Abstract
Background:
Periodontitis is a chronic inflammatory disease that causes tissue destruction due to the imbalance in the oxidant-antioxidant system. Melatonin has anti-inflammatory, antioxidant, and immune-modulatory properties and is considered to be a biomarker and diagnostic/therapeutic agent in the pathogenesis of periodontitis.Objectives:
The present study aimed to evaluate the salivary melatonin level and its changes following non-surgical periodontal therapy in patients with periodontitis.Methods:
In total, 90 salivary samples were collected from 60 patients, including 30 from patients with moderate chronic periodontitis (before periodontal treatment), and 30 from the same patients one month after the non-surgical periodontal therapy, and 30 from periodontally healthy subjects (control). Salivary melatonin levels were measured using the competitive immunoassay of the human melatonin ELISA kit.Results:
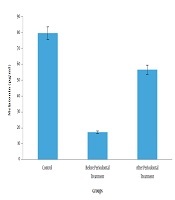
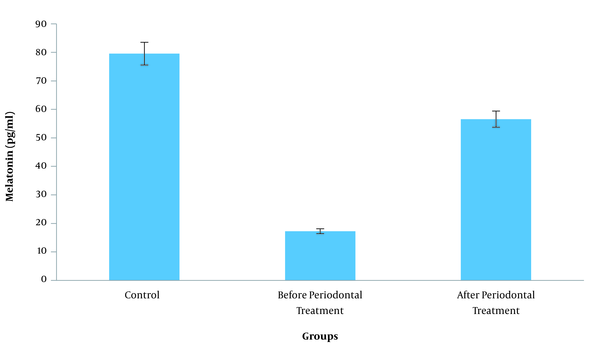
The highest melatonin concentration was observed in the control group (79.55 ± 59.22; P < 0.05), while the lowest concentration was observed in the pre-treatment group (P < 0.05). In addition, salivary melatonin concentration in the post-treatment group (56.58 ± 46.48) was significantly higher compared to the pre-treatment group (17.25 ± 5.79; P < 0.05).Conclusions:
According to the results, salivary melatonin levels improved after non-surgical periodontal therapy, which suggests the involvement of melatonin in the pathogenesis of periodontitis. However, the exact role of melatonin requires further investigation.Keywords
Non-surgical Periodontal Therapy Melatonin Chronic Periodontitis Saliva
1. Background
Melatonin (N-acetyl-5-methoxytryptamine) is an endogenous indolamine, which is responsible for the sleep/wake cycle. It is primarily secreted by the pineal gland with a circadian rhythm and a high level of secretion during the night and in darkness (1, 2). Other sources of melatonin secretion in the human body include the retina, gastrointestinal tract, leukocytes, bone marrow, and skin (3). Melatonin is recognized as a multitasking molecule with a wide variety of biological actions, including antioxidant effects (4, 5), anti-inflammatory effects, immunomodulatory properties (6), osteopromotion, bone loss inhibition (7, 8), oncostatic effects (9, 10), and neuroprotective properties (11). This biomarker enhances osteoblast differentiation and bone formation via stimulating type I collagen synthesis. Therefore, it exerts therapeutic effects on various injuries of the oral cavity through scavenging the free radicals produced during inflammation and reducing bone destruction (12). After the secretion of melatonin into the bloodstream, approximately 70% of the plasma melatonin binds to albumin, and 24% - 33% of free melatonin passively diffuses into the mouth through salivary glands. Therefore, melatonin is found in the saliva and crevicular fluid, representing the free fraction of melatonin in the plasma (13, 14).
Periodontitis is a highly prevalent inflammatory disorder worldwide, reported in 20% - 50% of the population. Periodontitis affects tooth-supporting structures, including the periodontal ligament, cementum, and the alveolar bone (15). This inflammatory disease is initiated due to the interactions between the host’s immune system, modifying environmental factors, and subgingival microbiota, particularly gram-negative organisms such as Porphyromonas gingivalis (16, 17). The activation of the immune response neutralizes the bacterial attack, while triggering the periodontal tissue destruction via releasing pro-inflammatory cytokines, prostaglandins, free radicals, and reactive oxygen species (ROS; oxidative damage). Previous studies have suggested that the presence of melatonin (direct scavenger of free radicals) in the saliva might affect the pathogenic process of periodontal diseases (18).
According to Drisko (19), non-surgical periodontal therapy, along with oral hygiene instructions, could help control the subgingival microbial plaque. In addition, the imbalance of the oxidant-antioxidant system due to the production of free radicals by bacteria and the host’s immune response during periodontitis could lead to oxidative damage to the periodontal tissue (20). Several studies have implied that salivary melatonin levels significantly decrease in patients with periodontal diseases, indicating that this biomarker could be used as a diagnostic and a potential therapeutic agent for periodontitis. Melatonin levels may change due to factors such as smoking, alcohol consumption, and aging, while no such effect has been attributed to gender (13). Melatonin is a direct scavenger of free radicals and promotes the anti-oxidative enzymes in the saliva, thereby indirectly affecting the pathogenesis of periodontal diseases (21).
2. Objectives
The present study aimed to investigate salivary melatonin level before and after non-surgical periodontal therapies to determine the treatment efficiency.
3. Methods
3.1. Subjects and Study Design
This case-control study was conducted to measure melatonin level before and after non-surgical periodontal therapies and treatment efficiency. Sample size was calculated based on the study by Lodhi et al. (22). In total, 60 participants (30 patients with moderate chronic periodontitis and 30 healthy controls) were recruited from the patients referring to the Periodontal Department of Babol University of Medical Sciences, Iran. The participants were informed about the study procedures and provided informed consent. The Ethics Committee of approved the research protocol in December 2017.
The inclusion criteria were as follows:
Patients aged 30 - 60 years;
Minimum of 20 natural teeth
The exclusion criteria were as follows:
Pregnancy and breastfeeding;
Systemic diseases (e.g., diabetes mellitus, kidney failure);
Using antioxidant or melatonin supplement therapies/medications (e.g., aspirin, diazepam) that modify the melatonin level within the past three months;
Immunosuppression (pathological/drug-induced) or autoimmune diseases;
History of periodontal treatment within the past six months;
Night work shifts.
3.2. Periodontal Evaluations
The participants were enquired about their medical and dental history, followed by the careful examination of the periodontal status using a standard periodontal probe (UNC-15, Hu-Fridey Instruments, Chicago, IL, USA). Each subject was motivated to observe oral hygiene and plaque control. clinical examinations and non-surgical periodontal therapy (scaling and root planing) were carried out by one periodontist. Measurements were obtained at four sites per teeth, including mesiofacial, buccal, distofacial, and lingual. The diagnostic criteria for moderate chronic periodontitis were as follows: Probing depth (≥ 5 and < 7 mm), clinical attachment loss (CAL) of 3 - 4 millimeters, and presence of bleeding on probing (23). The healthy subjects were confirmed by Leo and Silness gingival index (no CAL).
3.3. Sample Collection
In total, 90 saliva samples were collected, including 30 samples from the patients with moderate chronic periodontitis (pre-treatment), 30 samples from the same patients one month after scaling and root planing, and 30 samples from periodontally healthy subjects. Sample collection was performed at 9:00 AM - 12:00 AM. The participants were asked not to chew gum, eat or drink (except water) after waking. In addition, unstimulated saliva samples were harvested by the spitting method into sterile polypropylene tubes and sent to the Biochemistry Department of Babol University of Medical Sciences. The subjects were seated with their head bowed slightly forward for sample collection. Afterwards, one milliliter of saliva was centrifuged at 3,500 rpm for eight minutes to remove debris and preserved at the temperature of 4°C until evaluation.
3.4. Intervention
The patients diagnosed with periodontitis underwent non-surgical periodontal therapy (scaling and root planing) following a full mouth protocol using an ultrasonic device (DTE D5, China) and standard Gracey periodontal curettes (Hu-Friedy Instruments, Chicago, IL, USA). The patients were contacted one month after the treatment for sample collection.
3.5. Biochemical Assessment
The melatonin level of the saliva samples was measured using a competitive immunoassay. The kit uses the enzyme-linked immunosorbent assay (ELISA) technique based on the double-antibody sandwich technology to measure melatonin. In this study, melatonin was added to the wells, which were pre-coated with melatonin monoclonal antibody, and incubated. Afterwards, anti-melatonin antibodies labeled with biotin were added to the unit with streptavidin-HRP, forming an immune complex. The unbound enzymes were removed after incubation and washing. Following that, A and B substrates were added, and the solution turned blue and changed to yellow due to the acid effect. The shades of the solution and concentration of human melatonin were positively correlated.
3.6. Blinding
The operators in the Biochemistry Department and the data analyst were blinded from the beginning of the study to the completion of statistical analysis.
3.7. Statistical Analysis
Data analysis was performed in SPSS (Statistical Package for the Social Sciences V.24, Chicago, IL, USA) at the test power of 80% and significance level of 5% for the analysis of variance (ANOVA) and 1% for Tukey’s post-hoc test. Data were expressed as mean and standard deviation (SD). Continuous parameters were compared using paired t-test and one-way ANOVA, and categorical data were expressed as numbers and percentages. In addition, Tukey’s post-hoc test was used to compare the three study groups (healthy controls, before treatment, after treatment).
4. Results
In total, 90 saliva samples collected from 60 subjects were examined. The highest and lowest concentration of melatonin was observed in the control group and patients with periodontitis before the treatment, respectively. These data indicated that the melatonin concentration of the saliva increased after the non-surgical periodontal therapy (Table 1 and Figure 1). The significance level of F-test in ANOVA was 0.001, demonstrating that the difference in the melatonin level of the saliva was statistically significant between the three study groups. According to the information in Table 2, Tukey’s post-hoc test indicated that the discrepancy between the salivary melatonin level of the control group and patients with periodontitis before the treatment was the main cause of the difference in this regard (P = 0.001).
Comparison of melatonin between three study groups (control, before periodontal treatment, after periodontal treatment)
General Characteristics and Biochemical Parameters of Participants
Tukey’s Post-hoc Test to Compare Means between Groups
| Variable 1 | Variable 2 | Mean Difference ± SE | P-Value |
|---|---|---|---|
| Control | Before periodontal treatment | 67.30 ± 10.70 | 0.001a |
| After periodontal treatment | 22.97 ± 10.70 | 0.086 |
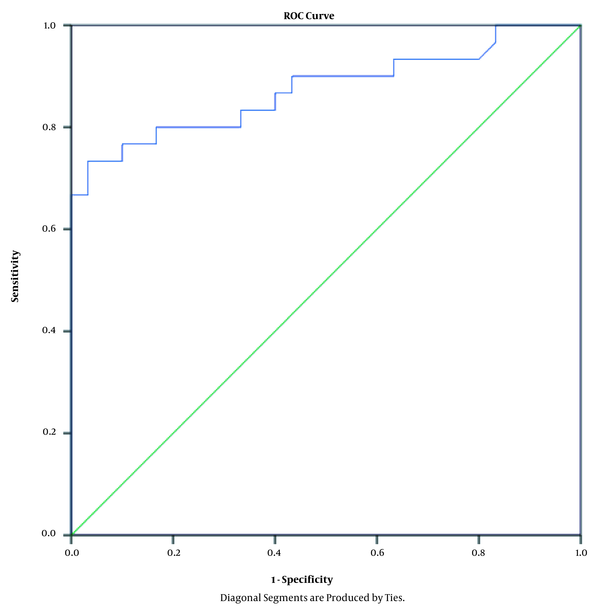
Table 3 shows the linear regression coefficients and significance levels. Accordingly, the non-surgical periodontal therapy enhanced the salivary melatonin level by 39.32 units (B = 39.32). Using the ROC curve, we also evaluated the specificity and sensitivity of the non-surgical periodontal therapies for the salivary melatonin level, and the obtained data suggested that the non-surgical periodontal therapies changed the salivary melatonin level to 23.79 ng/l with the specificity and sensitivity of 80% (area under ROC curve = 0.87) (Table 4 and Figure 2).
ROC curve showing specificity and sensitivity of non-surgical periodontal therapies on salivary melatonin
| Variable | B | SE | Beta | T | P-Value |
|---|---|---|---|---|---|
| Stage | 32.39 | 7.41 | 0.57 | 5.30 | 0.001b |
Results of ROC Curve Analysis
| Variable | Sensitivity, % | Specificity, % | Cutoff | AUC | P-Value |
|---|---|---|---|---|---|
| Melatonin | 80 | 80 | 23.79 | 0.87 | 0.001a |
5. Discussion
The present study indicated the increased level of salivary melatonin in the patients with periodontitis following the non-surgical periodontal therapy. Periodontitis is a chronic inflammatory disease, which destroys the tooth-supporting tissue due to the interaction between the subgingival flora and the host’s immune system. This is initiated by the release of pro-inflammatory cytokines, free radicals, and ROS (17, 18). Melatonin could exhibit antioxidant, anti-inflammatory, and bone-preserving properties, thereby playing a pivotal role in periodontal diseases (24). Several studies have reported that salivary melatonin decreases in patients with periodontitis, which was confirmed in the present study (20, 25). Therefore, it could be concluded that the reduction of salivary melatonin could be a potential indicator of periodontitis.
Following the successful non-surgical periodontal therapy in the current research, the mean salivary melatonin levels significantly increased by approximately 32.36 units. In a study in this regard, Baydas et al. (26) explained this phenomenon based on the theory of the destructive effects of periodontal inflammation on salivary glands, which causes a reduction in melatonin secretion. Our findings are consistent with the study by Bertl et al. (20), who reported a 25% increase in melatonin after a non-surgical periodontal therapy. Therefore, improved salivary melatonin could rebalance the oxidant/antioxidant system and reduce local inflammation in the oral cavity. According to the study by Balaji et al. (25), salivary glands produce melatonin as an extrapineal site of melatonin biosynthesis. On the other hand, melatonin 1 receptors in the buccal mucosa may exert receptor-mediated effects (27).
Melatonin secretion follows a circadian rhythm, with the maximal secretion occurring at 12:00 AM - 2:00 AM (3). In the current research, the samples were collected at 9:00 AM - 12:00 AM to achieve viable results, and sample collection was performed at the same time before and after the therapy to avoid the impact of the biorhythm on the melatonin level of the subjects.
Balaji et al. (25) reported no significant difference in the salivary or plasma melatonin levels of patients with chronic periodontitis. However, gingival crevicular fluid exhibited a significantly decreased level of melatonin (25). In another research, Ghallab et al. (28) demonstrated that melatonin levels in the gingival crevicular fluid were significantly higher in healthy controls compared to patients with generalized aggressive periodontitis and chronic periodontitis. Furthermore, the mentioned study indicated significantly lower melatonin levels in the patients with generalized aggressive periodontitis compared to those with chronic periodontitis (28). In contrast, Lodhi et al. stated that salivary melatonin levels increased with increased disease severity from control to periodontitis. They also observed that patients with periodontitis had the highest salivary melatonin levels, followed by gingivitis, while the lowest levels were reported in the healthy controls (22).
Given the conflicting results in this regard, we aimed to compare the salivary melatonin levels of patients with periodontitis with healthy subjects and its changes due to non-surgical periodontal therapy. According to our findings, the patients with chronic periodontitis had the lowest melatonin concentration before the treatment. However, we did not assess the difference in the melatonin levels between the patients with varied periodontal diseases (e.g., chronic/aggressive periodontitis, gingivitis).
According to the results of the present study, the healthy subjects had the highest melatonin level. After the periodontal treatment, the salivary melatonin level was observed to improve as well, which confirms the local involvement of melatonin and its immune-modulating effects. The usefulness of melatonin as a biomarker of periodontal disease was also confirmed by the sensitivity and specificity analysis using the ROC curve in our research. Correspondingly, the area under the curve (0.87) indicated an excellent performance. Furthermore, the ROC curve was used to assess the correlation between melatonin and the periodontal therapy.
Our findings demonstrated that melatonin changed with 80% sensitivity and 80% specificity from the pretreatment to the post-treatment stage. As a result, we decided to introduce a potentially significant cutoff for this association. Considering the ROC curve analysis and linear logistic regression, we established the sensitive cutoff of 23.75 from the pretreatment to the post-treatment stage in the periodontal patients. Melatonin could control the immune response of the periodontal tissue during local inflammation due to bacterial invasion (29). Therefore, it could be employed as an adjuvant therapy in the treatment of periodontal diseases (30-32). Further investigations are required to determine the effective dose of melatonin and its potential side-effects on the human body.
5.1. Conclusions
According to the results, salivary melatonin may be associated with periodontal inflammation. The levels of melatonin in the saliva were significantly lower in the patients with chronic periodontitis before the treatment compared to the healthy subjects. After the therapy, a significant increase was observed in the salivary melatonin levels. The lower melatonin in the patients with periodontitis could be due to the lack of protective or antioxidative role of melatonin in the oral cavity. It is recommended that further investigation be conducted to determine the underlying mechanism of melatonin in the pathogenesis of periodontal diseases.
References
-
1.
Pandi-Perumal SR, BaHammam AS, Brown GM, Spence DW, Bharti VK, Kaur C, et al. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res. 2013;23(3):267-300. [PubMed ID: 22739839]. https://doi.org/10.1007/s12640-012-9337-4.
-
2.
Annamalai S, Mohanam L, Alwin D, Prabhu V. Effect of combination therapy of melatonin and orlistat on high fat diet induced changes in lipid profiles and liver function parameters in serum of rats. Obes Med. 2016;2:41-5. https://doi.org/10.1016/j.obmed.2016.05.003.
-
3.
Carpentieri AR, Peralta Lopez ME, Aguilar J, Sola VM. Melatonin and periodontal tissues: Molecular and clinical perspectives. Pharmacol Res. 2017;125(Pt B):224-31. [PubMed ID: 28918172]. https://doi.org/10.1016/j.phrs.2017.09.003.
-
4.
Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51(1):1-16. [PubMed ID: 21752095]. https://doi.org/10.1111/j.1600-079X.2011.00916.x.
-
5.
Bonnefont-Rousselot D, Collin F, Jore D, Gardes-Albert M. Reaction mechanism of melatonin oxidation by reactive oxygen species in vitro. J Pineal Res. 2011;50(3):328-35. [PubMed ID: 21244479]. https://doi.org/10.1111/j.1600-079X.2010.00847.x.
-
6.
Mauriz JL, Collado PS, Veneroso C, Reiter RJ, Gonzalez-Gallego J. A review of the molecular aspects of melatonin's anti-inflammatory actions: recent insights and new perspectives. J Pineal Res. 2013;54(1):1-14. [PubMed ID: 22725668]. https://doi.org/10.1111/j.1600-079X.2012.01014.x.
-
7.
Renn TY, Huang YK, Feng SW, Wang HW, Lee WF, Lin CT, et al. Prophylactic supplement with melatonin successfully suppresses the pathogenesis of periodontitis through normalizing RANKL/OPG ratio and depressing the TLR4/MyD88 signaling pathway. J Pineal Res. 2018;64(3). [PubMed ID: 29274168]. https://doi.org/10.1111/jpi.12464.
-
8.
Maria S, Witt-Enderby PA. Melatonin effects on bone: potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J Pineal Res. 2014;56(2):115-25. [PubMed ID: 24372640]. https://doi.org/10.1111/jpi.12116.
-
9.
Mir SM, Yousefi B, Marjani A, Rahimi M, Qujeq D. The Sensitization of Melatonin in Osteosarcoma Cells by Suppression of Anti-Apoptotic Proteins. Pharm Sci. 2020;26(2):159-64. https://doi.org/10.34172/ps.2020.3.
-
10.
Niu G, Yousefi B, Qujeq D, Marjani A, Asadi J, Wang Z, et al. Melatonin and doxorubicin co-delivered via a functionalized graphene-dendrimeric system enhances apoptosis of osteosarcoma cells. Mater Sci Eng C Mater Biol Appl. 2021;119:111554. [PubMed ID: 33321618]. https://doi.org/10.1016/j.msec.2020.111554.
-
11.
Joshi N, Biswas J, Nath C, Singh S. Promising Role of Melatonin as Neuroprotectant in Neurodegenerative Pathology. Mol Neurobiol. 2015;52(1):330-40. [PubMed ID: 25159482]. https://doi.org/10.1007/s12035-014-8865-8.
-
12.
Czesnikiewicz-Guzik M, Konturek SJ, Loster B, Wisniewska G, Majewski S. Melatonin and its role in oxidative stress related diseases of oral cavity. J Physiol Pharmacol. 2007;58 Suppl 3:5-19. [PubMed ID: 17901579].
-
13.
Srinath R, Acharya AB, Thakur SL. Salivary and gingival crevicular fluid melatonin in periodontal health and disease. J Periodontol. 2010;81(2):277-83. [PubMed ID: 20151807]. https://doi.org/10.1902/jop.2009.090327.
-
14.
Cutando A, Galindo P, Gomez-Moreno G, Arana C, Bolanos J, Acuna-Castroviejo D, et al. Relationship between salivary melatonin and severity of periodontal disease. J Periodontol. 2006;77(9):1533-8. [PubMed ID: 16945031]. https://doi.org/10.1902/jop.2006.050287.
-
15.
Sanz M, D'Aiuto F, Deanfield J, Fernandez-Aviles F. European workshop in periodontal health and cardiovascular disease--scientific evidence on the association between periodontal and cardiovascular diseases: a review of the literature. Eur Heart J Suppl. 2010;12(Suppl B):B3-B12. https://doi.org/10.1093/eurheartj/suq003.
-
16.
Babaei H, Forouzandeh F, Maghsoumi-Norouzabad L, Yousefimanesh HA, Ravanbakhsh M, Zare Javid A. Effects of Chicory Leaf Extract on Serum Oxidative Stress Markers, Lipid Profile and Periodontal Status in Patients With Chronic Periodontitis. J Am Coll Nutr. 2018;37(6):479-86. [PubMed ID: 29558323]. https://doi.org/10.1080/07315724.2018.1437371.
-
17.
Bartold PM, Van Dyke TE. Host modulation: controlling the inflammation to control the infection. Periodontol 2000. 2017;75(1):317-29. [PubMed ID: 28758299]. https://doi.org/10.1111/prd.12169.
-
18.
Akalin FA, Baltacioglu E, Alver A, Karabulut E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J Clin Periodontol. 2007;34(7):558-65. [PubMed ID: 17555410]. https://doi.org/10.1111/j.1600-051X.2007.01091.x.
-
19.
Drisko CH. Nonsurgical periodontal therapy. Periodontol 2000. 2001;25:77-88. [PubMed ID: 11155183]. https://doi.org/10.1034/j.1600-0757.2001.22250106.x.
-
20.
Bertl K, Schoiber A, Haririan H, Laky M, Steiner I, Rausch WD, et al. Non-surgical periodontal therapy influences salivary melatonin levels. Clin Oral Investig. 2013;17(4):1219-25. [PubMed ID: 22847856]. https://doi.org/10.1007/s00784-012-0801-6.
-
21.
Reiter RJ, Rosales-Corral SA, Liu XY, Acuna-Castroviejo D, Escames G, Tan DX. Melatonin in the oral cavity: physiological and pathological implications. J Periodontal Res. 2015;50(1):9-17. [PubMed ID: 24665831]. https://doi.org/10.1111/jre.12176.
-
22.
Lodhi K, Saimbi CS, Khan MA, Nath C, Shukla R. Evaluation of melatonin levels in saliva in gingivitis and periodontitis cases: A pilot study. Contemp Clin Dent. 2016;7(4):519-23. [PubMed ID: 27994421]. [PubMed Central ID: PMC5141668]. https://doi.org/10.4103/0976-237X.194115.
-
23.
Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol. 2018;89 Suppl 1:S159-72. [PubMed ID: 29926952]. https://doi.org/10.1002/JPER.18-0006.
-
24.
Bae WJ, Park JS, Kang SK, Kwon IK, Kim EC. Effects of Melatonin and Its Underlying Mechanism on Ethanol-Stimulated Senescence and Osteoclastic Differentiation in Human Periodontal Ligament Cells and Cementoblasts. Int J Mol Sci. 2018;19(6). [PubMed ID: 29895782]. [PubMed Central ID: PMC6032161]. https://doi.org/10.3390/ijms19061742.
-
25.
Balaji TM, Vasanthi HR, Rao SR. Gingival, plasma and salivary levels of melatonin in periodontally healthy individuals and chronic periodontitis patients: a pilot study. J Clin Diagn Res. 2015;9(3):ZC23-5. [PubMed ID: 25954699]. [PubMed Central ID: PMC4413149]. https://doi.org/10.7860/JCDR/2015/11311.5652.
-
26.
Baydas G, Canatan H, Turkoglu A. Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin-induced diabetes mellitus. J Pineal Res. 2002;32(4):225-30. [PubMed ID: 11982791]. https://doi.org/10.1034/j.1600-079x.2002.01856.x.
-
27.
Madapusi BT, Rao SR. Preliminary Evaluation of Human Gingiva as an Extrapineal Site of Melatonin Biosynthesis in States of Periodontal Health and Disease. J Clin Diagnos Res. 2018. https://doi.org/10.7860/jcdr/2018/32451.11078.
-
28.
Ghallab NA, Hamdy E, Shaker OG. Malondialdehyde, superoxide dismutase and melatonin levels in gingival crevicular fluid of aggressive and chronic periodontitis patients. Aust Dent J. 2016;61(1):53-61. [PubMed ID: 25581300]. https://doi.org/10.1111/adj.12294.
-
29.
Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50(4):1129-46. [PubMed ID: 14740000].
-
30.
Tinto M, Sartori M, Pizzi I, Verga A, Longoni S. Melatonin as host modulating agent supporting nonsurgical periodontal therapy in patients affected by untreated severe periodontitis: A preliminary randomized, triple-blind, placebo-controlled study. J Periodontal Res. 2020;55(1):61-7. [PubMed ID: 31407353]. https://doi.org/10.1111/jre.12686.
-
31.
Bazyar H, Gholinezhad H, Moradi L, Salehi P, Abadi F, Ravanbakhsh M, et al. The effects of melatonin supplementation in adjunct with non-surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: a double-blind, placebo-controlled trial. Inflammopharmacology. 2019;27(1):67-76. [PubMed ID: 30328031]. https://doi.org/10.1007/s10787-018-0539-0.
-
32.
El-Sharkawy H, Elmeadawy S, Elshinnawi U, Anees M. Is dietary melatonin supplementation a viable adjunctive therapy for chronic periodontitis?-A randomized controlled clinical trial. J Periodontal Res. 2019;54(2):190-7. [PubMed ID: 30298556]. https://doi.org/10.1111/jre.12619.