Abstract
Background:
Obesity is a multifactorial disorder, and gut microbiota has a fundamental role in its pathophysiology. Bacteroides spp. has significant roles in gut microbiota- host interactions that determine health and disease development. Since the gut microbiota pattern changes based on different criteria in each population, we studied the abundance of two important Bacteroides strains, Bacteroides fragilis, and Bacteroides thetaiotaomicron, in Iranian obese and normal-weight subjects for the first time.Methods:
In this study, 100 participants were recruited and classified based on their body mass index (BMI). The subjects were divided into normal (average BMI, 22.37 kg/m2) and obese (average BMI, 29.10 kg/m2) groups. Bacterial DNA was extracted from the samples, and quantitative polymerase chain reaction (qPCR) was conducted based on 16s rDNA universal primers. Finally, the correlation between bacterial abundance and obesity was investigated.Results:
The results of qPCR showed that the relative abundance means of B. fragilis in normal weight and obese subjects was 8.68 × 1012 and 9.27 × 1012 cfu/mL, respectively. Also, the relative abundance mean of B. thetaiotaomicron in normal weight and obese subjects was 2.32 × 1012 and 5.39 × 1012 cfu/mL, respectively. Although obese subjects had more B. fragilis and B. thetaiotaomicron abundance compared to subjects with normal weight, no significant difference was identified between relative abundance of B. fragilis (P = 0.79) and B. thetaiotaomicron (P = 0.18) in the two groups.Conclusions:
Although obese subjects had more B. fragilis and B. thetaiotaomicron abundance compared to normal-weight subjects, no significant difference was identified between the two groups. Since Bacteroides spp. have significant role in gut microbiota-host interaction, determination of their abundance in obesity development and targeting restoration of gut microbiota pattern could be valuable in controlling obesity. In this regard, dietary intervention could be based on determination of gut microbiota pattern in certain populations.Keywords
Gut Microbiota Bacteroides fragilis Bacteroides thetaiotaomicron Obesity Metabolic Syndrome
1. Background
The gastrointestinal tract (GIT) is colonized with a complex and dynamic microbial community known as gut microbiota (1). The gut microbiota plays significant role in human health and diseases (2). It has important effects on host functions, including homeostasis of the GI, metabolism, immune, and nervous system (3). This complex community is composed of diverse microorganisms dominated by bacteria. Most of these bacterial species belong to phyla Firmicutes and Bacteroidetes. Moreover, other phyla such as Verrucomicrobia and Actinobacteria are present in gut microbiota (4). The composition of gut microbiota is established during 2 - 3 first years of life under control of many factors, including mode of delivery, genetic background, geography, nutrition, physical activity, and gender (5).
In normal conditions, symbiosis relationship between gut microbiota and host, the gut microbiota-host interaction is balanced. Conversely, in dysbiosis, alternation of gut microbiota pattern, putative interaction is disturbed. Dysbiosis resulted from high fat diet (HFD) induces low-grade inflammation, which leads to insulin resistance (IR). This condition is considered as turning point of various disorders and diseases such as obesity, metabolic syndrome, type 2 diabetes, inflammatory diseases (inflammatory bowel disease), and colorectal cancer (CRC) (6, 7).
Recent studies consider the gut microbiota as an environmental factor that has important role in the pathophysiology of obesity (8). Energy harvest increase changes in microbial components and metabolites are attributed to the alternation of gut microbiota composition in obesity. These events are parallel with increase of Firmicutes to Bacteroidetes ratio in obese subjects (9, 10). Therefore, this study aimed to investigate the gut microbiota composition.
It seems that Bacteroides spp. among the gut microbiota members has an important role in pathophysiology of obesity. Bacteroidetes are Gram-negative rods, anaerobic and non-spore-forming bacteria. Bacteroidetes spp. extract energy from protein and carbohydrates by fermentation (11). High enzymatic potentials of B. fragilis and B. thetaiotaomicron contribute in maintenance of homeostasis (12). Also, these bacteria have immunomodulatory effects that induce tolerance to gut microbiota (13). Hence, due to metabolic and immune potentials of B. fragilis and B. thetaiotaomicron, their frequency could be important in obesity treatment. Population criteria (genetic background, ethnicity, diet, lifestyle, geography distribution) could affect the gut microbiota pattern. Accordingly, in this study, for the first time, we focused on B. fragilis and B. thetaiotaomicron relative abundance in Iranian population.
2. Methods
2.1. Study Population
A total of 100 healthy adults (age range: 20 - 60 years) were recruited. These subjects were assigned into two equal groups of normal and obese based on body mass index (BMI). Subjects with BMI between 18.5 and 24.9 kg/m2 were considered as normal group. Participants with significant infection, chronic diseases, and the use of antibiotics and corticosteroids were excluded. This study was performed in accordance with the ethical rules of the Helsinki Declaration.
2.2. DNA Extraction from Stool Samples
The fresh stool samples were collected from subjects and stored immediately at -20°C. DNA was extracted using a QIAamp DNA stool mini kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. The quality of extracted DNA was assayed by agarose gel electrophoresis and spectrophotometric analysis. All DNA samples were stored at -20°C.
2.3. Quantitative Polymerase Chain Reaction (qPCR)
The abundance of bacteria was estimated by universal primers, which amplified a conserved region of the 16S rRNA gene. SYBR Green qPCR was conducted using Light Cycler® 96 SW 1.1 (Rocsh, Germany). Each reaction mixture of 20 μL was composed of SYBR Premix Ex Tag II (RR820L-Takara), 0.5 μL of each of the specific primers, and 5 μL of template DNA. The amplification program was designed according to appropriate annealing temperature consisting of 1 cycle of 95°C for 60 s, followed by 40 cycles of denaturation at 95°C for 5 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s (14).
2.3.1. Standard Curve
The abundance of bacteria was calculated through DNA concentration. For this purpose, serial dilutions of DNA from standard strain Escherichia coli were prepared. The standard curve allows to calculate DNA concentration of each bacteria in stool samples (15).
2.4. Statistical Analyses
In this study, categorical variables are presented as number (percent) and continuous variables as mean ± SD. Independent t-test was used to assess mean differences between normal and obese groups. Statistical analyses were performed using SPSS software version 21.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were 2-tailed, and a P ≤ 0.05 was considered statistically significant.
3. Results
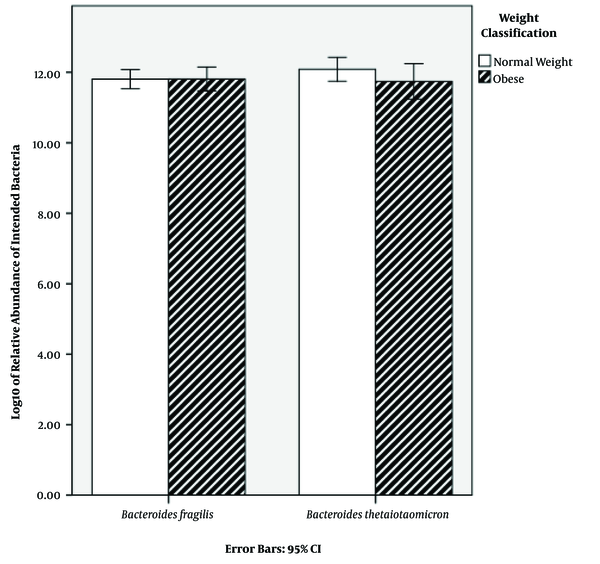
Characteristics of the subjects are shown in Table 1. BMI classification consisted of two groups: normal weight (50%) and obese (50%) (Table 1). The results of qPCR showed that the relative abundance mean of B. fragilis in normal weight and obese subjects was 8.68 × 1012 and 9.27 × 1012 CFU mL-1, respectively. Additionally, the relative abundance mean of B. thetaiotaomicron was 2.32 × 1012 and 5.39 × 1012 CFU mL-1 in normal weight and obese subjects, respectively (Table 2). In order to find a correlation between B. fragilis and B. thetaiotaomicron abundance and obesity, the bacterial concentrations were analyzed between normal and obese subjects. Although the obese subjects had more B. fragilis and B. thetaiotaomicron abundance compared to subjects with normal weight, no significant difference was identified between relative abundance of B. fragilis (P = 0.79) and B. thetaiotaomicron (P = 0.18) in the two groups (Figure 1).
Characteristics of Obese and Normal-Weight Adults
| Obese | Normal-weight | |
|---|---|---|
| Subjects (male/female) | 26 (12/14) | 28 (12/16) |
| Age (y) | 43.56 ± 1.92 | 30.55 ± 1.42 |
| Weight, kg | 81.38 ± 2.74 | 63 ± 1.32 |
| Height, m | 1.68 ± 0.025 | 1.68 ± 0.010 |
| BMI, kg/m2 | 28.42 ± 0.59 | 22 ± 0.36 |
| BMI s.d. score | 3.01 | 1.89 |
The Relative Abundance of Bacteroides thetaiotaomicron and B. fragilis
| Relative Abundance of B. thetaiotaomicron | Relative Abundance of B. fragilis | |
|---|---|---|
| Mean | 3.8611×1012 | 8.9777×1012 |
| Std. error of mean | 1.16127×1012 | 1.12594×1012 |
| Std. deviation | 1.16127×1013 | 1.12594×1013 |
| Minimum | 5.80×105 | 9.22×107 |
| Maximum | 7.75×1013 | 5.20×1013 |
Comparison of relative abundance in Bacteroides thetaiotaomicron and B. fragilis in Normal and Obese Subjects
4. Discussion
The gut microbiota is a diverse and complex microbial community which affects metabolism and energy homeostasis. The gut microbiota composition is associated with health and diseases (3). The pattern of gut microbiota has been established during the first 2-3 years of life and affects many aspects in the host, including immune cell functions, glucose and lipid metabolism, energy homeostasis, and susceptibility to disease (16, 17). The alteration of gut microbiota could be spotted as a biomarker in several disorders and diseases such as obesity (18-21). Bacteroidets has significant roles in gut microbiota-host interaction. Therefore, the determination of abundance could be valuable in controlling obesity, in this study, for the first time, the relative abundance of B. fragilis and B. thetaiotaomicron was determined in Iranian population.
Recently, a correlation between gut microbiota composition and obesity was identified (22-24). Various studies have reported that obese and non-obese subjects have different gut microbial compositions (14). Bacteroidets and Frimicutes are two important phyla that constitute gut microbial community. Bervoets et al. showed an elevated Firmicutes to Bacteroidetes ratio in obese children (14). B. fragilis and B. thetaiotaomicron are anaerobic intestinal commensal that belong to Bacteroidetes phylum. They have significant metabolic and immune potentials in gut microbiota-host interaction (13). Hence, their relative abundance could influence the host's metabolism.
There are various reports about abundance of B. fragilis and B. thetaiotaomicron. For example, Vael et al. proved that high intestinal B. fragilis abundance in infants between the age of 3 weeks and 1 year was associated with a higher risk of obesity in their adulthood (25). Karlsson et al. did not observe a significant difference between B. fragilis abundance in obese children compared to normal-weight subjects (26).
Kasai et al. demonstrated greater bacterial diversity and different gut microbial composition in obese compared with non-obese subjects. Also, their results showed that some bacterial species, including B. thetaiotaomicron, had significantly greater concentration in the non-obese group (27).
The establishment of gut microbiota composition is influenced by various factors including, mode of delivery, genetic background, lifestyle, geography, ethnicity, diet, etc. Therefore, these factors affect the results of gut microbiota and obesity studies.
4.1. Conclusion
Although the obese Iranian subjects had more fecal B. fragilis and B. thetaiotaomicron compared with normal-weight subjects, no significant difference was identified between the relative abundance of B. fragilis and B. thetaiotaomicron in obese and non-obese individuals. Since B. fragilis and B. thetaiotaomicron have important roles in metabolism and energy homeostasis, determining the bacterial relative abundance could be valuable in controlling obesity. In total, determination of gut microbiota pattern is essential regarding introducing some effective prevention and treatment strategies in obesity as well as other disorders.
Acknowledgements
References
-
1.
Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859-904. [PubMed ID: 20664075]. https://doi.org/10.1152/physrev.00045.2009.
-
2.
Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718-23. [PubMed ID: 15505215]. [PubMed Central ID: PMC524219]. https://doi.org/10.1073/pnas.0407076101.
-
3.
Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242-9. [PubMed ID: 22972297]. https://doi.org/10.1038/nature11552.
-
4.
Fouhy F, Ross RP, Fitzgerald GF, Stanton C, Cotter PD. Composition of the early intestinal microbiota: knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes. 2012;3(3):203-20. [PubMed ID: 22572829]. [PubMed Central ID: PMC3427213]. https://doi.org/10.4161/gmic.20169.
-
5.
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220-30. [PubMed ID: 22972295]. [PubMed Central ID: PMC3577372]. https://doi.org/10.1038/nature11550.
-
6.
Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260-70. [PubMed ID: 22411464]. [PubMed Central ID: PMC3418802]. https://doi.org/10.1038/nrg3182.
-
7.
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. [PubMed ID: 25651997]. [PubMed Central ID: PMC4315779]. https://doi.org/10.3402/mehd.v26.26191.
-
8.
Woting A, Blaut M. The intestinal microbiota in metabolic disease. Nutrients. 2016;8(4):202. [PubMed ID: 27058556]. [PubMed Central ID: PMC4848671]. https://doi.org/10.3390/nu8040202.
-
9.
Wallace TC, Guarner F, Madsen K, Cabana MD, Gibson G, Hentges E, et al. Human gut microbiota and its relationship to health and disease. Nutr Rev. 2011;69(7):392-403. [PubMed ID: 21729093]. https://doi.org/10.1111/j.1753-4887.2011.00402.x.
-
10.
Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34(1):39-58. [PubMed ID: 23159341]. https://doi.org/10.1016/j.mam.2012.11.001.
-
11.
Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18(1):2. [PubMed ID: 28061847]. [PubMed Central ID: PMC5219689]. https://doi.org/10.1186/s12865-016-0187-3.
-
12.
Elhenawy W, Debelyy MO, Feldman MF. Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. mBio. 2014;5(2):e00909-14. [PubMed ID: 24618254]. [PubMed Central ID: PMC3952158]. https://doi.org/10.1128/mBio.00909-14.
-
13.
Maier E, Anderson RC, Roy NC. Understanding how commensal obligate anaerobic bacteria regulate immune functions in the large intestine. Nutrients. 2014;7(1):45-73. [PubMed ID: 25545102]. [PubMed Central ID: PMC4303826]. https://doi.org/10.3390/nu7010045.
-
14.
Bervoets L, Van Hoorenbeeck K, Kortleven I, Van Noten C, Hens N, Vael C, et al. Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog. 2013;5(1):10. [PubMed ID: 23631345]. [PubMed Central ID: PMC3658928]. https://doi.org/10.1186/1757-4749-5-10.
-
15.
Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology (Reading). 2002;148(Pt 1):257-66. [PubMed ID: 11782518]. https://doi.org/10.1099/00221287-148-1-257.
-
16.
Bergstrom A, Skov TH, Bahl MI, Roager HM, Christensen LB, Ejlerskov KT, et al. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol. 2014;80(9):2889-900. [PubMed ID: 24584251]. [PubMed Central ID: PMC3993305]. https://doi.org/10.1128/AEM.00342-14.
-
17.
Rampelli S, Candela M, Turroni S, Biagi E, Pflueger M, Wolters M, et al. Microbiota and lifestyle interactions through the lifespan. Trends Food Sci Technol. 2016;57:265-72. https://doi.org/10.1016/j.tifs.2016.03.003.
-
18.
Tilg H, Moschen AR. Gut microbiome, obesity, and metabolic syndrome. Metabolic Syndrome. 2016. p. 447-59. https://doi.org/10.1007/978-3-319-11251-0_26.
-
19.
Newsholme P, Homem de Bittencourt PJ. Gut associated bacteria are critical to metabolism, inflammation and health. Curr Opin Clin Nutr Metab Care. 2016;19(4):245-9. [PubMed ID: 27213282]. https://doi.org/10.1097/MCO.0000000000000293.
-
20.
de Clercq NC, Groen AK, Romijn JA, Nieuwdorp M. Gut microbiota in obesity and undernutrition. Adv Nutr. 2016;7(6):1080-9. [PubMed ID: 28140325]. [PubMed Central ID: PMC5105041]. https://doi.org/10.3945/an.116.012914.
-
21.
Cani PD, Delzenne NM. Gut microbiota, obesity and associated metabolic disorders. WGO Handbook on Gut Microbes. World Digestive Health Day (WDHD); 2016.
-
22.
Scarpellini E, Campanale M, Leone D, Purchiaroni F, Vitale G, Lauritano EC, et al. Gut microbiota and obesity. Intern Emerg Med. 2010;5 Suppl 1:S53-6. [PubMed ID: 20865475]. https://doi.org/10.1007/s11739-010-0450-1.
-
23.
Gerard P. Gut microbiota and obesity. Cell Mol Life Sci. 2016;73(1):147-62. [PubMed ID: 26459447]. https://doi.org/10.1007/s00018-015-2061-5.
-
24.
Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol. 2013;27(1):73-83. [PubMed ID: 23768554]. https://doi.org/10.1016/j.bpg.2013.03.007.
-
25.
Vael C, Verhulst SL, Nelen V, Goossens H, Desager KN. Intestinal microflora and body mass index during the first three years of life: an observational study. Gut Pathog. 2011;3(1):8. [PubMed ID: 21605455]. [PubMed Central ID: PMC3118227]. https://doi.org/10.1186/1757-4749-3-8.
-
26.
Karlsson CL, Onnerfalt J, Xu J, Molin G, Ahrne S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring). 2012;20(11):2257-61. [PubMed ID: 22546742]. https://doi.org/10.1038/oby.2012.110.
-
27.
Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100. [PubMed ID: 26261039]. [PubMed Central ID: PMC4531509]. https://doi.org/10.1186/s12876-015-0330-2.