Abstract
Background:
Medicinal plants are considered as a useful source of antioxidants against diseases due to reducing free radicals. Caralluma tuberculata is one of such sources utilized by Iranian population as a traditional herbal medicine.Objectives:
The present study aimed at investigating antioxidant activity, total phenolic content (TPC), and total flavonoid content (TFC) of C. tuberculata root hydroalcoholic extract (CRE) and aerial parts hydroalcoholic extract (CAE).Methods:
Antioxidant properties were assessed using DPPH (1,1-dipheny l,2-picryl hydrazyl), superoxide anion (O2˙ˉ), nitric oxide (NO), ferric reducing antioxidant power, and anti-lipid peroxidation methods. TPC (mg pyrogallol/g extract) and TFC (mg quercetin/g extract) were measured by colorimetric methods.Results:
The findings showed that both CAE and CRE were rich in phenolic and flavonoid compounds. TPC (40.12 ± 1.2) and TFC (20.22 ± 0.62) were higher in CAE compared to CRE (TPC = 25.01 ± 0.85, TFC = 15.11 ± 0.71). Likewise, both CAE and CRE showed significant scavenging effects on DPPH, O2˙ˉ, and NO radicals and the effects of CAE was more potent (P < 0.05) compared to that of CRE. Also, plant extracts significantly inhibited lipid peroxidation in a dose-dependent manner (P < 0.05). Furthermore, CAE exhibited a more potent ferric reducing activity compared to CRE (510.21 ± 8.2 vs. 450.2 ± 9.3 μM Fe+2/g extract, respectively) (P < 0.05).Conclusions:
Based on the study findings, both CAE and CRE could be considered as potential sources of antioxidants and CAE was more effective than CRE.Keywords
1. Background
Although free radicals play important roles such as intracellular signaling and physiological functions in human being lives, endogenous antioxidants scavenge them when their production increases to provide an intracellular balance (1). In disease states, endogenous antioxidants are inadequate. Therefore, exogenous antioxidants are required to avoid oxidative damage (2). In spite of the presence of some synthetic antioxidants, these drugs are not fully safe and have some side effects such as liver damage or carcinogenesis in laboratory animals (3). Instead, herbal materials are considered as important sources of antioxidants, which are more effective, safer, and cheaper than synthetic drugs, and also have better compatibility with the human body. The herb-derived antioxidants such as phenolic and flavonoid compounds are useful sources against disease risk by reducing free radicals (4).
Caralluma tuberculata (C. tuberculata), belonging to Asclepiadaceae family, is a succulent plant with no leaves, and in Iran only grows in some regions of Sistan and Baluchistan province. C. tuberculata in the local Baluch language is called Marmootk and as a traditional medicinal herb is used to manage different human diseases such as diabetes (5). Review of literature on C. tuberculata showed that composition and antioxidant activities of this plant are not yet investigated in Iran.
2. Objectives
Therefore, the present in vitro study aimed at assessing antioxidant and free radical scavenging activities of hydroalcoholic extracts of root and aerial parts of C. tuberculata.
3. Methods
3.1. Chemicals
DPPH (1,1-dipheny l,2-picryl hydrazyl), nitro blue tetrazolium, pyrogallol, quercetin, sodium nitroprusside, and folin-ciocalteu reagent were purchased from Sigma (St. Louis, MO). Butylated hydroxytoluene and thiobarbituric acid were purchased from Merck (Darmstadt, Germany). All other chemicals used were of analytical grade.
3.2. Plant Material and Preparation of Extracts
Aerial parts and root of the plant were collected in April 2012 from the elevation of Irandegan district, Iran. The plant was identified and validated as C. tuberculata and voucher specimen was also deposited at the Herbarium of the Sistan and Baluchistan University. In order to prepare C. tuberculata root hydroalcoholic extract (CRE) and aerial parts hydroalcoholic extract (CAE), 100 g of each plant sample was extracted via macerating in 0.3 L of 70% ethanol for four days. The macerated samples were filtered and concentrated via rotary evaporator. Then, samples were lyophilized via freeze drying and the products were used for extraction yield (mass of extract/mass of dry matter). Then the dried extracts were stored at -70ºC until use. For experiments, 1 mg of each dried extract was dissolved in 1 mL of methanol/water (50:50 v/v) and the resulting solution was used in the experiments.
3.3. Estimation of Total Phenolic Content
According to Slinkard protocol, folin-ciocalteu reagent (FCR) was used to determine total phenolic content (TPC) for each dried extract (6). Pyrogallol was used as a standard for phenolic compounds. Findings were expressed as pyrogallol equivalents (mg pyrogallol/g dried extract).
3.4. Estimation of Total Flavonoid Contents
Total flavonoid content (TFC) was determined for each dried extract by colorimetric method (7). Quercetin was used as a standard for flavonoid compounds. Findings were expressed as quercetin equivalents (mg quercetin/g dried extract).
3.5. In vitro Antioxidant Assays
3.5.1. DPPH Radical Scavenging Activity
DPPH radical scavenging activity was evaluated according to procedure described by Blois (8). This experiment is relied on proton donating ability of plant extracts to remove DPPH as a source of free radicals. L-ascorbic acid was utilized as a standard for comparison. The DPPH radical scavenging activity was computed using the following formula:
Where, A blank is the absorbance of the control, and A sample is the absorbance of plant extracts or the standard sample.
3.5.2. Superoxide Radical Scavenging Activity
Superoxide anion (O2˙ˉ) was generated by oxidation of NADH in NADH-phenazine methosulphate (PMS) system and determined by reduction of nitro blue tetrazolium (NBT). L-ascorbic acid was utilized as a standard antioxidant. The percentage inhibition of O2˙ˉ generation, as an index of superoxide radical scavenging activity, was calculated using the same formula given in DPPH assay (9).
3.5.3. Nitric Oxide Radical Scavenging Activity
Nitric oxide (NO) radical was generated from sodium nitroprusside (SNP), and the ability of extracts to scavenge it was determined according to Griess reaction with slight modifications (10). The percentage inhibition of extracts was calculated using the same formula given in DPPH assay.
3.5.4. Anti-Lipid Peroxidation Assay
Anti-lipid peroxidation activity was evaluated in the liver of rat as a source of polyunsaturated fatty acid. This experiment is relied on determining malondialdehyde (MDA) levels, as an index of lipid peroxidation, by reaction between thiobarbituric acid (TBA) and MDA (11). In order to prepare liver homogenates, male Wistar rats weighing 200 - 230 g were anesthetized with diethyl ether and then decapitated. Immediately, their livers were removed and washed with normal saline. The study protocol for the rats was in accordance with the Guide for the Care and Use of Laboratory Animals (ethical code: 7182). The quantity of thiobarbituric acid reaction substrate (TBARS) was computed using absorption coefficient of 1.56 ×105 cm-1.M-1. The results were exhibited as a percentage inhibition, computed from a control measurement of the reaction combination without extracts.
3.5.5. Ferric Reducing Antioxidant Power
The method described by Benzie and Strain was followed to perform FRAP assay (12). This test is relied on the ability of the antioxidants to reduce non-colored form of a ferric-tripyridyltriazine complex to the colored form of ferrous-tripyridyltriazine. Findings were expressed as μM Fe+2 /g of dry mass and compared with that of butylated hydroxytoluene (BHT) as a standard antioxidant.
3.6. Statistical Analyses
The data were expressed as mean ± standard deviation (SD) for at least three independent tests performed on different days. One-way analysis of variance was used for statistical analysis followed by Tukey’s post-hoc test using SPSS version 21. A P value of less than 0.05 was considered the level of significance.
4. Results
As shown in Table 1, extraction yield of the CRE and CAE was 9.82 and 10.01, respectively.
4.1. Total Phenolic and Flavonoid Contents of Extracts
TPC (mg pyrogallol/g dried of extract) and TFC (mg quercetin/g dried of extract) of the CRE and CAE are represented in Table 1. As shown, in the studied samples (plants were collected during spring), TPC (40.12 ± 1.2) was higher in CAEs compared to CREs (25.01 ± 0.85). Similarly, CAEs were richer in TFC (20.22 ± 0.62) compared to CREs (15.11 ± 0.71).
4.2. DPPH Radical Scavenging Activity
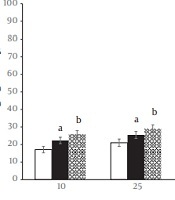
As shown in Figure 1, both CAE and CRE samples at all tested concentrations had significantly higher DPPH radical quenching activities than the control (P < 0.01). Concerning DPPH scavenging effects (Table 2), ascorbic acid had the lowest IC50 value (147.319 µg/mL), followed by CAE (180.797 µg/mL) and CRE (222.14 µg/mL).
Inhibitory effects of different concentrations (10 - 500 μg/mL) of Caralluma tuberculata root extract (CRE); C. tuberculata aerial parts extract (CAE), and ascorbic acid against DPPH (1,1-dipheny l,2-picryl hydrazyl) radicals. Values are expressed as mean ± SD of independent experiments repeated in triplicate (n = 9). aP < 0.01 versus CRE; bP < 0.01 versus CAE and CER

IC50 Values of CAE, CRE, BHT, and Ascorbic Acid
| Antioxidant Activity | IC50 Valuea, μg/mL | |||
|---|---|---|---|---|
| CRE | CAE | Ascorbic Acid | BHT | |
| DPPH radical scavenging activity | 222.14 | 180.79 | 147.31 | - |
| Superoxide radical scavenging activity | 307.62 | 218.58 | 327.69 | - |
| Nitric oxide radical scavenging activity | 281.78 | 196.57 | 130.82 | - |
| Anti-lipid peroxidation activity | 729.92 | 592.69 | - | 408.25 |
4.3. Superoxide Radical Scavenging Activity
In Figure 2, O2˙ˉ scavenging activity of different doses (10 - 500 μg/mL) of CAE, CRE, and ascorbic acid were compared. CAE, in all tested concentrations, exhibited greater O2˙ˉ quenching activity (P < 0.05) compared to both CRE and ascorbic acid as the reference control. The IC50 values concerning O2˙ˉ scavenging effects are shown in Table 2. According to data shown on this table, IC50 value had the following order: CAE (218.58) < CRE (307.62) < ascorbic acid (327.69).
Inhibitory effects of different concentrations (10 - 500 μg/mL) of Caralluma tuberculata root extract (CRE); C. tuberculata aerial parts extract (CAE), and ascorbic acid against superoxide radicals. Values are expressed as mean ± SD of independent experiments repeated in triplicate (n = 9). aP < 0.05 versus CRE and ascorbic acid; bP < 0.05 versus ascorbic acid

4.4. Nitric Oxide Radical Scavenging Activity
As shown in Figure 3, different concentrations of extracts as well as ascorbic acid removed nitric oxide radicals in a dose-dependent manner. Both CAE (IC50 = 196.57 μg/mL) and CRE (IC50 = 281.78 μg/mL) exhibited nitric oxide radical scavenging effects, but the effect of ascorbic acid (IC50 = 130.82 μg/mL) as a standard antioxidant was more potent (Table 2).
Inhibitory effects of different concentrations (10 - 500 μg/mL) of Caralluma tuberculata root extract (CRE); C. tuberculata aerial parts extract (CAE), and ascorbic acid against nitric oxide radicals. Values are expressed as mean ± SD for independent experiments repeated in triplicate (n = 9). aP < 0.01 versus CRE; bP < 0.01 versus CAE and CER; cP < 0.05 versus CAE

4.5. Anti-Lipid Peroxidation Activity
As shown in Figure 4 and Table 2, CRE (IC50 = 729.92 μg/mL) exhibited less anti-lipid peroxidation activity as compared to CAE (IC50 = 592.69 μg/mL). Anti-lipid peroxidation activity of BHT (IC50 = 408.25 μg/mL), as a standard antioxidant, was significantly higher than those of CRE and CAE.
Inhibitory effects of different concentrations (50 - 1000 μg/mL) of Caralluma tuberculata root extract (CRE); C. tuberculata aerial parts extract (CAE), and butylated hydroxytoluene (BHT) against lipid peroxidation induced by Fe2+ ascorbate system. Values are expressed as mean ± SD for independent experiments repeated in triplicate (n = 9). aP < 0.01 versus CRE; bP < 0.01 versus CAE and CRE

4.6. FRAP Assay
Ferric reducing antioxidant power of CAE, CRE, and BHT are shown on Table 1. The ferric reducing potential of the extracts (µM Fe+2/g dried extract) had the following order: BHT (650.14 ± 7.84) > CAE (510.21 ± 8.2) > CRE (450.2 ± 9.3).
5. Discussion
In the authors’ previous study, C. tuberculata was shown as a good source of antioxidants against diabetes-induced oxidative stress in animal model, which could reinstate the activity of antioxidant enzymes and oxidative markers to near-normal levels (13). The present in-vitro study focused on anti-oxidant activity of hydroalcoholic extracts of C. tuberculata.
Phenolic and flavonoid compounds are the major antioxidants in medicinal herbs. The antioxidant properties of phenolic compounds may be related to their capacity to act as reducing factors, hydrogen atom donors, unstable radical scavengers, and metal chelator (14-16). Therefore, the consumption of these natural compounds is useful for the prevention of some diseases, such as diabetes mellitus, tissue inflammation, hemolysis, nervous anxiety and hypertension, which are associated with oxidative stress (14, 17, 18). The current study results revealed that both CAE and CRE are rich in phenolic and flavonoid compounds and CAE with a higher TPC and TFC showed better antioxidant activities than CRE. In the study by Rehman et al., on different solvents of C. tuberculata, polar solvents with the highest degree of purity were found to extract phenolic and flavonoid compounds. The current study also used a polar solvent (ethanol 70%) for extraction (19).
DPPH is a stable free radical that produces a violet solution in ethanol. The antioxidant compounds reduce these radicals and produce a neutral complex. Formation of a colorless complex depends on the hydrogen donating potential of antioxidant compounds (20). Based on the results of the current study, both CAE and CRE showed notable DPPH radical quenching effects at all tested concentrations. The present study findings were in line with those of the previous studies reporting significant antioxidant activity of C. flava and C. arabica using the DPPH test (21, 22). Also, Rehman et al., reported that the polar fractions of the callus of C. tuberculata had more DPPH scavenging effects compared to non-polar fractions (19). Furthermore, Saeed et al., reported that extracts with high flavonoid and phenol compounds showed potent DPPH scavenging activities (23). The present study results also showed that CAE with higher TPC and TFC had greater inhibitory effects against DPPH radicals compared to CRE.
The superoxide radical is an important reactive oxygen species, produced through the normal aerobic metabolism (24). In disease states, overproduction of O2˙ˉ may give rise to more potent and dangerous free radicals such as OH, which later leads to lipid peroxidation (25). The finding showed that CAE in all tested concentration exhibited greater O2˙ˉ quenching activity compared to CRE and ascorbic acid as a reference control. Flavonoids are shown to have good superoxide anion quenching activities (9). As showed on Table 1, both CAE and CRE are rich in flavonoids. Thus, superoxide anion quenching activities observed in the present study could be attributable to flavonoid contents.
It is postulated that reactive nitrogen species such as NO or peroxynitrite (ONOO-) contribute to the development of a number of diseases (26). In the present study, both plant extracts exhibited nitric oxide scavenging activity. The present study results regarding nitric oxide radical scavenging effects were consistent with those of the study by Karthishwaran et al., which reported radical scavenging activity of Caralluma flava extract (21).
Oxidative stress can damage cell membrane via oxidizing polyunsaturated fatty acids and inducing lipid peroxidation (27). In biological systems, lipid peroxidation generates several products, among which MDA is believed to be the most considerable end product. MDA and other lipid peroxidation products can oxidize thiols in proteins and cause proteins dysfunction. As a result of protein dysfunction, several disorders including inflammation, hepatic and heart diseases may develop (28, 29). In the present study, plant extracts significantly inhibited lipid peroxidation in a dose-dependent manner. Similar results were obtained in a study by Tatiya et al., which evaluated anti-lipid peroxidation activities of C. adscendens (30).
It is reported that plant extracts with greater total phenolic and flavonoid contents exhibited the highest antioxidant activities (31). The current study findings showed that both extracts have reducing potentials, and CAE with higher TPC and TFC, exhibited better antioxidant activities than CRE. Tatiya et al., indicated that the reducing potential of C. adscendens had a dose-dependent manner (30). Also, Maheshu et al., showed that methanolic extract of C. adscendens had a strong ferric reducing potential (32).
5.1. Conclusions
In sum, the current study findings revealed that ethanolic extracts of root and aerial parts of C. tuberculata acted as anti-free radical and antioxidant agents against oxidative stress, which is probably considering at least partly to the phenolic and flavonoid contents present in the plant. In addition, aerial parts, in most experiments, were more effective than the root. According to the current study findings, C. tuberculata could be considered as a good source of antioxidant and bioactive substances.
References
-
1.
Gopalakrishnan VK, Starlin T. Enzymatic and non-enzymatic antioxidant properties of Tylophora pauciflora Wight and Arn.–an in vitro study. Asian J Pharm Clin Res. 2013;4(68-71).
-
2.
Upadhyay R, Chaurasia JK, Tiwari KN, Singh K. Antioxidant property of aerial parts and root of Phyllanthus fraternus Webster, an important medicinal plant. ScientificWorldJournal. 2014;2014:692392. [PubMed ID: 24587744]. [PubMed Central ID: PMC3921995]. https://doi.org/10.1155/2014/692392.
-
3.
Pavithra K, Vadivukkarasi S. Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.) Cogn. Food Sci Hum Wellness. 2015;4(1):42-6. https://doi.org/10.1016/j.fshw.2015.02.001.
-
4.
Vouldoukis I, Lacan D, Kamate C, Coste P, Calenda A, Mazier D, et al. Antioxidant and anti-inflammatory properties of a Cucumis melo LC. extract rich in superoxide dismutase activity. J Ethnopharmacol. 2004;94(1):67-75. [PubMed ID: 15261965]. https://doi.org/10.1016/j.jep.2004.04.023.
-
5.
Poodineh J, Nakhaee A. Hypoglycemic and hypolipidemic effects of Caralluma tuberculata and its safety on liver and kidneys of diabetic rats. Turkish J Biochem. 2016;41(3). https://doi.org/10.1515/tjb-2016-0023.
-
6.
Slinkard K, Singleton VL. Total phenol analysis: Automation and comparison with manual methods. Am J Enol Viticulture. 1977;28(1):49-55.
-
7.
Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555-9. https://doi.org/10.1016/s0308-8146(98)00102-2.
-
8.
Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199-200. https://doi.org/10.1038/1811199a0.
-
9.
Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;37(5):837-41. [PubMed ID: 2830882]. https://doi.org/10.1016/0006-2952(88)90169-4.
-
10.
Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994;201(2):748-55. [PubMed ID: 8003011]. https://doi.org/10.1006/bbrc.1994.1764.
-
11.
Mahakunakorn P, Tohda M, Murakami Y, Matsumoto K, Watanabe H. Antioxidant and free radical-scavenging activity of Choto-san and its related constituents. Biol Pharm Bull. 2004;27(1):38-46. [PubMed ID: 14709896]. https://doi.org/10.1248/bpb.27.38.
-
12.
Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": The FRAP assay. Anal Biochem. 1996;239(1):70-6. [PubMed ID: 8660627]. https://doi.org/10.1006/abio.1996.0292.
-
13.
Poodineh J, Khazaei Feizabad A, Nakhaee A. Antioxidant activities of Caralluma tuberculata on streptozotocin-induced diabetic rats. Drug Dev Res. 2015;76(1):40-7. [PubMed ID: 25620374]. https://doi.org/10.1002/ddr.21239.
-
14.
Ojiako OA, Chikezie PC, Ogbuji AC. Radical scavenging potentials of single and combinatorial herbal formulations in vitro. J Tradit Complement Med. 2016;6(2):153-9. [PubMed ID: 27114938]. [PubMed Central ID: PMC4833459]. https://doi.org/10.1016/j.jtcme.2014.11.037.
-
15.
Pracheta SV, Paliwal R, Sharma S. In vitro free radical scavenging and antioxidant potential of ethanolic extract of Euphorbia neriifolia Linn. Int J Pharm Pharm Sci. 2011;3(1):238-42.
-
16.
Sharma SK, Singh AP. In vitro antioxidant and free radical scavenging activity of Nardostachys jatamansi DC. J Acupunct Meridian Stud. 2012;5(3):112-8. [PubMed ID: 22682272]. https://doi.org/10.1016/j.jams.2012.03.002.
-
17.
Kalantari H, Larki A, Latifi SM. The genotoxicity study of garlic and pasipy herbal drops by peripheral blood micronucleus test. Acta Physiol Hung. 2007;94(3):261-6. [PubMed ID: 17853777]. https://doi.org/10.1556/APhysiol.94.2007.3.10.
-
18.
Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63(7):1035-42. [PubMed ID: 10924197].
-
19.
Rehman R, Chaudhary M, Khawar K, Lu G, Mannan A, Zia M. In vitro propagation of Caralluma tuberculata and evaluation of antioxidant potential. Biologia. 2014;69(3):341-9. https://doi.org/10.2478/s11756-013-0322-z.
-
20.
Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53(6):1841-56. [PubMed ID: 15769103]. https://doi.org/10.1021/jf030723c.
-
21.
Karthishwaran K, Shamisi SOSOA, Kurup SS, Sakkir S, Cheruth AJ. Free-radical-scavenging and antioxidant capacities with special emphasis on enzyme activities and in vitro studies in Caralluma flava N. E. Br. Biotechnol Biotechnol Equip. 2017;32(1):156-62. https://doi.org/10.1080/13102818.2017.1379362.
-
22.
Khasawneh M, Elwy HM, Fawzi NM, Hamza AA, Chevidenkandy AR, Hassan AH. Antioxidant activity and lipoxygenase inhibitory effect of Caralluma arabica and related polyphenolic constituents. Am J Plant Sci. 2014;5(11):1623-31. https://doi.org/10.4236/ajps.2014.511176.
-
23.
Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:221. [PubMed ID: 23153304]. [PubMed Central ID: PMC3524761]. https://doi.org/10.1186/1472-6882-12-221.
-
24.
Kalantar H, Sabetkasaei M, Shahriari A, Haj Molla Hoseini M, Mansouri S, Kalantar M, et al. The effect of rapamycin on oxidative stress in MCF-7 and MDA MB-231 human breast cancer cell lines. Jundishapur J Nat Pharm Prod. 2016;11(3). https://doi.org/10.17795/jjnpp-38177.
-
25.
Liu W, Wang H, Pang X, Yao W, Gao X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int J Biol Macromol. 2010;46(4):451-7. [PubMed ID: 20153767]. https://doi.org/10.1016/j.ijbiomac.2010.02.006.
-
26.
Wang B, Lin S, Hsiao W, Fan J, Fuh L, Duh P. Protective effects of an aqueous extract of Welsh onion green leaves on oxidative damage of reactive oxygen and nitrogen species. Food Chem. 2006;98(1):149-57. https://doi.org/10.1016/j.foodchem.2005.05.057.
-
27.
Goudarzi M, Kalantar M, Kalantar H. The hepatoprotective effect of gallic acid on mercuric chloride-induced liver damage in rats. Jundishapur J Nat Pharm Prod. 2017;12(4). https://doi.org/10.5812/jjnpp.12345.
-
28.
Bahramikia S, Ardestani A, Yazdanparast R. Protective effects of four Iranian medicinal plants against free radical-mediated protein oxidation. Food Chem. 2009;115(1):37-42. https://doi.org/10.1016/j.foodchem.2008.11.054.
-
29.
Bajpai VK, Sharma A, Kang SC, Baek KH. Antioxidant, lipid peroxidation inhibition and free radical scavenging efficacy of a diterpenoid compound sugiol isolated from Metasequoia glyptostroboides. Asian Pac J Trop Med. 2014;7(1):9-15. [PubMed ID: 24418075]. https://doi.org/10.1016/S1995-7645(13)60183-2.
-
30.
Tatiya AU, Kulkarni AS, Surana SJ, Bari ND. Antioxidant and hypolipidemic effect of Caralluma adscendens Roxb. in alloxanized diabetic rats. Int J Pharmacol. 2010;6(4):400-6. https://doi.org/10.3923/ijp.2010.400.406.
-
31.
Mahboubi M, Taghizadeh M, Kazempour N. Antimicrobial and antioxidant activities of pycnocycla spinosa extracts. Jundishapur J Nat Pharm Prod. 2014;9(3). e13859. [PubMed ID: 25237641]. [PubMed Central ID: PMC4165177].
-
32.
Maheshu V, Priyadarsini DT, Sasikumar JM. Antioxidant capacity and amino acid analysis of Caralluma adscendens (Roxb.) Haw var. fimbriata (wall.) Grav. & Mayur. aerial parts. J Food Sci Technol. 2014;51(10):2415-24. [PubMed ID: 25328180]. [PubMed Central ID: PMC4190237]. https://doi.org/10.1007/s13197-012-0761-5.