Abstract
Context:
The unprecedented rise in antibiotic-resistant bacterial infections is a challenging dilemma, urging the development of novel and effective antibiotics for the treatment of such infections. Anti-virulence therapy is a promising solution in this regard. Unlike antibiotics, these drugs could lower the selective evolutionary pressure on a bacterial population and target the virulence factor rather than the growth pathways, thereby targeting and repressing the propagation of antibiotic resistance and virulence genes in bacteria. The present study aimed to investigate anti-virulence therapy against bacterial infections, as well as the mechanisms of action and challenges.Evidence Acquisition:
This literature review discusses the issues of antibiotic resistance developed in bacterial infections, the difficulties in antibiotic treatment, the mechanisms involved in anti-virulence therapy, and the approved anti-virulence drug therapeutics.Results:
We outlined the success in overcoming antibiotic resistance by using anti-virulence therapy as an alternate treatment option, while also discussing the drawbacks associated with their use and safety against bacterial infections.Conclusions:
According to the results, anti-virulence therapy combined with antibiotic treatment is effective in the treatment of several bacterial infections.Keywords
Bacterial Infections Drugs Antibiotics Anti-virulence Therapy Antibiotic Resistance Virulence Genes
1. Context
Antimicrobial resistance has become a prominent public health crisis worldwide. Although antibiotics have served as life-saving drugs since the previous century, questions remain to be addressed regarding the effectiveness of these agents in the treatment of various infections. A report by the Centre for Disease Control and Prevention (CDC) estimates a minimum of 2.8 million patients with antibiotic-resistant infections and more than 35,000 annual deaths in the United States (1). Over the past decades, antibiotic resistance has continued to spread at an unprecedented rate, which is a multifactorial phenomenon. Genetic components encoding antibiotic resistance are transferred between different bacteria via horizontal gene transfer (2). In addition, conventional approaches to combating bacterial infections have relied solely on the disruption of bacterial cellular activities, which leads to bacterial death. This approach imposes an evolutionary pressure within the bacterial population, thereby leading to the selection of resistant bacteria (3).
Compared to conventional antibiotic treatment, anti-virulence compounds could dismantle bacterial virulence factors, providing a different treatment approach in the battle against antimicrobial resistance (3, 4). Anti-virulence therapeutic strategies have emerged as a promising alternative targeting virulence factors. The principle of anti-virulence therapy is interference with pathogen-host interactions and reducing damage to the host by avoiding the death of the pathogen directly (4, 5). Since anti-virulence therapy does not kill pathogens, there is a low selective evolutionary pressure to develop resistant mutants. Recently, anti-virulence drugs have been identified and approved for humans. For instance, 5-fluorouracil (5-FU) has been reported to inhibit biofilm production in Pseudomonas aeruginosa (6).
Virulence factors are bacterial products used by pathogens to invade the host and cause disease. Some of these factors include adhesions, toxins, and specialized secretion systems to deliver effectors and facilitate the gene regulation of virulence traits. Bacterial adhesion to the host is the primary phase of infection. Therefore, several anti-virulence strategies have been adopted to interfere with bacterial adhesion or biofilm formation (7). Furthermore, various toxins are secreted by pathogens to contribute to the development of a diseased condition in the system of the host. Therefore, toxin neutralization is considered an effective strategy for diminishing the virulence of pathogens (8).
Since the proper folding and assembly of virulence factors are essential to their biological activities, they might become potential targets for anti-virulence therapeutics to disturb virulence in pathogens (9). Recently, bacterial functional membrane microdomains (FMMs) have been reported to play a pivotal role in the assembly of several virulence factors, and targeting FMMs may be another possible therapeutic strategy in this regard (10). Another factor is quorum-sensing systems (QSs), which regulate the production of several virulence factors. QSs are among the most exploited targets for the development of anti-virulence drugs (11). Overall, anti-virulence therapy offers a promising solution for the treatment of various bacterial infections.
The present study aimed to provide an overview of the mechanisms of action of anti-virulence therapy and discuss promising anti-virulence therapeutic drugs, as well as the possible challenges associated with anti-virulence treatment.
2. Evidence Acquisition
The primary objective of this literature review was to assess antibiotic resistance in various bacterial infections and the use of anti-virulence drugs for the effective treatment of antibiotic-resistant bacterial infections. A literature search was conducted using terminologies such as antibiotic resistance in bacterial infections and anti-virulence therapy. In addition, we have discussed the mechanisms of action associated with the anti-virulence strategies used for the treatment of bacterial infections.
2.1. Mechanisms of Action
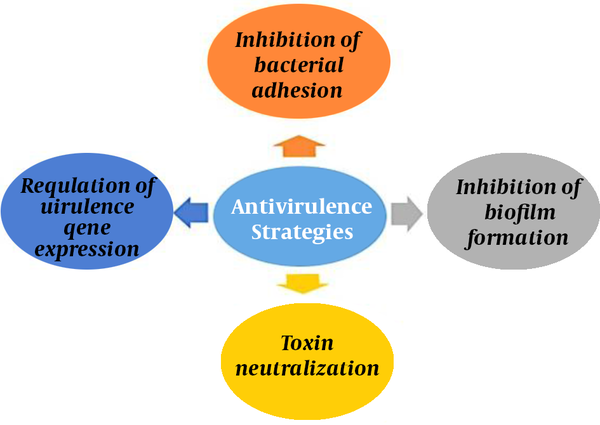
Traditional antibiotics cause bacterial death by interfering with the essential cellular activities of bacteria. The virulence properties of any pathogen show the capability of the pathogen to cause disease in the host. Some important anti-virulence strategies include targeting the adhesion mechanism of bacterial pathogens to host cells, biofilm formation, toxin neutralization, and regulation of virulence expressions. Since bacterial virulence targeting is specific, normal microflora remains safe in contrast to antibiotic therapy (4, 12). (Figure 1)
Different anti-virulence strategies against bacterial infections
2.2. Inhibition of Adhesions and Biofilm Formation
Bacterial infections begin with the pathogen attaching to the host cell, which leads to disease development. Adhesins are the key virulence factors involved in this adhesion mechanism, which aid in the attachment to further cause the colonization of bacteria within the system of the host.
Bacteria have developed various adaptation mechanisms, such as the pili and flagella that allow their adhesion to the host (13, 14). Pili subunits are assembled into a multi-subunit structure known as the pilus. Uropathogenic E. coli (UPEC) is the leading cause of urinary infection, which requires two pili (type I and P) to adhere to the host tissue and cause infection (15). Therefore, the anti-virulence agents that inhibit the pili/fimbriae or adhesins restrict the interaction of bacterial cells with the host tissues, thereby contributing to the clearance of the bacteria from the system of the host. This strategy provides an edge by avoiding the release and translocation of other virulence factors, such as toxins (16).
Research has shown multiple potential inhibitors (eg, bicyclic 2-pyridones and N-substituted amino acid derivatives) for pili formation (17). Furthermore, bacterial lectins such as FimH or PapG are located on the pili, contributing to pathogenesis through invasion, colonization, and biofilm formation (18). Lectins also recognize a specific type of mannose receptors on the host cell surface. Therefore, researchers have been investigating the use of lectins as the potential therapeutic targets of anti-virulence therapy (19, 20).
According to the literature, biphenyl mannosides could effectively inhibit bacterial lectins (19). An alternate strategy in this regard is to inhibit the attachment of bacteria by preventing the binding of bacterial surface proteins to the host receptors. Several pathogens use glycosphingolipids on the cell surface of the host as their binding receptors (21). Therefore, previous findings have shown success in mouse cells through blocking the ceramide-specific glycosyltransferase enzyme to reduce the colonization of UPEC E. coli (22).
Sortase enzyme is found in numerous Gram-positive pathogens such as staphylococci, streptococci, enterococci, and Listeria monocytogenes, playing a key role in virulence mechanisms. In Staphylococcus aureus, sortase A contributes to bacterial adhesion and the further invasion of host tissues, along with biofilm formation. It also aids in immune evasion by inhibiting opsonization and phagocytosis. Synthetic compounds such as methyl (2E)-2,3-bis(4-methoxyphenyl) prop-2-enoate and (Z)-3-(2,5-dimethoxy phenyl)-2-(4-methoxyphenyl) acrylonitrile also show sortase A inhibitory activity in S. aureus infections (23, 24).
Biofilm formation is another cause of bacterial infections. Biofilm is a thin mucilage layer containing a population of bacteria and contributes to the adherence of the bacteria to the host cell. This evolutionary mechanism increases the fitness of the bacteria from antibiotics and other defense mechanisms. Therefore, the formation of biofilms on surfaces may cause long-term infections and antibiotic resistance (25). Pseudomonas aeruginosa and S. aureus are such examples of biofilm formation contribution to the higher resistance and prevalence of bacterial infection. Additionally, biofilm formation has been shown to increase the occurrence of processes such as horizontal gene transfer, which also contribute to developing resistance traits in bacteria (26).
The current strategies to combat biofilm formation include interference with the formation of biofilms or breaking down the existing biofilms. Two lectin proteins are present in pathogenic P. aeruginosa, which are LecA and LecB. These lectins help bacteria in biofilm formation through host-cell adhesion, playing a key role in bacterial virulence (20, 27, 28). Therefore, the inhibitors of these lectins could inhibit biofilm formation in P. aeruginosa. Furthermore, compounds such as C-glycosidic sulfonamides could inhibit LacB and restrict biofilm formation by 80% (29, 30).
Studies have shown that treatment with D-amino acids is associated with the breakage of bacterial linkage within the biofilm in pathogenic B. subtilis (31). At the transcriptional level, the genes related to flagellar movement have been down-regulated. Other findings in this regard have demonstrated diminished the biofilm formation process and fimbriae production in E. coli 0157:H7 through a compound known as coumarin (32).
Damaging the component of the extracellular matrix is another strategy to prevent biofilm formation. For instance, biofilm formation is inhibited in pathogenic B. subtilis by a compound known as 3, 3′, 4′, 5-tetrachloro-salicylanilide (TCS), which targets the extracellular matrix (33). Moreover, DNAse I has the potential to degrade the extracellular matrix of Campylobacter jejuni, thereby preventing biofilm formation in these bacteria (34, 35). Meanwhile, 5-FU is a uracil analogue, which diminishes virulence and biofilm formation in P. aeruginosa. This anticancer uracil analogue could also repress biofilm formation by altering the QS pathways of P. aeruginosa without affecting bacterial growth (6). In a study in this regard, this compound was reported to inhibit biofilm formation in EHEC E. coli by affecting the virulence genes. AriR is also a global regulator that controls acid resistance in E. coli, and 5-FU has been reported to act through these global regulators to inhibit virulence and biofilm formation in E. coli K-12 (36).
2.3. Toxin Neutralization
Toxins are released by the pathogens that cause considerable damage to the host and could disrupt important cellular processes of the host. Antibodies and various molecules could be used to inhibit bacterial toxins and are important approaches to anti-virulence treatment (37). A commonly studied example in this regard is the Shiga toxin from E. coli, which contains two subunits (A and B). Subunit A is the active subunit, and subunit B is the receptor-binding subunit. The Shiga toxin is responsible for a multitude of factors that contribute to disease development; such examples are diarrhea, kidney damage, and destruction of the red blood cells and platelets. Furthermore, subunit A interferes with the protein synthesis process and cleaves a glycosidic bond in the 60S subunit of the ribosome, while subunit B binds to globosyl ceramide 3 (Gb3) ganglioside (38). Synsorb-Pk is the inhibitor of the Shiga toxin, which mimics the Gb3 receptor. Therefore, both synsorb-Pk and Gb3 receptors compete for binding to subunit B (39). Previous studies have partially succeeded in using molecules to mimic Gb3 (40). For instance, Silberstein et al. reported that in a rat model (C-9), an inhibitor of glucosylceramide synthase could effectively decrease the interaction of subunit B of the toxin through the Gb3 receptors, thereby reducing disease progression (41).
The administration of antibodies plays a pivotal role in neutralizing these toxins and reducing their detrimental effects on the host system. The antibodies that target the Shiga toxin have been isolated in rats, showing the capability of inhibiting E. coli attachment to enterocytes and contributing to neutralizing the Shiga toxin (41). Additionally, Hashish et al. administered a multiepitope fusion protein to mice, observing immunity against enterotoxic E. coli (42).
A murine model study showed that biaryl hydroxyketone compounds such as F12 and F 19 could be used as effective antiviral agents in the cases where they could act as ArgA inhibitors through preventing AgrA binding to promoter P3, which, in turn, inhibits the formation of the toxins that are responsible for disease development; as a result, antibiotic efficacy improves in-vivo. The model system has proven effective in the treatment of staphylococcal infections. Since all Gram-positive bacteria have Arg, operons containing the homologs of ArgA F12 and F 19 could be used against various Gram-positive bacteria, including staphylococci, streptococci, and bacilli, as well as drug-resistant strains (43).
In S. aureus infections, bacterial toxin α-hemolysin plays a key role in virulence. This pore-forming toxin causes hemolysis during an infection. Monoclonal antibody MEDI4893 remains a subject of clinical trials and acts as a blocker of S. aureus α-hemolysin toxin (44). Leukotoxin is another S. aureus toxin and a group of bi-component Luk toxin family, consisting of Panton-Valentine leukocidin (PVL) cytotoxin. It is responsible for the lysis of white blood cells in the host. Polyclonal antibodies such as intravenous polyclonal immunoglobulin preparations are PVL-specific antibodies, which act by interfering with the binding of PVL to neutrophils, thereby causing anti-PVL leukotoxicity (45).
Current advancements have underlined liposomes as a therapeutic strategy to neutralize toxins. Since liposomes are made of lipids and are mostly inactive against bacteria, using therapies combined with antibiotics provides an effective treatment strategy against bacterial infections (46). In a study in this regard, Henry et al. reported that in-vivo, the administration of liposomes to mice protected the animals against pulmonary infections and septicemia, which are caused by S. aureus and S. pneumonia, respectively. The researchers also observed that combined therapy with liposomes and antibiotics increased survival rates compared to single therapy (47). Moreover, Wei et al. suggested developing vaccines using nano-toxoids (8). The authors also proposed the use of nano-sponges to reduce bacterial infection by extracting toxins from the environment (8). This method is advantageous as it does not solely combat infections, but also lowers the evolutionary pressure for the selection of resistant strains. Further investigation is required to recognize the mechanism and efficacy of nano-sponges in toxin neutralization.
Scaffold proteins play a key role in regulating the assembly of interacting protein components, as well as in biological processes. In S. aureus, the scaffold protein FloA contributes to the interaction of protein complexes, thereby resulting in the progression of S. aureus infections. Therefore, the scaffold activity of FloA is essential to the interaction of other protein complexes that are related to multidrug resistance and enhance the virulence of S. aureus. According to the literature, anti-FMM compounds such as zaragozic acid, miltefosine, and 5-doxyl-stearic acid could disrupt the correct assembly of type VII secretion system (T7SS). These compounds may also alter FloA oligomerization, thereby affecting the scaffold protein activity and further impairing the secretion of T7SS-associated virulence factors in S. aureus infections (48, 49).
2.4. Targeting Virulence Gene Expression
Another strategy in anti-virulence therapy involves the targeting of the regulatory pathways that express virulence factors. Prior research has demonstrated success through the mechanism of targeting specific regulatory steps in the regulation of virulence factors (50, 51). Recent advances have also been directed toward the inhibition of QS cell-cell communication by the bacteria that regulate these processes. QS is the process through which bacteria release signaling molecules to increase their rapid proliferation until reaching a specific population size (52). Once the bacteria are ‘quorate’, QS could enhance the expression of virulence factors in the expression of other genes. Moreover, bacteria could begin to express such factors only after reaching a specific threshold that could cause an infection. Therefore, QS pathways have become an important research area, and the inhibition of such processes could reduce bacterial proliferation, while also interfering with the expression of virulence factors (11).
One large area of target regarding the inhibition of QS pathways relies on the notion of down-regulating the expression of autoinducers (53). Autoinducers are the signaling molecules used by bacteria to sense the population density and promote bacterial proliferation to reach a specific threshold. Gram-positive bacteria produce autoinducing peptides that require cleavage, while Gram-negative bacteria produce autoinducers such as N-acyl-homoserine lactones (AHLs) (54). Notably, both Gram-positive and Gram-negative bacteria use the signaling molecule autoinducer-2.
Gram-negative bacteria have been shown to produce AHLs from s-adenosyl methionine. In a study, Kalaiarasan et al. observed the inhibition of AHLs in P. aeruginosa by s-adenosyl-homocysteine (55). Additionally, other molecules that prevent the production of AHLs have been identified and could be used to reduce the expression of virulence factors such as biofilm formation. On the other hand, Gram-positive bacteria such as S. aureus produce autoinducers, while they require cleavage for activation (56). In this regard, Wright et al. reported that in the early stages of infections, administering inhibitory autoinducing peptides could inhibit S. aureus-induced abscess formation (Table 1) (57).
Targets, Inhibitors, and Mechanisms of Action of Anti-virulence Agents
| Targets | Inhibitors | Mechanism of Action |
|---|---|---|
| Biofilm formation and adhesion Pilin chaperones | Bicyclic 2-pyridones and N-substituted amino acid derivatives | Inhibition of pilus assembly |
| B. subtilis | 3, 3′, 4′, 5-tetrachlorosalicylanilide | Inhibition of biofilm formation |
| Toxin delivery Shiga toxin in E. coli | Synsorb-Pk | Binding of synsorb-Pk to subunit B of Shiga toxin prevents toxin binding to host receptor |
| Folding and assembly Staphylococcus aureus | Functional membrane microdomains | Inhibition of FloA oligomerization and scaffold activity |
| Regulation of virulence expression Quorum sensing (LuxR homologs) | Structural analogues of AHLs (eg, halogenated furanones) | Accelerate turnover of LuxR homologs; inhibited expression of quorum sensing-regulated genes; inhibited production of carbapenem in E. carotovora and virulence factors in P. aeruginosa |
| Quorum sensing (S. aureus AgrC) | Inhibitory autoinducing peptides | Inhibition of Agr locus activation |
2.5. Potential Anti-virulence Therapeutics as Effective Treatments
Currently, the US Food and Drug Administration (FDA) has approved anti-virulence drugs for three pathogens (B. anthracis, C. botulinum, and C. difficile) although they do not confer antibiotic resistance (58) (Table 2). However, these pathogens are considered to cause a life-threatening state in infections and contribute to a high rate of mortality and morbidity in the patients.
FDA-approved Anti-virulence Therapies
| Compound | Type | Molecular Target |
|---|---|---|
| Clostridium botulinum baby BIG (botulism immune globulin intravenous) | Human | Botulinum (BoNT) serotypes A and B |
| BAT (botulism antitoxin heptavalent [A, B, C, D, E, F, G]) | Equine | BoNT serotypes A-G |
| Bacillus anthracis raxibacumab | Human | Protective antigen of anthrax toxin; inhibited binding of antigen to host cell receptor |
| Obiltoxaximab | Human | |
| Clostridium difficile bezlotoxumab | Human | TcdB |
Bacillus anthracis is a Gram-positive bacterium, which causes diseases in warm-blooded livestock and could also be transmitted to humans. Enzyme penicillinase and β-lactamase enzymes in B. anthracis are responsible for the development of antibiotic resistance against regular antibiotics. Therefore, B. anthracis is considered a hazardous pathogen, classified as a bioweapon. Anti-virulence therapies target two main virulence factors, which are the anti-phagocytic poly-γ-d-glutamic acid polypeptide capsule and tripartite anthrax toxin (59). There are two anthrax toxins, which are known as the lethal toxin and the edema toxin (60). These toxins interfere with cellular processes and may persist for a long time even after toxin neutralization. Therefore, it is crucial to address the concerns raised regarding the length of interventions. An anthrax vaccine, known as the anthrax vaccine adsorbed (BioThrax), has been produced. However, it should be administered at multiple doses and may not be effective in protecting the immune system against fatal effects (61).
The FDA has approved two drugs under the animal efficacy rule; these agent are raxibacumab (Abthrax; GlaxoSmithKline) and obiltoxaximab (Anthim, ETI 204; Elusys Therapeutics), which prevent the toxic effects of the lethal factor and edema factor of toxins (62). The protective antigen (PA) of B. anthracis binds to the host cell receptors and eases the transport of the lethal factor and the edema factor in the cytosol, thereby contributing to the pathogenesis of anthrax (63).
Raxibacumab is a recombinant human monoclonal antibody, which is capable of neutralizing anthrax toxins by inhibiting the binding of the toxin component PA to the host cell receptors (64). Obiltoxaximab is another monoclonal antibody, which could also neutralize the free PA of B. anthracis and inhibits the lethal effects of anthrax toxins (65). These agents have proven effective in eliminating the inhalational anthrax infection after one treatment intervention conducted on rabbits and monkeys (66).
Clostridium botulinum is a Gram-positive bacterium and a causative agent of botulism, which leads to weakness and a possible paralytic condition in the patients. Botulinum (BoNT) neurotoxin interferes with the neurons at the neuromuscular junction, thereby preventing the release of neurotransmitters at the synaptic cleft (67). C. botulinum is similar to B. anthracis and classified as a potent bioweapon due to the practicality of culturing this pathogen from the environment. To date, the FDA has approved immunoglobulins as potential anti-virulence therapies for the treatment of botulism. Botulism immune globulin intravenous (BIG) has been used for the treatment of infant botulism (types A and B) (68), showing promising outcomes and causing a reduction in the length of hospital stay and other associated factors. Equine-derived botulism antitoxin heptavalent (BAT; Cangene) is the second-type drug that has been approved under the animal efficacy rule and is used for the treatment of types A-G in children and adults (69).
Clostridium difficile is a Gram-positive bacterium, which causes weakness, abdominal pain, and diarrhea in infections. The rate of C. difficile infections has increased exponentially, and recurrent infections have become a major concern as a leading cause of mortality compared to other pathogens.
TcdA and TcdB are two toxins that could disrupt the cytoskeleton structure (70). They are targeted by anti-virulence therapies to prevent C. difficile infections. Bezlotoxumab targets TcdA and TcdB, and its use is associated with a lower rate of C. difficile infection recurrence in those who are at a higher risk of infection recurrence. Since its FDA approval in 2016, bezlotoxumab has become one of the most efficacious anti-virulence therapies in the treatment of life-threatening and prevalent bacteria (71). Currently, multiple studies are investigating potential toxin inhibitors at the preclinical stages.
2.6. Potential, Challenges, and Future Direction
Anti-virulence therapy is known as a promising treatment for various bacterial infections. First, anti-virulence treatment lowers the selection pressure for resistance genes by targeting virulence factors rather than the growth pathway. Moreover, this type of therapy does not kill the pathogen, but rather, allows it to pass through the host without any damage. As a result, it provides an advantage over regular antibiotic treatment against numerous bacterial infections. For a more effective treatment, combined therapy with antibiotics helps eliminate the infection and prevent recurrence (72). The development of therapeutics to target prevalent virulence factors in numerous bacteria could be broadly used for the treatment of multiple bacterial infections.
Although anti-virulence therapies are promising alternatives to antibiotics, they have certain drawbacks as well (Table 3). Since anti-virulence therapy targets specific factors or pathways, it may target other non-related pathways that control important functions. Furthermore, these therapies could affect the normal microflora. Among Gram-negative bacteria, LED209 could potentially impact the healthy microbiome in the gastrointestinal tract (73). It is essential to maintain the balance of normal microbiota as the disturbance of this balance could be harmful to the respective host organism. Therefore, the therapy should only target a specific factor rather than metabolic processes in some cases.
Advantages and Disadvantages of Anti-virulence Therapy
| Advantages | Disadvantages |
|---|---|
| Imposing a lower evolutionary pressure than antibiotics | Possibly diminished therapeutic effects compared to antibiotics |
| Possible disarming of pathogen without killing it | Possible persistence of residual bacteria after discontinuation of therapy |
| Supplemented with antibiotics to eliminate residual infection or prevent recurrent infections | May require constant combination therapy with antibiotics to prevent recurrent infections. |
| Quorum sensing inhibitors may help regulate virulence gene expression and reduce production of multiple virulence factors. | May not be effective in all disease forms caused by same pathogen. |
Despite the lower evolutionary pressure for resistance, studies have shown that it is possible to develop resistance over time since bacteria have evolved the mechanisms that allow their adaptation to their environment; these mechanisms mainly involve the modification or overexpression of the target. For instance, target modification in L133P amino acid renders its resistant to virstatin, which is a cholera toxin inhibitor (74). Further investigations in this regard should be focused on the prospective trials aimed at assessing the potential side-effects of these anti-virulence therapies. Moreover, it is paramount to properly select the virulence factors that should be targeted as a treatment option in particular bacteria.
As anti-virulence therapies begin to rise in popularity, there is an urgent need to develop an assessment mechanism to recognize the factors that are required to evaluate the effectiveness of these treatments against bacterial infections. Organism sequencing could provide the necessary tools to determine the factors that should be targeted and whether the process should be applied in different host cells as well (5). Since anti-virulence therapeutics are mostly specific to bacteria, the development of broad-spectrum anti-virulence therapeutics remains challenging.
3. Conclusions
The increasing prevalence of bacterial infections and antibiotic resistance poses a great risk to global health. Therefore, novel therapies must be investigated that do not involve conventional antibiotic therapies. Anti-virulence therapy is an emerging field that lowers the selection pressure toward resistance, does not kill the pathogen, and focuses on targeting virulence factors rather than growth. The development of anti-virulence therapeutics is mainly focused on the pathogen and its interaction with the host and environment. Therefore, recognizing the pathogen-host interaction is essential. Furthermore, the development of broad-spectrum drugs provides an edge for the treatment of multiple bacterial infections.
Despite the potential of three FDA-approved drugs, anti-virulence therapy remains challenging, and the obstacles in this regard should be overcome. Avoidance of the misbalance of the normal microflora in the host system due to the use of these drugs and their potential side-effects on the mammalian system are prominent issues that should be addressed as well. This is essential to approve the safety of anti-virulence therapies as an alternative treatment option against bacterial infections. In conclusion, vigilant and extensive research alongside clinical trials are recommended regarding anti-virulence therapeutics to ensure their safe and effective use.
References
-
1.
Centre for Disease Control and Prevention. Antibiotic / Antimicrobial Resistance (AR / AMR). Centre for Disease Control and Prevention; 2020. Available from: https://www.cdc.gov/drugresistance/index.html.
-
2.
Canton R. Antibiotic resistance genes from the environment: a perspective through newly identified antibiotic resistance mechanisms in the clinical setting. Clin Microbiol Infect. 2009;15 Suppl 1:20-5. [PubMed ID: 19220348]. https://doi.org/10.1111/j.1469-0691.2008.02679.x.
-
3.
Dickey SW, Cheung GYC, Otto M. Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov. 2017;16(7):457-71. [PubMed ID: 28337021]. https://doi.org/10.1038/nrd.2017.23.
-
4.
Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol. 2007;3(9):541-8. [PubMed ID: 17710100]. https://doi.org/10.1038/nchembio.2007.24.
-
5.
Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol. 2008;6(1):17-27. [PubMed ID: 18079741]. [PubMed Central ID: PMC2211378]. https://doi.org/10.1038/nrmicro1818.
-
6.
Ueda A, Attila C, Whiteley M, Wood TK. Uracil influences quorum sensing and biofilm formation in Pseudomonas aeruginosa and fluorouracil is an antagonist. Microb Biotechnol. 2009;2(1):62-74. [PubMed ID: 21261882]. [PubMed Central ID: PMC3815422]. https://doi.org/10.1111/j.1751-7915.2008.00060.x.
-
7.
Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95-108. [PubMed ID: 15040259]. https://doi.org/10.1038/nrmicro821.
-
8.
Wei X, Gao J, Wang F, Ying M, Angsantikul P, Kroll AV, et al. In Situ Capture of Bacterial Toxins for Antivirulence Vaccination. Adv Mater. 2017;29(33). [PubMed ID: 28656663]. [PubMed Central ID: PMC5581250]. https://doi.org/10.1002/adma.201701644.
-
9.
Calvert MB, Jumde VR, Titz A. Pathoblockers or antivirulence drugs as a new option for the treatment of bacterial infections. Beilstein J Org Chem. 2018;14:2607-17. [PubMed ID: 30410623]. [PubMed Central ID: PMC6204809]. https://doi.org/10.3762/bjoc.14.239.
-
10.
Garcia-Fernandez E, Koch G, Wagner RM, Fekete A, Stengel ST, Schneider J, et al. Membrane Microdomain Disassembly Inhibits MRSA Antibiotic Resistance. Cell. 2017;171(6):1354-1367 e20. [PubMed ID: 29103614]. [PubMed Central ID: PMC5720476]. https://doi.org/10.1016/j.cell.2017.10.012.
-
11.
Defoirdt T. Quorum-Sensing Systems as Targets for Antivirulence Therapy. Trends Microbiol. 2018;26(4):313-28. [PubMed ID: 29132819]. https://doi.org/10.1016/j.tim.2017.10.005.
-
12.
Mellbye B, Schuster M. The sociomicrobiology of antivirulence drug resistance: a proof of concept. mBio. 2011;2(5). [PubMed ID: 21990612]. [PubMed Central ID: PMC3190357]. https://doi.org/10.1128/mBio.00131-11.
-
13.
Lundmark C. Molecular Adaptations in Bacteria. BioScience. 2006;56(10). https://doi.org/10.1641/0006-3568(2006)56[872:Maib]2.0.Co;2.
-
14.
Thanassi DG, Bliska JB, Christie PJ. Surface organelles assembled by secretion systems of Gram-negative bacteria: diversity in structure and function. FEMS Microbiol Rev. 2012;36(6):1046-82. [PubMed ID: 22545799]. [PubMed Central ID: PMC3421059]. https://doi.org/10.1111/j.1574-6976.2012.00342.x.
-
15.
Pinkner JS, Remaut H, Buelens F, Miller E, Aberg V, Pemberton N, et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc Natl Acad Sci U S A. 2006;103(47):17897-902. [PubMed ID: 17098869]. [PubMed Central ID: PMC1693844]. https://doi.org/10.1073/pnas.0606795103.
-
16.
Krachler AM, Orth K. Targeting the bacteria-host interface: strategies in anti-adhesion therapy. Virulence. 2013;4(4):284-94. [PubMed ID: 23799663]. [PubMed Central ID: PMC3710331]. https://doi.org/10.4161/viru.24606.
-
17.
Pemberton N, Pinkner JS, Jones JM, Jakobsson L, Hultgren SJ, Almqvist F. Functionalization of bicyclic 2-pyridones targeting pilus biogenesis in uropathogenic Escherichia coli. Tetrahedron Letters. 2007;48(26):4543-6. https://doi.org/10.1016/j.tetlet.2007.04.142.
-
18.
Wright KJ, Hultgren SJ. Sticky fibers and uropathogenesis: bacterial adhesins in the urinary tract. Future Microbiol. 2006;1(1):75-87. [PubMed ID: 17661687]. https://doi.org/10.2217/17460913.1.1.75.
-
19.
Han Z, Pinkner JS, Ford B, Obermann R, Nolan W, Wildman SA, et al. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J Med Chem. 2010;53(12):4779-92. [PubMed ID: 20507142]. [PubMed Central ID: PMC2894565]. https://doi.org/10.1021/jm100438s.
-
20.
Diggle SP, Stacey RE, Dodd C, Camara M, Williams P, Winzer K. The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ Microbiol. 2006;8(6):1095-104. [PubMed ID: 16689730]. https://doi.org/10.1111/j.1462-2920.2006.001001.x.
-
21.
Aerts J, Artola M, van Eijk M, Ferraz MJ, Boot RG. Glycosphingolipids and Infection. Potential New Therapeutic Avenues. Front Cell Dev Biol. 2019;7:324. [PubMed ID: 31867330]. [PubMed Central ID: PMC6908816]. https://doi.org/10.3389/fcell.2019.00324.
-
22.
Svensson M, Platt FM, Svanborg C. Glycolipid receptor depletion as an approach to specific antimicrobial therapy. FEMS Microbiol Lett. 2006;258(1):1-8. [PubMed ID: 16630247]. https://doi.org/10.1111/j.1574-6968.2006.00175.x.
-
23.
Oh KB, Kim SH, Lee J, Cho WJ, Lee T, Kim S. Discovery of diarylacrylonitriles as a novel series of small molecule sortase A inhibitors. J Med Chem. 2004;47(10):2418-21. [PubMed ID: 15115384]. https://doi.org/10.1021/jm0498708.
-
24.
Tian BX, Eriksson LA. Catalytic mechanism and roles of Arg197 and Thr183 in the Staphylococcus aureus sortase A enzyme. J Phys Chem B. 2011;115(44):13003-11. [PubMed ID: 21950672]. https://doi.org/10.1021/jp2058113.
-
25.
Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318-22. [PubMed ID: 10334980]. https://doi.org/10.1126/science.284.5418.1318.
-
26.
Madsen JS, Burmolle M, Hansen LH, Sorensen SJ. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol. 2012;65(2):183-95. [PubMed ID: 22444301]. https://doi.org/10.1111/j.1574-695X.2012.00960.x.
-
27.
Tielker D, Hacker S, Loris R, Strathmann M, Wingender J, Wilhelm S, et al. Pseudomonas aeruginosa lectin LecB is located in the outer membrane and is involved in biofilm formation. Microbiology (Reading). 2005;151(Pt 5):1313-23. [PubMed ID: 15870442]. https://doi.org/10.1099/mic.0.27701-0.
-
28.
Chemani C, Imberty A, de Bentzmann S, Pierre M, Wimmerova M, Guery BP, et al. Role of LecA and LecB lectins in Pseudomonas aeruginosa-induced lung injury and effect of carbohydrate ligands. Infect Immun. 2009;77(5):2065-75. [PubMed ID: 19237519]. [PubMed Central ID: PMC2681743]. https://doi.org/10.1128/IAI.01204-08.
-
29.
Pertici F, de Mol NJ, Kemmink J, Pieters RJ. Optimizing divalent inhibitors of Pseudomonas aeruginosa lectin LecA by using a rigid spacer. Chemistry. 2013;19(50):16923-7. [PubMed ID: 24307357]. https://doi.org/10.1002/chem.201303463.
-
30.
Sommer R, Wagner S, Rox K, Varrot A, Hauck D, Wamhoff EC, et al. Glycomimetic, Orally Bioavailable LecB Inhibitors Block Biofilm Formation of Pseudomonas aeruginosa. J Am Chem Soc. 2018;140(7):2537-45. [PubMed ID: 29272578]. https://doi.org/10.1021/jacs.7b11133.
-
31.
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328(5978):627-9. [PubMed ID: 20431016]. [PubMed Central ID: PMC2921573]. https://doi.org/10.1126/science.1188628.
-
32.
Zhang Y, Sass A, Van Acker H, Wille J, Verhasselt B, Van Nieuwerburgh F, et al. Coumarin Reduces Virulence and Biofilm Formation in Pseudomonas aeruginosa by Affecting Quorum Sensing, Type III Secretion and C-di-GMP Levels. Front Microbiol. 2018;9:1952. [PubMed ID: 30186266]. [PubMed Central ID: PMC6110822]. https://doi.org/10.3389/fmicb.2018.01952.
-
33.
Feng X, Guo W, Zheng H, Du J, Luo H, Wu Q, et al. Inhibition of biofilm formation by chemical uncoupler, 3, 3′, 4′, 5-tetrachlorosalicylanilide (TCS): From the perspective of quorum sensing and biofilm related genes. Biochemical Engineering Journal. 2018;137:95-9. https://doi.org/10.1016/j.bej.2018.05.010.
-
34.
Okshevsky M, Regina VR, Meyer RL. Extracellular DNA as a target for biofilm control. Curr Opin Biotechnol. 2015;33:73-80. [PubMed ID: 25528382]. https://doi.org/10.1016/j.copbio.2014.12.002.
-
35.
Brown HL, Hanman K, Reuter M, Betts RP, van Vliet AH. Campylobacter jejuni biofilms contain extracellular DNA and are sensitive to DNase I treatment. Front Microbiol. 2015;6:699. [PubMed ID: 26217328]. [PubMed Central ID: PMC4498105]. https://doi.org/10.3389/fmicb.2015.00699.
-
36.
Attila C, Ueda A, Wood TK. 5-Fluorouracil reduces biofilm formation in Escherichia coli K-12 through global regulator AriR as an antivirulence compound. Appl Microbiol Biotechnol. 2009;82(3):525-33. [PubMed ID: 19172264]. https://doi.org/10.1007/s00253-009-1860-8.
-
37.
Garland M, Loscher S, Bogyo M. Chemical Strategies To Target Bacterial Virulence. Chem Rev. 2017;117(5):4422-61. [PubMed ID: 28234447]. https://doi.org/10.1021/acs.chemrev.6b00676.
-
38.
Melton-Celsa AR, Sperandio V, Hovde CJ. Shiga Toxin (Stx) Classification, Structure, and Function. Microbiology Spectrum. 2014;2(4). https://doi.org/10.1128/microbiolspec.EHEC-0024-2013.
-
39.
Lingwood CA, Law H, Richardson S, Petric M, Brunton JL, De Grandis S, et al. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem. 1987;262(18):8834-9. https://doi.org/10.1016/s0021-9258(18)47490-x.
-
40.
Kitov PI, Sadowska JM, Mulvey G, Armstrong GD, Ling H, Pannu NS, et al. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature. 2000;403(6770):669-72. [PubMed ID: 10688205]. https://doi.org/10.1038/35001095.
-
41.
Silberstein C, Lucero MS, Zotta E, Copeland DP, Lingyun L, Repetto HA, et al. A glucosylceramide synthase inhibitor protects rats against the cytotoxic effects of shiga toxin 2. Pediatr Res. 2011;69(5 Pt 1):390-4. [PubMed ID: 21270676]. https://doi.org/10.1203/PDR.0b013e318211dd57.
-
42.
Hashish EA, Zhang C, Ruan X, Knudsen DE, Chase CC, Isaacson RE, et al. A multiepitope fusion antigen elicits neutralizing antibodies against enterotoxigenic Escherichia coli and homologous bovine viral diarrhea virus in vitro. Clin Vaccine Immunol. 2013;20(7):1076-83. [PubMed ID: 23697572]. [PubMed Central ID: PMC3697457]. https://doi.org/10.1128/CVI.00249-13.
-
43.
Greenberg M, Kuo D, Jankowsky E, Long L, Hager C, Bandi K, et al. Small-molecule AgrA inhibitors F12 and F19 act as antivirulence agents against Gram-positive pathogens. Sci Rep. 2018;8(1):14578. [PubMed ID: 30275455]. [PubMed Central ID: PMC6167350]. https://doi.org/10.1038/s41598-018-32829-w.
-
44.
Yu X, Robbie GJ, Wu Y, Esser MT, Jensen K, Schwartz HI, et al. Safety, Tolerability, and Pharmacokinetics of MEDI4893, an Investigational, Extended-Half-Life, Anti-Staphylococcus aureus Alpha-Toxin Human Monoclonal Antibody, in Healthy Adults. Antimicrob Agents Chemother. 2017;61(1). e01020. https://doi.org/10.1128/aac.01020-16.
-
45.
Gauduchon V, Cozon G, Vandenesch F, Genestier AL, Eyssade N, Peyrol S, et al. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. J Infect Dis. 2004;189(2):346-53. [PubMed ID: 14722901]. https://doi.org/10.1086/380909.
-
46.
Fang RH, Luk BT, Hu CM, Zhang L. Engineered nanoparticles mimicking cell membranes for toxin neutralization. Adv Drug Deliv Rev. 2015;90:69-80. [PubMed ID: 25868452]. [PubMed Central ID: PMC4547889]. https://doi.org/10.1016/j.addr.2015.04.001.
-
47.
Henry BD, Neill DR, Becker KA, Gore S, Bricio-Moreno L, Ziobro R, et al. Engineered liposomes sequester bacterial exotoxins and protect from severe invasive infections in mice. Nat Biotechnol. 2015;33(1):81-8. [PubMed ID: 25362245]. https://doi.org/10.1038/nbt.3037.
-
48.
Koch G, Wermser C, Acosta IC, Kricks L, Stengel ST, Yepes A, et al. Attenuating Staphylococcus aureus Virulence by Targeting Flotillin Protein Scaffold Activity. Cell Chem Biol. 2017;24(7):845-857 e6. [PubMed ID: 28669526]. [PubMed Central ID: PMC5536197]. https://doi.org/10.1016/j.chembiol.2017.05.027.
-
49.
Mielich-Suss B, Wagner RM, Mietrach N, Hertlein T, Marincola G, Ohlsen K, et al. Flotillin scaffold activity contributes to type VII secretion system assembly in Staphylococcus aureus. PLoS Pathog. 2017;13(11). e1006728. [PubMed ID: 29166667]. [PubMed Central ID: PMC5718613]. https://doi.org/10.1371/journal.ppat.1006728.
-
50.
Ross-Gillespie A, Weigert M, Brown SP, Kummerli R. Gallium-mediated siderophore quenching as an evolutionarily robust antibacterial treatment. Evol Med Public Health. 2014;2014(1):18-29. [PubMed ID: 24480613]. [PubMed Central ID: PMC3935367]. https://doi.org/10.1093/emph/eou003.
-
51.
Gao P, Ho PL, Yan B, Sze KH, Davies J, Kao RYT. Suppression of Staphylococcus aureus virulence by a small-molecule compound. Proc Natl Acad Sci U S A. 2018;115(31):8003-8. [PubMed ID: 30012613]. [PubMed Central ID: PMC6077739]. https://doi.org/10.1073/pnas.1720520115.
-
52.
Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319-46. [PubMed ID: 16212498]. https://doi.org/10.1146/annurev.cellbio.21.012704.131001.
-
53.
Gordon CP, Olson SD, Lister JL, Kavanaugh JS, Horswill AR. Truncated Autoinducing Peptides as Antagonists of Staphylococcus lugdunensis Quorum Sensing. J Med Chem. 2016;59(19):8879-88. [PubMed ID: 27585401]. [PubMed Central ID: PMC5234682]. https://doi.org/10.1021/acs.jmedchem.6b00727.
-
54.
Galloway WR, Hodgkinson JT, Bowden SD, Welch M, Spring DR. Quorum sensing in Gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem Rev. 2011;111(1):28-67. [PubMed ID: 21182299]. https://doi.org/10.1021/cr100109t.
-
55.
Kalaiarasan E, Thirumalaswamy K, Harish BN, Gnanasambandam V, Sali VK, John J. Inhibition of quorum sensing-controlled biofilm formation in Pseudomonas aeruginosa by quorum-sensing inhibitors. Microb Pathog. 2017;111:99-107. [PubMed ID: 28818490]. https://doi.org/10.1016/j.micpath.2017.08.017.
-
56.
Wang B, Zhao A, Novick RP, Muir TW. Key driving forces in the biosynthesis of autoinducing peptides required for staphylococcal virulence. Proc Natl Acad Sci U S A. 2015;112(34):10679-84. [PubMed ID: 26261307]. [PubMed Central ID: PMC4553796]. https://doi.org/10.1073/pnas.1506030112.
-
57.
Wright J3, Jin R, Novick RP. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc Natl Acad Sci U S A. 2005;102(5):1691-6. [PubMed ID: 15665088]. [PubMed Central ID: PMC547845]. https://doi.org/10.1073/pnas.0407661102.
-
58.
D'Angelo F, Baldelli V, Halliday N, Pantalone P, Polticelli F, Fiscarelli E, et al. Identification of FDA-Approved Drugs as Antivirulence Agents Targeting the pqs Quorum-Sensing System of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2018;62(11). [PubMed ID: 30201815]. [PubMed Central ID: PMC6201120]. https://doi.org/10.1128/AAC.01296-18.
-
59.
Jang J, Cho M, Chun JH, Cho MH, Park J, Oh HB, et al. The poly-gamma-D-glutamic acid capsule of Bacillus anthracis enhances lethal toxin activity. Infect Immun. 2011;79(9):3846-54. [PubMed ID: 21690241]. [PubMed Central ID: PMC3165481]. https://doi.org/10.1128/IAI.01145-10.
-
60.
Liu S, Moayeri M, Leppla SH. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol. 2014;22(6):317-25. [PubMed ID: 24684968]. [PubMed Central ID: PMC4041834]. https://doi.org/10.1016/j.tim.2014.02.012.
-
61.
Ovsyannikova IG, Pankratz VS, Vierkant RA, Pajewski NM, Quinn CP, Kaslow RA, et al. Human leukocyte antigens and cellular immune responses to anthrax vaccine adsorbed. Infect Immun. 2013;81(7):2584-91. [PubMed ID: 23649091]. [PubMed Central ID: PMC3697592]. https://doi.org/10.1128/IAI.00269-13.
-
62.
Baldari CT, Tonello F, Paccani SR, Montecucco C. Anthrax toxins: A paradigm of bacterial immune suppression. Trends Immunol. 2006;27(9):434-40. [PubMed ID: 16861036]. https://doi.org/10.1016/j.it.2006.07.002.
-
63.
Chen Z, Moayeri M, Purcell R. Monoclonal antibody therapies against anthrax. Toxins (Basel). 2011;3(8):1004-19. [PubMed ID: 22069754]. [PubMed Central ID: PMC3202866]. https://doi.org/10.3390/toxins3081004.
-
64.
Kummerfeldt CE. Raxibacumab: potential role in the treatment of inhalational anthrax. Infect Drug Resist. 2014;7:101-9. [PubMed ID: 24812521]. [PubMed Central ID: PMC4011807]. https://doi.org/10.2147/IDR.S47305.
-
65.
Greig SL. Obiltoxaximab: First Global Approval. Drugs. 2016;76(7):823-30. [PubMed ID: 27085536]. https://doi.org/10.1007/s40265-016-0577-0.
-
66.
Krishnan V, Andersen BH, Shoemaker C, Sivko GS, Tordoff KP, Stark GV, et al. Efficacy and immunogenicity of single-dose AdVAV intranasal anthrax vaccine compared to anthrax vaccine absorbed in an aerosolized spore rabbit challenge model. Clin Vaccine Immunol. 2015;22(4):430-9. [PubMed ID: 25673303]. [PubMed Central ID: PMC4375354]. https://doi.org/10.1128/CVI.00690-14.
-
67.
Frick CG, Richtsfeld M, Sahani ND, Kaneki M, Blobner M, Martyn JA. Long-term effects of botulinum toxin on neuromuscular function. Anesthesiology. 2007;106(6):1139-46. [PubMed ID: 17525589]. https://doi.org/10.1097/01.anes.0000267597.65120.16.
-
68.
Arnon SS, Schechter R, Maslanka SE, Jewell NP, Hatheway CL. Human botulism immune globulin for the treatment of infant botulism. N Engl J Med. 2006;354(5):462-71. [PubMed ID: 16452558]. https://doi.org/10.1056/NEJMoa051926.
-
69.
Yu PA, Lin NH, Mahon BE, Sobel J, Yu Y, Mody RK, et al. Safety and Improved Clinical Outcomes in Patients Treated With New Equine-Derived Heptavalent Botulinum Antitoxin. Clin Infect Dis. 2017;66(suppl_1):S57-64. [PubMed ID: 29293928]. [PubMed Central ID: PMC5866099]. https://doi.org/10.1093/cid/cix816.
-
70.
Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18(2):247-63. [PubMed ID: 15831824]. [PubMed Central ID: PMC1082799]. https://doi.org/10.1128/CMR.18.2.247-263.2005.
-
71.
Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N Engl J Med. 2017;376(4):305-17. [PubMed ID: 28121498]. https://doi.org/10.1056/NEJMoa1602615.
-
72.
Rezzoagli C, Archetti M, Mignot I, Baumgartner M, Kummerli R. Combining antibiotics with antivirulence compounds can have synergistic effects and reverse selection for antibiotic resistance in Pseudomonas aeruginosa. PLoS Biol. 2020;18(8). e3000805. [PubMed ID: 32810152]. [PubMed Central ID: PMC7433856]. https://doi.org/10.1371/journal.pbio.3000805.
-
73.
Rasko DA, Moreira CG, Li de R, Reading NC, Ritchie JM, Waldor MK, et al. Targeting QseC signaling and virulence for antibiotic development. Science. 2008;321(5892):1078-80. [PubMed ID: 18719281]. [PubMed Central ID: PMC2605406]. https://doi.org/10.1126/science.1160354.
-
74.
Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science. 2005;310(5748):670-4. [PubMed ID: 16223984]. https://doi.org/10.1126/science.1116739.