Abstract
Background:
Tamoxifen (TAM) is an effective agent for the treatment of breast cancer and has antifungal activity against various fungi. However, the antifungal effects of TAM in-vivo and in patients under treatment remain unclear.Objectives:
The present study aimed to investigate the antifungal effects of TAM on yeast oral flora in-vitro and in breast cancer patients.Methods:
In this case-control study, the antifungal effects of TAM were assessed on 50 breast cancer patients receiving TAM treatment, 50 breast cancer patients without TAM treatment, and 50 healthy controls. The disc-diffusion method was used to determine the antifungal effects of TAM on six clinical yeast isolates in-vitro.Results:
The number and species count of the yeasts were extremely low in the patients undergoing TAM treatment compared to the other subjects. On the other hand, the absence of the isolates was more evident in the patients receiving TAM treatment (96%). Candida albicans was frequently isolated from all the subjects. In the in-vitro tests, all the yeasts were susceptible to the two concentrations of TAM (5 and 10 µg/mL) at varying degrees. In addition, C. intermedia was the most susceptible yeast species to TAM with a low minimal inhibitory concentration (3.8 µg/mL).Conclusions:
According to the results, TAM exerted significant antifungal effects on the yeasts of the oral cavity in the breast cancer patients, showing superior inhibitory effects compared to clotrimazole. Therefore, TAM is recommended as a promising antifungal, while further investigation is required regarding its safety.Keywords
1. Background
Tamoxifen (TAM) has a molecular weight of 371.524 g/mol and is classified as a selective estrogen receptor modulator (SERM) drug (1). TAM exhibits anti-estrogenic effects through the inhibition of estrogen binding to the mammary cells (1, 2). Breast cancer may develop under the effect of estrogen (3, 4), and TAM is commonly used for the treatment of breast cancer or as an adjuvant drug for the protection of the women who are at the risk of developing breast cancer (5, 6). The therapeutic activity of TAM in breast cancer often encompasses both the positive and negative types of breast cancer estrogen receptor-α (7).
According to the literature, TAM has potential activity against various fungi (8-11). The first indication of the antifungal effects of TAM was reported by Wiseman et al. (1989) after testing the activity of this agent against Saccharomyces cerevisiae (12). After a few years, this activity was recorded against other types of fungi as well (4).
Candida albicans is the most common type of yeast used to investigate the antifungal effects of TAM (9-11, 13). Low concentrations of TAM (15 - 20 µM) have shown fungicidal effects against C. albicans (especially at the stationary phase) compared to imidazole (10, 14). Furthermore, the growth and biofilm development of C. albicans have been reported to be significantly inhibited in the presence of TAM (1 mg/mL) (11), while the other isolates of Candida spp. and Cryptococcus neoformans require a lower minimal inhibitory concentration (MIC; 8 - 64 µg/mL) to induce growth inhibition (15). In addition, the clinical isolates of fluconazole-sensitive and C. albicans-resistant collected from patients with periodontal disease have been reported to be susceptible to TAM (13). TAM may also be involved in improving the antifungal effects of other standard antifungal agents against yeast growth, such as terbinafine and azole (8).
2. Objectives
The present study aimed to investigate the antifungal effects of TAM against the yeasts obtained from oral cavity flora in-vitro and in breast cancer patients.
3. Methods
3.1. Patients
This case-control study aimed to assess the antifungal effects of TAM on the yeasts of the oral cavity of breast cancer patients. A total, 100 women with breast cancer (aged 29 - 62 years) and 50 healthy controls (aged 28 - 60 years) were enrolled in the study.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Local Ethics Committee of the College of Medicine of the University of Karbala (No. 157) in January 2018. Written informed consent was obtained from the subjects prior to participation.
The breast cancer patients were divided into two groups of TAM treatment and treatment with other agents than TAM. The subjects voluntarily attended the oncology unit of Al-Ammam Al-Hussein Medical City Hospital in Karbala province during December 2018 - March 2019, and those undergoing chemotherapy were excluded from the study.
3.2. Yeast Isolation
Yeast samples were collected from the oral cavity of all the subjects using sterilized cotton swabs and cultured on Sabouraud dextrose agar (SDA; HiMedia, India). Following that, inoculated media were incubated at the temperature of 37°C for 24 hours for the growth of suspected yeasts. The colony count of the isolated yeasts was performed as colony forming unit (CFU), and the diagnosis of the isolated yeasts was carried out using the VITEK® 2 system and VITEK® 2 YST ID diagnostic cards for yeasts (BioMérieux, France).
3.3. In-Vitro Antifungal Effects of TAM
The disc-diffusion method was applied to assess the antifungal effects of TAM against the isolated yeasts based on the CLSI-M44-3rd method (2018) (16). Initially, an inoculum of the isolated yeasts was prepared by culturing the yeasts in a sterilized test tube with Sabouraud dextrose broth (SDB; HiMedia, India) and incubated at the temperature of 37°C for 24 hours.
At the next stage, the turbidity of the growth suspension was adjusted at 0.5 McFarland standard using sterilized normal saline to obtain the approximate concentration of 1.5 × 108 CFU/mL. Following that, 100 microliters of the standard fungal count was inoculated on SDA through spreading by a sterilized cotton swab and left to fix on the media for three minutes. Some discs with the diameter of six millimeters were also prepared from sterilized filtered paper. In addition, a stock solution of TAM was also provided (up to 20 μg/mL) and dissolved in DMSO. Afterwards, filter discs were impregnated in a solution containing different concentrations of TAM citrate (5 and 10 μg/mL; Ebewe Pharma, Austria) and placed on an inoculated plate using sterilized forceps. The plates were incubated at the temperature of 37°C for 24 hours. Moreover, discs containing various concentrations of clotrimazole (0.5 and 1 μg/mL) were dissolved in sterilized water as positive control, while a disc of sterilized distilled water was used as negative control. Inhibition zone was measured in millimeters around the effective disc.
3.4. Minimum Inhibitory Concentration (MIC) of TAM
The MIC of TAM in the yeast samples was determined using the broth dilution method of CLSI-M27-4th (2017) (17). Initially, a broth culture of the isolated yeasts was prepared from the inoculum of the grown yeast in SDB and incubated at the temperature of 37°C for 24 hours. Afterwards, the turbidity of the yeast cells was adjusted at 0.5 McFarland standards to contain approximately 1.5 × 108 CFU/mL. Serial concentrations of TAM (5, 4, 3.8, 3.5, and 3 µg/mL) were also prepared from the stock solution (10 µg/mL).
At the next stage, plastic microdilution plates (96-well) were used to determine the MIC value of TAM in the yeast samples. Each well was filled with 50 microliters of the standard count of each fungal suspension and 100 microliters of SDB, followed by 50 microliters of a specific concentration of TAM. In addition, several controls were used within each microdilution plate, including SDB with yeast only, SDB without yeast, and SDB with TAM only. The inoculated plates were incubated at the temperature of 37°C for 24 hours, and the obtained results were visually read as the presence or absence of fungal growth.
3.5. Statistical Analysis
Data analysis was performed in Microsoft Excel©, and the obtained data were expressed as mean and standard deviation (SD). One-way analysis of variance (ANOVA) was applied to analyze the variables, and the P-value of less than 0.05 was considered significant in all the statistical analyses.
4. Results
4.1. Effects of TAM on the Oral Cavity Yeasts of the Breast Cancer Patients
The successful isolation of yeasts from the oral cavity of the breast cancer patients and healthy controls was the positive outcome of the study. However, only few patients (4%) receiving TAM treatment showed the significant positive isolation of yeasts. Meanwhile, the patients without TAM treatment and the healthy controls showed the positive growth of yeasts (24 and 18%, respectively). Moreover, the majority of the subjects had negative results in terms of yeast isolation, especially those undergoing TAM treatment (96%) (Table 1).
| Subject Group | No. of Patients with Fungal Growth | Total No. | |
|---|---|---|---|
| Positive | Negative | ||
| Breast cancer with tamoxifen | 2 (4) b | 48 (96) | 50 |
| Breast cancer without tamoxifen | 12 (24) | 38 (76) | 50 |
| Healthy | 9 (18) | 41 (82) | 50 |
| Total No. | 23 | 127 | 150 |
4.2. Yeast Count and Species
The count and species of the isolated yeasts were determined after collecting the swab samples from all the subjects. The breast cancer patients under TAM treatment exhibited significantly lower yeast colony counts (2 CFU from each C. albicans and C. ciferrii species) compared to those without TAM treatment and the healthy controls. Moreover, only two yeast species were diagnosed in the patients under TAM treatment compared to the six species isolated from the patients without TAM treatment. Meanwhile, three yeast species were isolated from the healthy controls. According to the findings, C. albicans was the most common species isolated from all the subjects, followed by C. ciferrii, while C. famata was only isolated from the patients without TAM treatment (Table 2).
| Subject group/Type of yeast | Colony Count (cfu)* | No. of Subject |
|---|---|---|
| Breast cancer with tamoxifen | ||
| C. albicans | 2 A | 1 (50) |
| C. ciferrii | 2 B | 1 (50) |
| Breast cancer without tamoxifen | ||
| C. laurentii | 60 A, B | 1 (8.33) |
| C. albicans | 10 - 60 | 3 (25) |
| C. intermedia | 50 - 80 | 2 (16.66) |
| C. glabrata | 60 A, B | 3 (25) |
| C. ciferrii | 2 | 2 (16.66) |
| C. famata | 20 | 1 (8.33) |
| Healthy | ||
| C. albicans | 2 - 5 | 5 (55.55) |
| C. ciferrii | 3 | 1 (11.11) |
| C. intermedia | 2 | 1 (11.11) |
| C. glabrata | 3 - 6 | 2 (22.22) |
| Total No. | 23 |
The colony counts of C. albicans and C. ciferrii were higher in the patients without TAM treatment and the healthy controls, while a low count was also detected in the patients undergoing TAM treatment. Other isolated yeasts (Cryptococcus laurentii, Candida intermedia, and Candida glabrata) also had higher colony counts in the patients without TAM treatment (Table 2). Only C. albicans was isolated from the patients receiving TAM treatment for two years, while C. ciferrii was isolated from those undergoing the treatment for four years. However, no significant correlation was observed between the detected yeasts and TAM treatment period (Table 3).
Relationship of TAM Treatment Period and Isolated Yeasts
| Treatment Period (y) | Patient No. | Type of Yeast | Number of Colony |
|---|---|---|---|
| 2 | 1 | C. albicans | 2 |
| 4 | 1 | C. ciferrii | 2 |
| Total No. | 2 | - | 4 |
4.3. In-Vitro Antifungal Effects of TAM
The antifungal effects of TAM were investigated against six yeast isolates from the oral cavity of the breast cancer patients, and all the samples demonstrated varied susceptibility to the two concentrations of TAM. In addition, significant differences were observed between C. laurentii and five species of yeasts (P < 0.05), while no such difference was denoted in the case of C. albicans at neither concentrations of TAM.
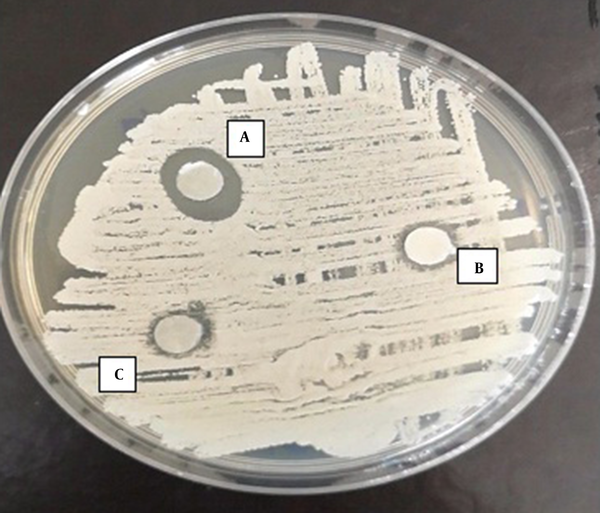
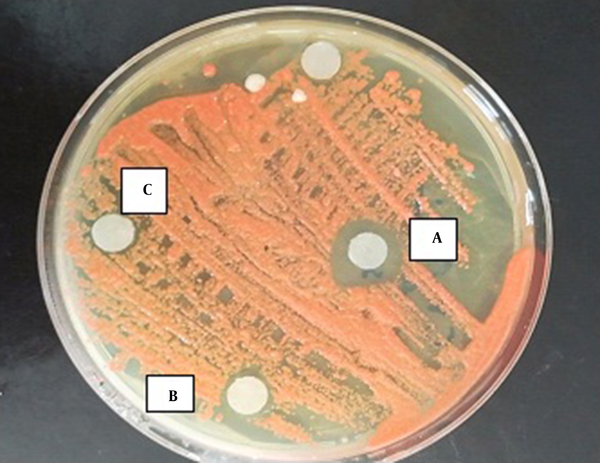
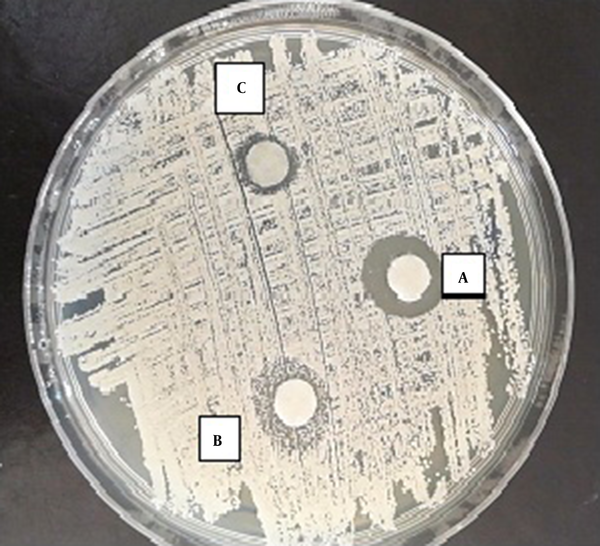
According to the results of the disc-diffusion method, C. albicans was the most susceptible yeast to both concentrations of TAM with a significant difference with the other yeast species in this regard (P < 0.05) (Figure 1). Furthermore, C. laurentii and C. glabrata were inhibited more significantly at the same concentrations of TAM (Figures 2 and 3). On the other hand, C. ciferrii and C. famata were the less susceptible species of the isolated yeasts at the two concentrations of TAM (Table 4).
Zone of inhibition of the effect of TAM on C. albicans. A, 10 µg/mL of TAM; B, 5 µg/mL of TAM; C, 2.5 µg/mL of TAM.
Zone of inhibition of the effect of TAM on Cryptococcus laurentii. A, 10 µg/mL of TAM; B, 5 µg/mL of TAM; C, 2.5 µg/mL of TAM.
Zone of inhibition of the effect of TAM on C. glabrata. A, 10 µg/mL of TAM; B. 5 µg/mL of TAM; C, 2.5 µg/mL of TAM.
| No. | Isolated Strain | Zone of Inhibition (mm) | |||
|---|---|---|---|---|---|
| TAM Concentration (µg/mL) | Clotrimazole Concentration (µg/mL) | ||||
| 10 | 5 | 1 | 0.5 | ||
| 1 | C. albicans | 11.5 A | 9 B | 10 | 8 |
| 2 | C.ciferrii | 7.6 A | 7.3 | R | R |
| 3 | C. laurentii | 11 A, B | 9 B | 14.5 | 12.5 |
| 4 | C. glabrata | 9.3 A | 7.3 | R | R |
| 5 | C. intermedia | 8 A | 7 | 13 | 11 |
| 6 | C. famata | 7.6 A | 7.3 | R | R |
Notably, three yeast species (C. ciferrii, C. glabrata, and C. famata) showed resistance to the two concentrations of clotrimazole (1 and 0.5 µg/mL) (Table 4). Therefore, it could be concluded that TAM was a more effective antifungal agent against the isolated yeasts compared to clotrimazole.
4.4. MIC of TAM in the Isolated Yeasts
Based on the estimated MIC of TAM in the isolated yeasts, C. intermedia was observed to be more susceptible to TAM with a lower MIC value (3.8 µg/mL) compared to the other yeast species. Meanwhile, three species of the isolated yeasts (C. albicans, C. laurentii, and C. glabrata) were inhibited by TAM with the MIC value of 4 µg/mL, while C. ciferrii and C. famata required a higher MIC of TAM to be inhibited (5 µg/mL) (Table 5).
Minimum Inhibitory Concentration (MIC) Value of TAM on Isolated Yeast
| No. | Isolated Strain | TAM Concentration (µg/mL) |
|---|---|---|
| 1 | C. albicans | 4 |
| 2 | C. ciferrii | 5 |
| 3 | C. laurentii | 4 |
| 4 | C. glabrata | 4 |
| 5 | C. intermedia | 3.8 |
| 6 | C. famata | 5 |
5. Discussion
The therapeutic effects of TAM on breast cancer mainly depends on the inhibition binding of estrogen to its cellular receptors, which ultimately prevents the changing effects of estrogen on the cellular genetic associated with the development of breast cancer (1, 5). Therefore, it could be concluded that TAM acts as a SERM (1), and the antitumor activities of TAM may encompass its ability to induce an oxidation reaction in breast cancer cells through increasing Nrf2 expression (7). Furthermore, TAM has been reported to have antioxidant effects through protecting the stability of the liposome membrane of the mammalian cells by decreasing the fluidity of the membrane (18). In 1977, the USFDA approved the use of TAM for the treatment of postmenopausal women experiencing advanced breast cancer, as well as a post-surgery adjuvant therapy to eradicate micrometastasis in primary breast cancer (19). However, the common period of using TAM as an anti-cancer agent should not be less than five years or last 10 years for the 20% reduction of cancer recurrence (3).
According to the literature, a large number of organisms live in the oral cavity of the human body either as resident or temporary flora (20). Yeasts represent the most common type of fungi of the normal flora in the oral cavity (21). Several factors may affect the diversity and residence of oral microflora, especially yeasts. Some of these factors also influence the survival and distribution of various yeast species within the complex community of normal flora; such examples are age, amount of saliva, pH, smoking habits, and denture wearing, while other factors are considered less significant given that taste disorders and gender are also correlated with the sensation of dryness or having a burning mouth (22, 23).
The findings of the current research demonstrated that TAM cause significant antifungal effects in-vitro against the isolated yeasts from the oral cavity of the breast cancer patients compared to clotrimazole (standard antifungal agent). On the other hand, C. albicans and C. laurentii were observed to be more susceptible to TAM compared to the other yeast species. Several studies have also confirmed the antifungal effects of TAM on various fungal species (8-11). For instance, a study in this regard indicated that C. albicans was the most susceptible yeast species to TAM compared to other standard antifungal agents (13-15). Furthermore, biofilm formation by C. albicans has been reported to be inhibited by TAM (1 mg/mL) (11). The fungicidal effects of TAM against the logarithmic growth of C. albicans are considered moderate and transient at 1 × 10-5 M, while they become almost negligible at 5 × 10-6 M (24). The fungicidal activity of TAM against C. albicans is significantly affected by pH as the notable inhibitory effects of TAM (> 99%; 10 µM) have been demonstrated at higher pH values than the neutral range (9). Moreover, filamentous fungi are affected by the antifungal activity of TAM as the growth of four fungal species (Aspergillus spp., Penicillium spp., Mucor spp., and Rhizopus spp.) have been reported to be inhibited in the presence of TAM (25).
In the present study, the normal yeast flora of the breast cancer patients had lower counts and species variability compared to the subjects receiving medication therapy with other agents and the healthy controls. The significant effects of TAM on the yeast content could be an indicator for possible future disturbances in the normal balance between various organisms of the oral cavity. In a similar study, the administration of TAM at the dosage of 200 mg/kg to a murine model revealed the in-vivo ability of this agent to limit the dissemination of candidiasis (15). In addition, the in-vitro half-maximal effective dose (EC50) of TAM against promastigotes and amastigotes of Leishmania amazonensis has been estimated at 13.3 and 4.5 μM, respectively, while the in-vivo value for promastigote in mice has been reported to be 13.2 mg/kg/day (26). In cancer patients, the interaction of antifungal agents and TAM is evident against fungal infections. Triazole agents (especially voriconazole) are known to enhance the pharmacokinetic parameters of TAM after the application of physiologically-based pharmacokinetic models (27). In addition, the antifungal properties of some azoles and terbinafine have been reported to improve through enhanced interaction with TAM (8). The therapeutic effects of fluconazole and amphotericin B (AmB) could also improve following their combined use with TAM for the treatment of cryptococcosis infection (e.g., cryptococcosis meningitis) (28, 29).
Similar to other drugs, TAM could cause side-effects in the breast cancer patients receiving treatment, especially when used at high doses (1). The most common side-effects of TAM include blood clot, dizziness, hyperreflexia, tremor, and acute neurotoxicity (1-3). As such, the preferred duration of TAM therapy is often two years (3). In a longitudinal clinical study, TAM was reported to be an effective antifungal agent against oral yeasts. According to the results of the present study, the use of TAM as an antifungal agent against oral fungal infections is not necessarily long-term compared to its use as an anticancer drug.
The exact mechanism of action of TAM as an antifungal agent remains unclear. The calcium-calcineurin signaling pathway plays a pivotal role in fungal growth, development, and reproduction and is also associated with the virulence of multiple pathogenic fungi (30). TAM has been shown to exert inhibitory effects on some components of the calcium-calcineurin pathway in fungal cells, thereby increasing their susceptibility to the inhibitory effects of various antifungal drugs, such as AmB and fluconazole (31). Another proposed mechanism of TAM against fungal cells depends on its blocking effects on the calmodulin site (32). Calmodulin normally activates the serine-threonine phosphatase calcineurin pathway, which is associated with the virulence of C. neoformans, to cause disease in the human body (33). Therefore, TAM could prevent some pathogenic fungi that cause infections by affecting their virulence ability.
5.1. Conclusion
According to the results, TAM had significant antifungal effects against the yeasts of the oral cavity in the breast cancer patients, and its inhibitory effects were also more significant compared to clotrimazole. This may be an indicator for the reduction of bacterial growth in the oral cavity, which is often controlled by oral yeasts through competitive mechanisms. Therefore, it is recommended that patients under TAM treatment be monitored in terms of fungal activity in the oral cavity and even low counts of fungi be taken into account as they increase the risk of bacterial infections. On the other hand, TAM could be used routinely for the long-term treatment of breast cancer as it is generally a safer antifungal agent; further investigation is required to confirm this finding.
References
-
1.
Drugbank Online. Tamoxifen. USA: Drugbank Online; 2005, [updated 27th Jun 2021; cited 13th May 2019]. Available from: https://go.drugbank.com/drugs/DB00675.
-
2.
National Center for Biotechnology Information. PubChem compound summary for CID 2733526, Tamoxifen. Bethesda, USA: National Center for Biotechnology Information; 2021.
-
3.
Ramani KV, Ramani H, Alurkar S, Ajaikumar BS, Trivedi RG. Breast cancer: Medical treatment, side effects, and complementary therapies. New York, USA: Momentum Press; 2017.
-
4.
Ingvarsson S. Breast cancer: introduction. Seminars in Cancer Biology. 2001;11(5):323-6. https://doi.org/10.1006/scbi.2001.0381.
-
5.
Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339(22):1609-18. [PubMed ID: 9828250]. https://doi.org/10.1056/NEJM199811263392207.
-
6.
Colleoni M, Gelber S, Goldhirsch A, Aebi S, Castiglione-Gertsch M; International Breast Cancer Study Group, et al. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13-93. J Clin Oncol. 2006;24(9):1332-41. [PubMed ID: 16505417]. https://doi.org/10.1200/JCO.2005.03.0783.
-
7.
Bekele RT, Venkatraman G, Liu RZ, Tang X, Mi S, Benesch MG, et al. Oxidative stress contributes to the tamoxifen-induced killing of breast cancer cells: Implications for tamoxifen therapy and resistance. Sci Rep. 2016;6:21164. [PubMed ID: 26883574]. [PubMed Central ID: PMC4756695]. https://doi.org/10.1038/srep21164.
-
8.
Zhang X, Fang Y, Jaiseng W, Hu L, Lu Y, Ma Y, et al. Characterization of tamoxifen as an antifungal agent using the yeast schizosaccharomyces pombe model organism. Kobe J Med Sci. 2015;61(2):E54-63. [PubMed ID: 26628015].
-
9.
Beggs WH. Drug protonation and pH in relation to the lethal action of tamoxifen on Candida albicans. J Antimicrob Chemother. 1996;37(4):841-2. [PubMed ID: 8722553]. https://doi.org/10.1093/jac/37.4.841.
-
10.
Beggs WH. Comparative activities of miconazole and the anticancer drug tamoxifen against Candida albicans. J Antimicrob Chemother. 1994;34(1):186-7. [PubMed ID: 7961210]. https://doi.org/10.1093/jac/34.1.186.
-
11.
Wakharde AA, Halbandge SD, Phule DB, Karuppayil SM. Anticancer drugs as antibiofilm agents in Candida albicans: Potential targets. Assay Drug Dev Technol. 2018;16(5):232-46. [PubMed ID: 29446984]. https://doi.org/10.1089/adt.2017.826.
-
12.
Wiseman H, Cannon M, Arnstein HR. Observation and significance of growth inhibition of Saccharomyces cerevisiae (A224A) by the anti-oestrogen drug tamoxifen. Biochem Soc Trans. 1989;17(6):1038-9. [PubMed ID: 2697611]. https://doi.org/10.1042/bst0171038.
-
13.
Muthular M, Balsamo F, Passero P, Jewtuchowicz V, Miozza V, Villalba MB, et al. Effects of tamoxifen on periodontal disease and Candida albicans of patients with breast cancer and other pathologies. Future Microbiol. 2019;14:129-37. [PubMed ID: 30672328]. https://doi.org/10.2217/fmb-2018-0272.
-
14.
Beggs WH. Growth phase in relation to the lethal action of tamoxifen on Candida albicans. Res Commun Mol Pathol Pharmacol. 1995;88(1):115-8. [PubMed ID: 7620832].
-
15.
Dolan K, Montgomery S, Buchheit B, Didone L, Wellington M, Krysan DJ. Antifungal activity of tamoxifen: In vitro and in vivo activities and mechanistic characterization. Antimicrob Agents Chemother. 2009;53(8):3337-46. [PubMed ID: 19487443]. [PubMed Central ID: PMC2715577]. https://doi.org/10.1128/AAC.01564-08.
-
16.
Clinical and Laboratory Standards Institute. Method for antifungal disk diffusion susceptibility testing of yeasts: Approved guidelines. 38. 3rd ed. Pennsylvania, USA: Clinical and Laboratory Standards Institute; 2018.
-
17.
Clinical and Laboratory Standards Institute. Method for antifungal disk diffusion susceptibility testing of yeasts: Approved guidelines. 37. 4th ed. Pennsylvania, USA: Clinical and Laboratory Standards Institute; 2017.
-
18.
Wiseman H, Quinn P, Halliwell B. Tamoxifen and related compounds decrease membrane fluidity in liposomes. Mechanism for the antioxidant action of tamoxifen and relevance to its anticancer and cardioprotective actions? FEBS Lett. 1993;330(1):53-6. [PubMed ID: 8370459]. https://doi.org/10.1016/0014-5793(93)80918-k.
-
19.
Manna S, Holz MK. Tamoxifen action in ER-negative breast cancer. Sign Transduct Insights. 2016;5:1-7. [PubMed ID: 26989346]. [PubMed Central ID: PMC4792287]. https://doi.org/10.4137/STI.S29901.
-
20.
Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69(1):137-43. [PubMed ID: 23201354]. https://doi.org/10.1016/j.phrs.2012.11.006.
-
21.
Stenderup A. Oral mycology. Acta Odontol Scand. 1990;48(1):3-10. [PubMed ID: 2181808]. https://doi.org/10.3109/00016359009012728.
-
22.
Shimizu C, Kuriyama T, Williams DW, Karasawa T, Inoue K, Nakagawa K, et al. Association of oral yeast carriage with specific host factors and altered mouth sensation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(4):445-51. [PubMed ID: 18329579]. https://doi.org/10.1016/j.tripleo.2007.11.030.
-
23.
Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6(1). e1000713. [PubMed ID: 20072605]. [PubMed Central ID: PMC2795202]. https://doi.org/10.1371/journal.ppat.1000713.
-
24.
Beggs WH. Anti-Candida activity of the anti-cancer drug tamoxifen. Res Commun Chem Pathol Pharmacol. 1993;80(1):125-8. [PubMed ID: 8488339].
-
25.
Pasrija R, Kundu D. First report of antifungal activity of tamoxifen against filamentous fungi. Int J Life Sci Res. 2018;6(4):296-8.
-
26.
Trinconi CT, Reimao JQ, Yokoyama-Yasunaka JK, Miguel DC, Uliana SR. Combination therapy with tamoxifen and amphotericin B in experimental cutaneous leishmaniasis. Antimicrob Agents Chemother. 2014;58(5):2608-13. [PubMed ID: 24550333]. [PubMed Central ID: PMC3993234]. https://doi.org/10.1128/AAC.01315-13.
-
27.
Chen L, Zhu L, Li M, Li N, Qi F, Wang N. Predicting the effects of different triazole antifungal agents on the pharmacokinetics of tamoxifen. AAPS PharmSciTech. 2019;20(1):24. [PubMed ID: 30604153]. https://doi.org/10.1208/s12249-018-1219-5.
-
28.
Butts A, Koselny K, Chabrier-Rosello Y, Semighini CP, Brown JC, Wang X, et al. Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. mBio. 2014;5(1):e00765-13. [PubMed ID: 24520056]. [PubMed Central ID: PMC3950514]. https://doi.org/10.1128/mBio.00765-13.
-
29.
Ngan NTT, Mai NTH, Tung NLN, Lan NPH, Tai LTH, Phu NH, et al. A randomized open label trial of tamoxifen combined with amphotericin B and fluconazole for cryptococcal meningitis. Wellcome Open Res. 2019;4:8. [PubMed ID: 30801037]. [PubMed Central ID: PMC6381443]. https://doi.org/10.12688/wellcomeopenres.15010.1.
-
30.
Rispail N, Soanes DM, Ant C, Czajkowski R, Grunler A, Huguet R, et al. Comparative genomics of MAP kinase and calcium-calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet Biol. 2009;46(4):287-98. [PubMed ID: 19570501]. https://doi.org/10.1016/j.fgb.2009.01.002.
-
31.
Liu S, Hou Y, Liu W, Lu C, Wang W, Sun S. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot Cell. 2015;14(4):324-34. [PubMed ID: 25636321]. [PubMed Central ID: PMC4385803]. https://doi.org/10.1128/EC.00271-14.
-
32.
Butts A, Martin JA, DiDone L, Bradley EK, Mutz M, Krysan DJ. Structure-activity relationships for the antifungal activity of selective estrogen receptor antagonists related to tamoxifen. PLoS One. 2015;10(5). e0125927. [PubMed ID: 26016941]. [PubMed Central ID: PMC4446328]. https://doi.org/10.1371/journal.pone.0125927.
-
33.
Odom AR. The triphenylethylenes, a novel class of antifungals. mBio. 2014;5(3):e01126-14. [PubMed ID: 24781746]. [PubMed Central ID: PMC4010834]. https://doi.org/10.1128/mBio.01126-14.