type lectin, and macrophage mannose receptor (MR) recognize mannose-rich fungal structures. Moreover, the NOD-like receptor; NLRP3 (nucleotide-binding domain, leucine-rich-repeat-containing family, pyrin domain-containing 3), forms an inflammasome complex in combination with ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and caspase 1, leading to interleukin-1β (IL-1β) and IL-18 production, contributing to the host’s immune defense against sporotrichosis.
The increased virulence of S. brasiliensis, as well as the decreased activity of the innate immune system and reduced Th1 response may promote the advanced stage of the infection. T cells favor Th17-mediated immune activity to control fungal infections while reducing excessive and damaging inflammatory responses through inducing T regulatory (Treg) cells (7). These results showed that S. brasiliensis had greater virulence compared with S. schenckii and S. globosa; on the other hand, S. mexicana and S. pallida (formerly S. albicans) showed low or no virulence (7).
2.2. Importance of Reactive Oxygen and Nitrogen Species
Macrophages are specialized cells against different infectious agents, including fungi. Macrophages’ functions involve phagocytosis, antigen processing and presentation, and ultimately linking the innate immunity with adaptive immunity (8). In addition to phagocytosis, macrophages produce substances such as enzymes, complement components, and coagulation factors (9), as well as potent inflammatory mediators like reactive oxygen species (ROS) and reactive nitrogen species (RNS) (10). The relevance of these ROS (mainly hydrogen peroxide, H2O2) in the microbicidal activity of macrophages has been suggested in previous studies (11); moreover, macrophage activity is suppressed by free radicals, as well as superoxide dismutase (related to ROS) and catalase and NO (related to RNS) (12).
An imbalance has been observed between immune responses to infections and the virulence of the agent. This imbalance is mediated through the NO released by macrophages; one consequence of which includes lower susceptibility to the infection (13).
2.3. Importance of Pattern Recognition Receptors
Importance of pattern recognition receptors (PRRs) are classes of innate immunity receptors, that are often produced during cellular damage or early phases of infections. These include Toll-like (TLRs) and NOD type receptors (RTNs). The primary function of PRRs is the recognition of various pathogens, modulating stress signaling (14).
2.4. Importance of TLRs and Phagocytosis
TLRs were first identified in Drosophila melanogaster and suggested to control the activity and progression of several mycoses (14). The engagement of TLRs with their agonists favors the dimerization of their extra and intracellular domains, leading to signal transduction through the myeloid differentiation primary response protein 88 (MyD88) and the activation of adaptive immune responses (15). Immune recognition of antigens on the surface of most fungi is mainly carried out by TLR4 and, albeit in a smaller proportion, TLR2/1, and TLR2/6 heterodimers. During fungal infections, the functions of TLRs can be antagonistic but complementary. TLR4 regulates the Th1 response and influences the development of Th17, while TLR2 promotes the differentiation of Treg cells by regulating Th1 and Th17 activities (16, 17).
Phagocytosis constitutes the first line of immune defense, allowing for the sampling of antigens and the processing of invading pathogens (8). Phagocytosis is directed by several fully identified receptors including FC receptors, integrins, and complement receptors. Moreover, other receptors may be involved in the binding of antigens, potentiating their internalization (8). Among PRRs, mannose receptor (MRs, like Dectin-1, are phagocytic receptors with no inflammatory signaling; however, they co-modulate these signals and enhance phagocytosis in combination with other PRRs (such as TLRs 3, 7, 8 and 9) (18). Recent studies have shown the role of TLR2 in potentiating S. schenckii phagocytosis and inducing the production of inflammatory mediators by macrophages (19, 20).
2.5. Importance of RTNs and the Inflammasome
RTNs are cytosolic PRRs forming pentameric/heptameric protein structures known as inflammasomes. Infectious agents that surpass the host’s surface receptors are recognized by RTNs (21, 22). Inflammasome acts as a regulator of IL-18-mediated inflammation, contributing to the defense against infectious diseases; these mechanisms may enhance the antimicrobial characteristics of inflammatory cells, mainly phagocytes, activating Th1/Th17 responses (23).
In a recent report by Goncalves et al. (24), the role of inflammasomes in S. schenckii infection was determined, indicating that the inflammasomes assembled with NLRP3, an intracellular sensor that detects a broad range of microbial motifs (25), play a protective role against the infection. This function is related to the activation of pro-inflammatory mediators and IL-1/IL-17 induced T helper responses, linking the recognition of the fungus via innate immunity receptors to subsequent adaptive immune responses (25).
2.6. Importance of Macrophage Activation Profile
Macrophages evolve into two types of phenotypes (26) essential to adequate immune responses against several pathogens. These include activated “classic” (M1) and “alternative” (M2) cell types. The first may cause tissue damage and directly influence sporotrichosis through “producing high levels of IL-12and regulating the activity of nitric oxide (NO) synthase (27). On the other hand, M2 macrophages produce high amounts of IL10, regulate the activity of the arginase I enzyme, and promote tissue repair by inducing angiogenesis and the remodeling of the affected tissue (28). In an experimental model of sporotrichosis, both M1 and M2 macrophages were identified during the active phase of the disease. After stimulation with a cell wall-derived peptide-polysaccharide, the production of IL-10 and TFG-β increased during the infection (29).
2.7. Importance of Dendritic Cell (DC) Activation Profile
Activation of DCs regulates the interaction between pro- and anti-inflammatory mechanisms necessary for immune regulation (30). The specific function of these cells in sporotrichosis has not been entirely elucidated. Uenotsuchi et al. (30) showed that DCs derived from human monocytes expressed a unique cellular function when they were exposed to yeasts, and the conidia of S. schenckii derived from patients with either visceral or cutaneous sporotrichosis.
In another study, bone marrow-derived dendritic cells (BMDCs) were stimulated with either complete fungi or the exoantigen of S. schenckii; the recognition of these antigens by DCs (plasticity) triggered T cell-mediated immune mechanisms (31).
2.8. Importance of Interactions Among Immune Responses in Sporotrichosis
The interplay between innate and adaptive immune responses is important for more robust and directed responses leading to the control of infectious agents. Many specific antifungal cellular and humoral immune pathways are directed against S. schenckii, playing vital roles in protecting the host against the fungus (32).
The severity of sporotrichosis depends mainly on the extent of immune responses; immunosuppressed subjects develop disseminated clinical forms whereas immunocompetent subjects generally develop localized manifestations (24). This is probably due to variable T-cell mediated immune responses, which are essential to define the clinical severity of sporotrichosis (33).
IL-12 induces the proliferation of T cells and natural killer (NK) cells and is an essential mediator of immune responses against not only viruses but also fungi as shown by the growing evidence on the effects of some fungi such as S. schenckii on the maturation of NK cells and the production of proinflammatory cytokines (34). The development of Th1 immunity is primarily mediated by IL-12 and IFNγ (27). On the other hand, TNFα is an essential cytokine with a critical function in several acute and chronic inflammatory diseases (35). This can be explained by its ability to induce proinflammatory cytokines, mainly IL-1 (36).
IFNγ is a potent trigger for activating macrophages, and its local production in pathological conditions prolongs the course of diseases (37). Also, the macrophages activated by IFNγ produce different cytokines that control the progression of infectious agents; among these are TNFα, as well as IL6 and 12. IFNγ also induces NO production by stimulating macrophages and modulates T-cell proliferation and function mainly through regulating IL-12 production (38). Also, IFNγ improves the antigen presentation activity of macrophages and promotes different stimuli to increase the activity of Th1 lymphocytes, thereby enhancing not only innate but also adaptive immunity. IL4 and IL10 augment the humoral immunity via stimulating specialized cells such as eosinophils and mast cells, induce the differentiation of B cells to plasma cells, and finally trigger the production of IgE. In the initial phase of sporotrichosis, increased levels of IL4 may delay the onset of the Th1 response by inhibiting the production of IFNγ while in the later stages of the disease, the infection can be drastically limited by the intervention of cytokines such as IL4 (39). This initial increase in IL4 levels is maintained during the advanced stages of the disease, which in combination with elevated IgG titers, it reflects the involvement of Th2 immunity in advanced stages of sporotrichosis, at least in animal models (40).
2.9. Importance of Apoptosis and Th17 Response in Sporotrichosis
Apoptosis is a mechanism for cellular death, in which cells are removed without an evident sign of inflammation. Apoptosis may follow an infectious process either as an escape mechanism for the infectious agent or a strategy to prevent the spread of the infection (41). Fernandes et al. (42) showed that the immunosuppression associated with sporotrichosis and susceptibility to a systemic disease in a murine model were probably related to an increase in NO production, leading to the apoptosis of splenic cells, the suppression of the immune response, and the high production of IL-10 and TNFα, as observed in several infectious diseases including sporotrichosis (43). The above-mentioned findings seem to explain the depletion of cellular immunity observed in the acute phase of sporotrichosis (44).
2.10. Importance of the Th17 Response in Sporotrichosis
Infection-induced apoptosis is involved in the induction and development of Th17 responses (45); however, the role of this pathway in sporotrichosis pathogenesis is not clear yet. The essential role of Th17 cells in the immunoprotection against agents such as bacteria and fungi, as well as the production of IL17, which is necessary for homeostasis of the mucosa, has been shown in previous studies. IL17 also increases the recruitment of neutrophils and Th1 cells and induces the production of proinflammatory cytokines by epithelial cells (46).
A previous study evaluated the immunogenic potential of the fungus (S. schenckii) and its exoantigen. The exoantigen was sufficient to induce the release of proinflammatory cytokines (31). The exposure of BMDCs to the entire fungus induced the release of significant levels of IL-12 and IL-6; however, the release of TGFβ was minimal. IL-23, which is mainly induced by the exoantigen of S. schenckii, in addition to inducing high levels of IL-6 and decreasing IL-12, which inhibits the differentiation of Th17 cells, results in an intense release of IL-17 in the culture medium of BMDCs and splenic lymphocytes (31).
3. Conclusions
The recognition of S. schenckii via TLR4 leads to potent inflammatory reactions. TLR2 regulates inflammation and may represent an escape mechanism of the fungus. Simultaneously, the activation of inflammasomes and apoptosis by sporotrichosis regulates immune responses, mainly Th17-induced reactions, against S. schenckii. Nevertheless, these aspects are still under study. Currently, the important points related to the innate and adaptive immune responses against the infectious agent are under evaluation both in vivo and in vitro to characterize the behavior of the fungus in different experimental models.
References
-
1.
Carlos IZ, Sassa MF, da Graca Sgarbi DB, Placeres MC, Maia DC. Current research on the immune response to experimental sporotrichosis. Mycopathologia. 2009;168(1):1-10. [PubMed ID: 19241140]. https://doi.org/10.1007/s11046-009-9190-z.
-
2.
Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11(4):275-88. [PubMed ID: 21394104]. https://doi.org/10.1038/nri2939.
-
3.
Hamad M. Innate and adaptive antifungal immune responses: partners on an equal footing. Mycoses. 2012;55(3):205-17. [PubMed ID: 21815944]. https://doi.org/10.1111/j.1439-0507.2011.02078.x.
-
4.
Casadevall A, Pirofski LA. What Is a Host? Attributes of Individual Susceptibility. Infect Immun. 2018;86(2). [PubMed ID: 29084893]. [PubMed Central ID: PMC5778373]. https://doi.org/10.1128/IAI.00636-17.
-
5.
Lei PC, Yoshiike T, Yaguchi H, Ogawa H. Histopathological studies of Sporothrix schenckii-inoculated mice. Possible functions of polymorphonuclear leukocytes in normal and immunocompromised (congenitally athymic nude) mice. Mycopathologia. 1993;122(2):89-93. [PubMed ID: 8327001]. https://doi.org/10.1007/BF01103604.
-
6.
Batista-Duharte A, Tellez-Martinez D, Roberto de Andrade C, Portuondo DL, Jellmayer JA, Polesi MC, et al. Sporothrix brasiliensis induces a more severe disease associated with sustained Th17 and regulatory T cells responses than Sporothrix schenckii sensu stricto in mice. Fungal Biol. 2018;122(12):1163-70. [PubMed ID: 30449354]. https://doi.org/10.1016/j.funbio.2018.08.004.
-
7.
Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, et al. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34(3):422-34. [PubMed ID: 21435589]. [PubMed Central ID: PMC3258585]. https://doi.org/10.1016/j.immuni.2011.03.002.
-
8.
Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825-52. [PubMed ID: 11861619]. https://doi.org/10.1146/annurev.immunol.20.103001.114744.
-
9.
Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL. Anti-TNF-alpha therapies: the next generation. Nat Rev Drug Discov. 2003;2(9):736-46. [PubMed ID: 12951580]. https://doi.org/10.1038/nrd1175.
-
10.
Laskin JD, Heck DE, Laskin DL. Multifunctional role of nitric oxide in inflammation. Trends Endocrinol Metab. 1994;5(9):377-82. [PubMed ID: 18407233]. https://doi.org/10.1016/1043-2760(94)90105-8.
-
11.
Sasada M, Johnston RJ. Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980;152(1):85-98. [PubMed ID: 7400757]. [PubMed Central ID: PMC2185903]. https://doi.org/10.1084/jem.152.1.85.
-
12.
Lee M, Yea SS. Hydrogen peroxide inhibits the immune response to lipopolysaccharide by attenuating signaling through c-Jun N-terminal kinase and p38 associated with protein kinase C. Immunopharmacology. 2000;48(2):165-72. [PubMed ID: 10936514]. https://doi.org/10.1016/s0162-3109(00)00202-2.
-
13.
Schaffner A, Davis CE, Schaffner T, Markert M, Douglas H, Braude AI. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J Clin Invest. 1986;78(2):511-24. [PubMed ID: 3734102]. [PubMed Central ID: PMC423588]. https://doi.org/10.1172/JCI112603.
-
14.
Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011;29:1-21. [PubMed ID: 20936972]. [PubMed Central ID: PMC3434799]. https://doi.org/10.1146/annurev-immunol-030409-101229.
-
15.
Sasai M, Yamamoto M. Pathogen recognition receptors: ligands and signaling pathways by Toll-like receptors. Int Rev Immunol. 2013;32(2):116-33. [PubMed ID: 23570313]. https://doi.org/10.3109/08830185.2013.774391.
-
16.
Bourgeois C, Kuchler K. Fungal pathogens-a sweet and sour treat for toll-like receptors. Front Cell Infect Microbiol. 2012;2:142. [PubMed ID: 23189270]. [PubMed Central ID: PMC3504294]. https://doi.org/10.3389/fcimb.2012.00142.
-
17.
Sassa MF, Saturi AE, Souza LF, Ribeiro LC, Sgarbi DB, Carlos IZ. Response of macrophage Toll-like receptor 4 to a Sporothrix schenckii lipid extract during experimental sporotrichosis. Immunology. 2009;128(2):301-9. [PubMed ID: 19740386]. [PubMed Central ID: PMC2767319]. https://doi.org/10.1111/j.1365-2567.2009.03118.x.
-
18.
Moretti J, Blander JM. Insights into phagocytosis-coupled activation of pattern recognition receptors and inflammasomes. Curr Opin Immunol. 2014;26:100-10. [PubMed ID: 24556406]. [PubMed Central ID: PMC3932007]. https://doi.org/10.1016/j.coi.2013.11.003.
-
19.
Carlos IZ, Sgarbi DB, Santos GC, Placeres MC. Sporothrix schenckii lipid inhibits macrophage phagocytosis: involvement of nitric oxide and tumour necrosis factor-alpha. Scand J Immunol. 2003;57(3):214-20. [PubMed ID: 12641649]. https://doi.org/10.1046/j.1365-3083.2003.01175.x.
-
20.
Negrini Tde C, Ferreira LS, Alegranci P, Arthur RA, Sundfeld PP, Maia DC, et al. Role of TLR-2 and fungal surface antigens on innate immune response against Sporothrix schenckii. Immunol Invest. 2013;42(1):36-48. [PubMed ID: 23231043]. https://doi.org/10.3109/08820139.2012.719982.
-
21.
Philpott DJ, Yamaoka S, Israel A, Sansonetti PJ. Invasive Shigella flexneri activates NF-kappa B through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J Immunol. 2000;165(2):903-14. [PubMed ID: 10878365]. https://doi.org/10.4049/jimmunol.165.2.903.
-
22.
Yang CS, Shin DM, Jo EK. The Role of NLR-related Protein 3 Inflammasome in Host Defense and Inflammatory Diseases. Int Neurourol J. 2012;16(1):2-12. [PubMed ID: 22500248]. [PubMed Central ID: PMC3321399]. https://doi.org/10.5213/inj.2012.16.1.2.
-
23.
van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32(3):110-6. [PubMed ID: 21333600]. https://doi.org/10.1016/j.it.2011.01.003.
-
24.
Goncalves AC, Ferreira LS, Manente FA, de Faria C, Polesi MC, de Andrade CR, et al. The NLRP3 inflammasome contributes to host protection during Sporothrix schenckii infection. Immunology. 2017;151(2):154-66. [PubMed ID: 28140444]. [PubMed Central ID: PMC5418459]. https://doi.org/10.1111/imm.12719.
-
25.
Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23-35. [PubMed ID: 12511873]. https://doi.org/10.1038/nri978.
-
26.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677-86. [PubMed ID: 15530839]. https://doi.org/10.1016/j.it.2004.09.015.
-
27.
Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J Exp Med. 1998;187(12):2103-8. [PubMed ID: 9625771]. [PubMed Central ID: PMC2212367]. https://doi.org/10.1084/jem.187.12.2103.
-
28.
Alegranci P, de Abreu Ribeiro LC, Ferreira LS, Negrini Tde C, Maia DC, Tansini A, et al. The predominance of alternatively activated macrophages following challenge with cell wall peptide-polysaccharide after prior infection with Sporothrix schenckii. Mycopathologia. 2013;176(1-2):57-65. [PubMed ID: 23686275]. https://doi.org/10.1007/s11046-013-9663-y.
-
29.
Segal BH. Role of macrophages in host defense against aspergillosis and strategies for immune augmentation. Oncologist. 2007;12 Suppl 2:7-13. [PubMed ID: 18039634]. https://doi.org/10.1634/theoncologist.12-S2-7.
-
30.
Uenotsuchi T, Takeuchi S, Matsuda T, Urabe K, Koga T, Uchi H, et al. Differential induction of Th1-prone immunity by human dendritic cells activated with Sporothrix schenckii of cutaneous and visceral origins to determine their different virulence. Int Immunol. 2006;18(12):1637-46. [PubMed ID: 17035348]. https://doi.org/10.1093/intimm/dxl097.
-
31.
Verdan FF, Faleiros JC, Ferreira LS, Monnazzi LG, Maia DC, Tansine A, et al. Dendritic cell are able to differentially recognize Sporothrix schenckii antigens and promote Th1/Th17 response in vitro. Immunobiology. 2012;217(8):788-94. [PubMed ID: 22656886]. https://doi.org/10.1016/j.imbio.2012.04.006.
-
32.
Nascimento RC, Almeida SR. Humoral immune response against soluble and fractionate antigens in experimental sporotrichosis. FEMS Immunol Med Microbiol. 2005;43(2):241-7. [PubMed ID: 15681154]. https://doi.org/10.1016/j.femsim.2004.08.004.
-
33.
Shiraishi A, Nakagaki K, Arai T. Role of cell-mediated immunity in the resistance to experimental sporotrichosis in mice. Mycopathologia. 1992;120(1):15-21. [PubMed ID: 1480205]. https://doi.org/10.1007/BF00578497.
-
34.
Ferreira LS, Portuondo DL, Polesi MC, Carlos IZ. Natural killer cells are pivotal for in vivo protection following systemic infection by Sporothrix schenckii. Immunology. 2018;155(4):467-76. [PubMed ID: 30030839]. [PubMed Central ID: PMC6231018]. https://doi.org/10.1111/imm.12986.
-
35.
Eigler A, Greten TF, Sinha B, Haslberger C, Sullivan GW, Endres S. Endogenous adenosine curtails lipopolysaccharide-stimulated tumour necrosis factor synthesis. Scand J Immunol. 1997;45(2):132-9. [PubMed ID: 9042424]. https://doi.org/10.1046/j.1365-3083.1997.d01-377.x.
-
36.
Okuda Y, Sakoda S, Shimaoka M, Yanagihara T. Nitric oxide induces apoptosis in mouse splenic T lymphocytes. Immunol Lett. 1996;52(2-3):135-8. [PubMed ID: 8905408]. https://doi.org/10.1016/0165-2478(96)02597-7.
-
37.
Chen GH, McDonald RA, Wells JC, Huffnagle GB, Lukacs NW, Toews GB. The gamma interferon receptor is required for the protective pulmonary inflammatory response to Cryptococcus neoformans. Infect Immun. 2005;73(3):1788-96. [PubMed ID: 15731080]. [PubMed Central ID: PMC1064966]. https://doi.org/10.1128/IAI.73.3.1788-1796.2005.
-
38.
Tachibana T, Matsuyama T, Mitsuyama M. Involvement of CD4+ T cells and macrophages in acquired protection against infection with Sporothrix schenckii in mice. Med Mycol. 1999;37(6):397-404. [PubMed ID: 10647120]. https://doi.org/10.1046/j.1365-280x.1999.00239.x.
-
39.
Antachopoulos C, Roilides E. Cytokines and fungal infections. Br J Haematol. 2005;129(5):583-96. [PubMed ID: 15916680]. https://doi.org/10.1111/j.1365-2141.2005.05498.x.
-
40.
Maia DC, Sassa MF, Placeres MC, Carlos IZ. Influence of Th1/Th2 cytokines and nitric oxide in murine systemic infection induced by Sporothrix schenckii. Mycopathologia. 2006;161(1):11-9. [PubMed ID: 16389479]. https://doi.org/10.1007/s11046-005-0142-y.
-
41.
Labbe K, Saleh M. Cell death in the host response to infection. Cell Death Differ. 2008;15(9):1339-49. [PubMed ID: 18566602]. https://doi.org/10.1038/cdd.2008.91.
-
42.
Fernandes KS, Neto EH, Brito MM, Silva JS, Cunha FQ, Barja-Fidalgo C. Detrimental role of endogenous nitric oxide in host defence against Sporothrix schenckii. Immunology. 2008;123(4):469-79. [PubMed ID: 18194265]. [PubMed Central ID: PMC2433314]. https://doi.org/10.1111/j.1365-2567.2007.02712.x.
-
43.
Navarre WW, Zychlinsky A. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2000;2(4):265-73. [PubMed ID: 11207583]. https://doi.org/10.1046/j.1462-5822.2000.00056.x.
-
44.
Carlos IZ, Zini MM, Sgarbi DB, Angluster J, Alviano CS, Silva CL. Disturbances in the production of interleukin-1 and tumor necrosis factor in disseminated murine sporotrichosis. Mycopathologia. 1994;127(3):189-94. [PubMed ID: 7808513]. https://doi.org/10.1007/BF01102920.
-
45.
Brereton CF, Blander JM. Responding to infection and apoptosis--a task for TH17 cells. Ann N Y Acad Sci. 2010;1209:56-67. [PubMed ID: 20958317]. https://doi.org/10.1111/j.1749-6632.2010.05747.x.
-
46.
Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10(7):479-89. [PubMed ID: 20559326]. https://doi.org/10.1038/nri2800.