Abstract
Background:
Triphala as a combination of three plant’s fruits including Emblica officinalis Gaertn, Terminalia chebula Retz, and Terminalia bellerica Roxb is valued for its antioxidant properties. It has been routinely used in various medicinal studies.Objective:
In this study, the protective effects of E. officinalis, T. chebula, T. bellerica, and Triphala methanolic extracts on cell viability and reactive oxygen species (ROS) generated in PC12 cells were studied under serum/glucose deprivation (SGD) induced cell injury. Synergistic activity of three extracts was also explored.Methods:
Cells were seeded overnight and then exposed to SGD condition for 18 h. Next, they were pretreated with different concentrations of the four extracts (3 - 250 µg/ml) for 4 h. Cell viability and ROS generation were evaluated by viability assay and flow cytometry, respectively. Synergistic activity of three extracts was analyzed with Compusyn software.Results:
SGD could induce cell toxicity after 18 h (P < 0.001). Pretreatment with extracts reduced SGD toxicity in PC12 cells. A significant raise in ROS generation was seen following SGD-induced toxicity. Pretreatment with T. chebula reversed the increased ROS production following ischemic insult.Conclusions:
These results demonstrated the neuroprotection of these extracts possibly by ROS decrement and synergistic activity of them against SGD-induced toxicity.Keywords
1. Background
Brain ischemia is a main cause of cell death leading to disability in the elderly population, in spite of developments in its prevention and treatment (1). It is assumed that reactive oxygen species (ROS) production is involved in ischemia-induced cell damage and neurodegenerative conditions (1, 2). According to previous studies, serum/glucose deprivation (SGD) has proven to be effective in vitro models in understanding the molecular mechanisms of neuronal damage during brain ischemia. In addition, it is very useful model for neuroprotective drug development against ischemia-induced brain injury (3, 4). Rat pheochromocytoma (PC12) cell line is a suitable cell structure for in vitro research of SGD insult (5).
Antioxidants suppress the effects of ROS. Production of free radicals probably is the base of many diseases and abnormal conditions (6, 7). Therefore, the use of antioxidants is a promising approach to manage several conditions including neuroprotection (8, 9).
Herbal extracts have been generally used in ayurvedic formulation and found to have beneficial effects against several diseases and conditions (10). Therefore, it is proposed that some of herbal extracts can be developed as antioxidant drugs.
Emblica officinalis Gaertn (Family Euphorbiaceae), Terminalia chebula Retz (Family Combretaceae), and Terminali bellerica Roxb (Family Combretaceae) are fruits of an annual plant that is native to India (11). Triphala is one of such ayurvedic formulations used by most health care practitioners in India. It is an equivalent combination of fruits of three medicinal plants, amalaki (Emblica officinalis), haritaki (Terminalia chebula), and bibhitaki (Terminalia bellerica) (9). Triphala has several beneficial uses including colon tonic, laxative, eye rejuvenator, anti-inflammatory, antiviral, etc. (9).
E. officinalis is extensively used in the medicine of India and supposed to increase protection against several diseases. It has beneficial effects on malignancy, diabetes mellitus, hepatic disease, cardiovascular trouble, anemia, and so on. It has been applied as antioxidant, immunomodulator, antipyretic, analgesic, cytoprotective, antitussive, and gastroprotective (12). The principle ingredients of E. officinalis are tannins and phenolic compounds. Therefore, E. officinalis has potent antioxidant activity (12). Rao et al. reported that E. officinalis is antioxidant and antiapoptotic and inhibits DNA fragmentation. It also showed immunosuppressive activity on proliferation of lymphocyte and restoration of IL-2 and g-IFN generation (13). Another study reported that E. officinalis improved cell survival, decreased free radical generation, inhibited immunosuppression, and restored both phagocytosis and g-IFN production by macrophages (14).
In another study, it was reported that antioxidant activity of T. bellerica was more than that of tocopherol and this activity was attributed to hydroxyl benzoic acid and hydroxyl cinnamic acid derivatives, flavonolaglycones, and their glycosides (15).
Aqueous extract of T. chebula was investigated for probable antioxidant effects by testing its capacity to inhibit γ radiation-induced lipid peroxidation and damage to superoxide dismutase enzyme in rat liver mitochondria. Several studies showed that T. chebula has many useful compounds including ascorbate, gallic acid, and ellagic acid. The T. chebula extract inhibits xanthine oxidase activity and it is a good radical scavenger (16). Another research reported that extract of T. cheubla modulated oxidative conditions and improved antioxidant status in the liver and kidney of rats (17).
To bring a new insight into the neuroprotective value of E. officinalis, T. chebula, and T. bellerica, the present study was carried out to determine the effects of extracts of these three fruits on protecting PC12 cells against SGD-induced cell death. Triphala as a mixture of E. officinalis, T. chebula, and T. bellerica has been routinely used in different studies. However, the type of this interaction in this model has not been clarified yet. Therefore, possible synergistic activity of these three medicinal plants was also considered as one of the objectives of this study.
2. Methods
2.1. Cells and Reagents
PC12 cell line was obtained from Pasteur Institute (Tehran, Iran). 4,5-Dimethylthiazol-2-yl, 2,5-diphenyl tetrazolium (MTT), 2,7-dichlorofluorescin diacetate (DCFH-DA), and dulbecco’sphosphate-buffered saline (PBS) were purchased from Sigma (St Louis, MO, USA). Glucose-high dulbecco’s modified eagle’s medium (DMEM), glucose-free DMEM, and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY). Dimethyl sulfoxide (DMSO) was purchased from Merck. E. officinalis, T. chebula, and T. bellerica fruits were authenticated by the Pharmacology Department, School of Pharmacy, Mashhad University of Medical Sciences, Iran.
2.2. Cell Culture
Cells were cultured in glucose DMEM (4.5 g/L) supplemented with 10% FBS and 100 unit/mL of penicillin/streptomycin. All cells were maintained in a humidified atmosphere (90%) containing 5% CO2 at 37˚C.
2.3. Preparation of E. officinalis, T. chebula, and T. bellerica Extracts
E. officinalis, T. chebula, and T. bellerica fruits were authenticated. The fruits of E. officinalis, T. chebula, and T.bellerica were dried and powdered. Herbs were extracted in a Soxhlet extractor with methanol (99.8%) (200 g of each herb). The extract was concentrated under reduced pressure and kept at -70ºC in sealed vials until use. Then, the extracts were dissolved separately in dimethyl sulfoxide (DMSO) (50 mg/ml) and kept at -20ºC. The percentages of yield of extracts were 21, 23, and 19 for E. officinalis, T. chebula, and T. bellerica, respectively.
2.4. Cell Proliferation (MTT) Assay
PC12 cells (5000/well) were seeded out in 96-well culture plate overnight. Then, the cells were pretreated with 10 μL of each of four extracts (3 - 250 µg/mL) for 4 h separately, and then incubated simultaneously for another 18 h in SGD condition. SGD condition was induced by glucose free DMEM without FBS. MTT solution in phosphate-buffered saline (5 mg/ml) was added to each well at a final concentration of 0.05% (18, 19). After 3 h, the formazan precipitate was dissolved in DMSO. The absorbance at 570 and 620 nm (background) was measured using a Stat FAX303 plate reader. All treatments were carried out in triplicate.
2.5. Measurement of Intracellular ROS Production
ROS was calculated by measurement of hydrogen peroxide production (20). Briefly, PC12 cells were seeded (100,000/well) in 24-well plates overnight. Then, the cells were pretreated with T. chebula (250 μg/ml), for 4 h followed by exposing to SGD condition. After 18 h, cells were incubated with DCFH-DA for 30 min (21). The medium was removed to a Falcon tube and the cells were trypsinized and collected into the same tube. After washing twice with PBS, the intensity of DCFH-DA fluorescence was determined by using a FACScan flow cytometer (Becton Dickinson), with an excitation wavelength of 480 nm and an emission wavelength of 530 nm.
2.6. Extract Combination Assay
The synergistic protective effects of three extracts were calculated by combination index (CI) method based on Chou equation and analyzed by CompuSyn software (ComboSyn, NJ, USA). CI equation is assessable quantity of the grade of drug interaction in terms of synergism and antagonism of a given endpoint of the effect measurement. Synergism is present when CI is less than 1.0, additive effect when CI equals 1.0, and antagonism when CI is greater than 1.0 (22).
2.7. Statistical Analysis
Data are expressed as mean ± SEM. Statistical differences between the groups were analyzed by one-way analysis of variance with subsequent Bonferroni test. A probability level of P < 0.05 was considered statistically significant.
3. Results
Effects of extracts against SGD-induced cytotoxicity: Exposure to SGD for 18 h could decrease cell viability to 5.40 ± 1.97 (P < 0.001). As shown in Figures 1-4, treatment with E. officinalis extract resulted in increased cell viability subsequent to ischemic insult after 18 h (P < 0.001 at concentration of 125 - 250 μg/ml). Pretreatment with T. Chebula (P < 0.001 at concentration of 62.5 - 250 μg/ml and P < 0.05 at concentration of 31.25 μg/ml), T. Bellerica and Triphala extracts (P < 0.001 at concentration of 31.25 - 250 μg/ml and P < 0.05 at concentration of 15.62 μg/ml) also significantly increased cell viability under SGD condition. The effects of extracts on the survival of cultured PC12 cells displayed a concentration-dependent pattern. As shown in Figure 1, the combination of three extracts (E. officinalis, T. bellerica, and T. chebula) under the name of Triphala significantly increased cell protection and survival as compared with each extract alone. The pattern of cell protection with Triphala extract showed synergistic activity and CI was calculated as 0.69 and 0.73 at concentrations of 125 and 250 μg/ml of Triphala, respectively. All MTT results were illustrated in Figures 1-4, respectively. There was no toxic effect when PC12 cells were incubated with E. officinalis, T. chebula, T. bellerica, and Triphala (data not shown).
Effect of Triphala extract on PC12 Cells Viability Exposed to SGD for 18 h Using MTT Assay
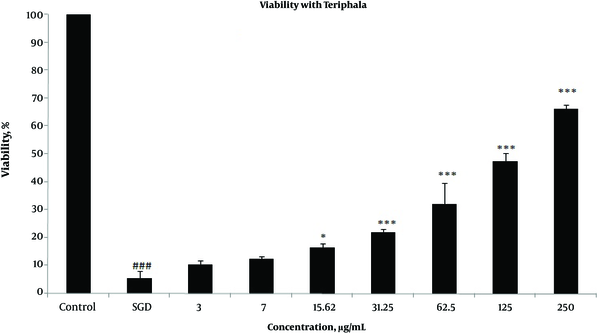
Effect of E. officinalis Extract on PC12 Cells Viability Exposed to SGD for 18 h Using MTT Assay
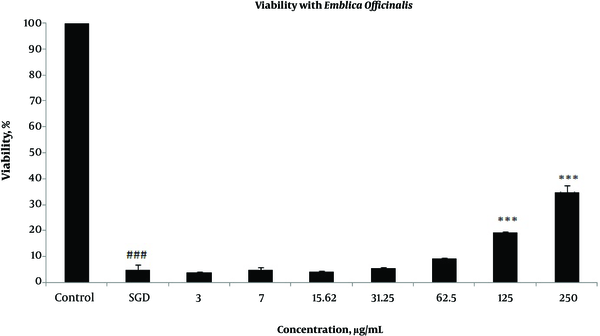
Effect of T. Chebula Extract on PC12 Cells Viability Exposed to SGD for 18 h Using MTT Assay
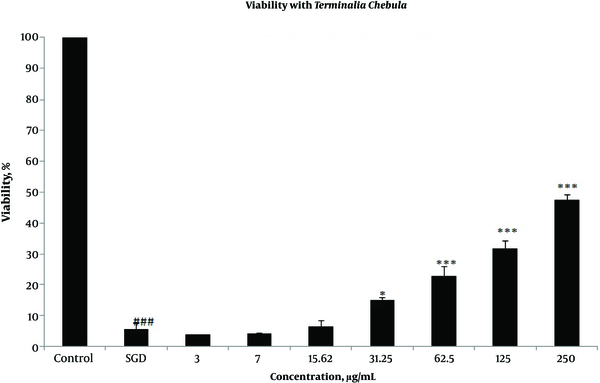
Effect of T. Bellerica Extract on PC12 Cells Viability Exposed to SGD for 18 h Using MTT Assay
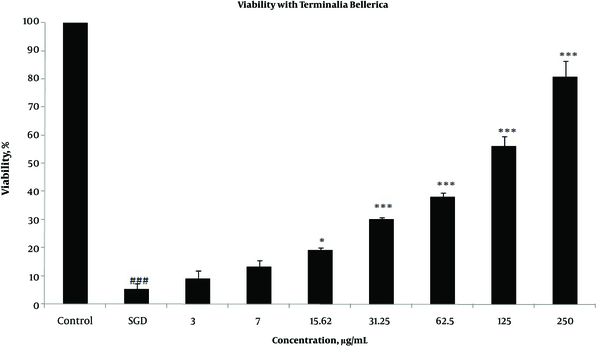
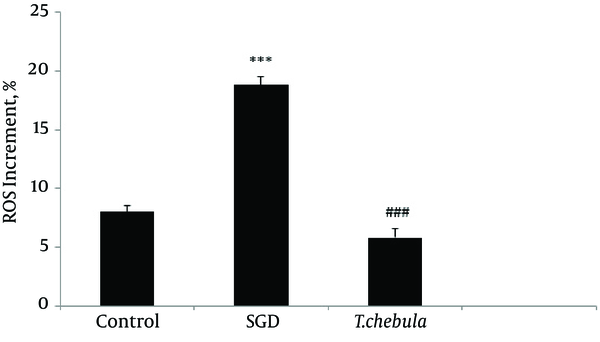
Role of intracellular ROS production: SGD caused a major increase in intracellular ROS production after 18 h as compared with control group. As shown in Figure 5, pretreatment with T. chebula decreased intracellular ROS versus SGD insult. Results were illustrated in Figures 5 and 6, respectively.
Flow Cytometry With DCFH-DA Staining for Measuring Intracellular ROS Production for Cultured PC12 Cells in Control Group (A), SGD Alone Group (B), Pretreatment With T. chebula (250 µg/ml) Plus Exposure to SGD (C), Respectively. The Efficacy of T. chebula in Decrement of Intracellular ROS Production in Comparison With Control Group (D).
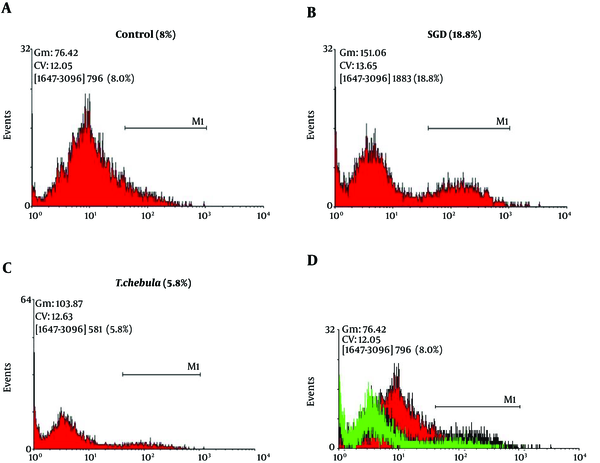
The Effects of T. chebula on Decrement of Intracellular ROS Production in PC12 Cells Using DCFH-DA Staining and Flow Cytometry
4. Discussion
Dysfunction of oxidative metabolism that occurs in brain ischemia is linked to neuron degeneration (23). Oxidative stress is a condition induced by oxygen-derived free radicals usually identified as ROS. Several processes have been implicated in the pathogenesis of cell insult. These are production of toxic lipid metabolites, proteases, endonucleases, and ROS. These events cause oxidative injury to macromolecules such as lipids, proteins, and nucleic acids (7). It has been documented that the basis of many neurological and neurodegenerative diseases including ischemia-reperfusion, convulsion, Parkinson, and Alzheimer’s disease, at least in part, is the generation of free radicals. It has been proven that treatment with antioxidants has protective role against CNS injuries (6, 7, 24). Increment of intracellular ROS production in SGD model has been reported in several studies. Mousavi et al. reported that intracellular ROS production significantly increased when PC12 cells were exposed to SGD condition (21). Other research also referred to the role of ROS production in SGD insult (25, 26). In this study, SGD caused a major increase in ROS production that is in agreement with other reports.
In the present research, SGD has been used to induce injury in PC12 cells as a suitable model of the pathological process of cerebral ischemia as an attempt to study the possible use of naturally occurring drugs with neuroprotective effects. The results of this investigation suggest that E. officinalis, T. chebula, T. bellerica, and Triphala extracts have significant protective effects and can reverse SGD insult. Therefore, protective effects of these three extracts and their combination were proven in this research for the first time. Results showed that the combination of three extracts (E. officinalis, T. chebula, and T. bellerica) increased cell viability and caused more protection percentage synergistically. To our knowledge, it is first time to show synergistic activity of three extracts. Since in folk medicine, combination of three extracts under the name of Triphala is used, the results of the present study clearly confirmed rational use of Triphala in comparison with single extract treatment.
The ROS generation increment may also be responsible for SGD-induced toxicity (27). It has been found that ROS generation mediates many damages during brain ischemia and in the penumbral region of infarcts caused by stable ischemia (7). Also, ROS generation was known as probable candidate for evaluating the acute CNS injury pathogenesis, thus it is becoming a suitable and important therapeutic target (28-30). Many herbs with antioxidant activity were explored with successful in SGD induced-toxicity model. Mousavi et al. reported that the extract of Nigella sativa decreased the amount of ROS production and refined the PC12 cell survival mediated via antioxidant properties (21). Another study reported that Punica granatum has defensive properties against SGD-induced toxicity and antioxidant activity is contributed to this protection (31). In addition, Bakhtiari et al. reported that methanolic extract of Hibiscus sabdariffa has protective effects against SGD insult in PC12 cells via antioxidant activity (32).
In the present study, pretreatment with T. chebula extract showed an effective block ROS generation by SGD, demonstrating the inhibition of intracellular ROS generation possibly implicated in the protective effects of this extract, thus, confirming its antioxidant activity in ischemia-induced cell insult.
To conclude, the present study indicated that pretreatment with E. officinalis, T. chebula, and T. bellerica extracts and combination of them improved SGD-induced cytotoxicity in cultured PC12 cells, and its protective effects might be mediated via inhibition of intracellular ROS generation. The present study on the protective activity of E. officinalis, T. chebula, T. bellerica, and Triphala suggests the probable use of these extracts in a clinical setting to prevent and treatment common neurological injuries.
Acknowledgements
References
-
1.
Amantea D, Marrone MC, Nistico R, Federici M, Bagetta G, Bernardi G, et al. Oxidative stress in stroke pathophysiology: Validation of hydrogen peroxide metabolism as a pharmacological target to afford neuroprotection. Int Rev Neurobiol. 2009;85:363-74.
-
2.
Behl C, Moosmann B. Antioxidant neuroprotection in Alzheimer's disease as preventive and therapeutic approach.(Abstracts). Free Radic Biol Med. 2002;33(2):182–91.
-
3.
Chu LF, Wang WT, Ghanta VK, Lin CH, Chiang YY, Hsueh CM. Ischemic brain cell-derived conditioned medium protects astrocytes against ischemia through GDNF/ERK/NF-kB signaling pathway. Brain Res. 2008;1239:24-35. [PubMed ID: 18804095]. https://doi.org/10.1016/j.brainres.2008.08.087.
-
4.
Hillion JA, Takahashi K, Maric D, Ruetzler C, Barker JL, Hallenbeck JM. Development of an ischemic tolerance model in a PC12 cell line. J Cereb Blood Flow Metab. 2005;25(2):154-62. [PubMed ID: 15647748]. https://doi.org/10.1038/sj.jcbfm.9600003.
-
5.
Woronowicz A, Amith SR, Davis VW, Jayanth P, De Vusser K, Laroy W, et al. Trypanosome trans-sialidase mediates neuroprotection against oxidative stress, serum/glucose deprivation, and hypoxia-induced neurite retraction in Trk-expressing PC12 cells. Glycobiology. 2007;17(7):725-34. [PubMed ID: 17389653]. https://doi.org/10.1093/glycob/cwm034.
-
6.
Sun AY, Chen YM. Oxidative stress and neurodegenerative disorders. J Biomed Sci. 1998;5(6):401-14.
-
7.
Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9(1):119-31. [PubMed ID: 9989455].
-
8.
Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of alpha-tocopherol. Neurosci Lett. 2004;362(1):61-4. [PubMed ID: 15147781]. https://doi.org/10.1016/j.neulet.2004.02.067.
-
9.
Naik GH, Priyadarsini KI, Mohan H. Free radical scavenging reactions and phytochemical analysis of triphala, an ayurvedic formulation. Curr Sci. 2006:1100-5.
-
10.
Ahmad I, Mehmood Z, Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol. 1998;62(2):183-93.
-
11.
Kumar Jain P, Ravichandran V, Sharma S. The antioxidant activity of some medicinal plants. Turk J Biol. 2008;32(2008):197-202.
-
12.
Khan KH. Roles of emblica officinalis in medicine- a review. Bot Res Internat. 2009;2(4):218-28.
-
13.
Rao CHV, Sairam K, Agarwal VK. Effect of Phyllanthus emblica Linn on gastric ulceration and secretion. Ind J Pharmacol. 2000;(32):81–7.
-
14.
Pandey M, Singh S, Patkit P. Efficasy and safety of bresol Tablets in the management of Bronchial Athma. India J Clinic Prac. 2010;20(10):707-13.
-
15.
Saleem A, Ahotupa M, Pihlaja K. Total phenolics concentration and antioxidant potential of extracts of medicinal plants of Pakistan. Z Naturforsch C. 2001;56(11-12):973-8. [PubMed ID: 11837686].
-
16.
Naik GH, Priyadarsini KI, Naik DB, Gangabhagirathi R, Mohan H. Studies on the aqueous extract of Terminalia chebula as a potent antioxidant and a probable radioprotector. Phytomedicine. 2004;11(6):530-8. [PubMed ID: 15500265]. https://doi.org/10.1016/j.phymed.2003.08.001.
-
17.
Mahesh R, Bhuvana S, Begum VM. Effect of Terminalia chebula aqueous extract on oxidative stress and antioxidant status in the liver and kidney of young and aged rats. Cell Biochem Funct. 2009;27(6):358-63. [PubMed ID: 19548245]. https://doi.org/10.1002/cbf.1581.
-
18.
Mortazavian SM, Ghorbani A, Hesari TG. Effect of hydro-alcoholic extract of Viola tricolor and its fractions on proliferation of uterine cervix carcinoma cells. Iran J Obstet Gynecol Infertil. 2012;15(22):9-16.
-
19.
Ghorbani A, Hadjzadeh MA, Rajaei Z, Zendehbad SB. Effects of fenugreek seeds on adipogenesis and lipolysis in normal and diabetic rats. Pak J Biol Sci. 2014;17(4):523-8. [PubMed ID: 25911840].
-
20.
Ghorbani A, Sadeghnia HR, Asadpour E. Mechanism of protective effect of lettuce against glucose/serum deprivation-induced neurotoxicity. Nutr Neurosci. 2015;18(3):103-9. [PubMed ID: 24621063]. https://doi.org/10.1179/1476830513Y.0000000107.
-
21.
Mousavi SH, Tayarani-Najaran Z, Asghari M, Sadeghnia HR. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2010;30(4):591-8. [PubMed ID: 20054635]. https://doi.org/10.1007/s10571-009-9484-1.
-
22.
Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621-81. [PubMed ID: 16968952]. https://doi.org/10.1124/pr.58.3.10.
-
23.
Johnson EJ, Greenlund LJ, Akins PT, Hsu CY. Neuronal apoptosis: current understanding of molecular mechanisms and potential role in ischemic brain injury. J Neurotrauma. 1995;12(5):843-52. [PubMed ID: 8594212]. https://doi.org/10.1089/neu.1995.12.843.
-
24.
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54(2):271-84. [PubMed ID: 12037143].
-
25.
Afsharzadeh M, Tayarani-Najaran Z, Zare A, Mousavi SH. Protective Effect of Scutellaria litwinowii Extract on Serum/Glucose-Deprived Cultured PC12 Cells and Determining the Role of Reactive Oxygen Species. J Toxicol. 2012;2012:413279. [PubMed ID: 22888343]. https://doi.org/10.1155/2012/413279.
-
26.
Shafaei-Bajestani N, Emami SA, Asili J, Tayarani-Najaran Z. Anti-apoptotic effect of taxodione on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2014;34(8):1103-9. [PubMed ID: 25187359]. https://doi.org/10.1007/s10571-014-0085-2.
-
27.
Liu Y, Song XD, Liu W, Zhang TY, Zuo J. Glucose deprivation induces mitochondrial dysfunction and oxidative stress in PC12 cell line. J Cell Mol Med. 2003;7(1):49-56. [PubMed ID: 12767261].
-
28.
Bromont C, Marie C, Bralet J. Increased lipid peroxidation in vulnerable brain regions after transient forebrain ischemia in rats. Stroke. 1989;20(7):918-24. [PubMed ID: 2749850].
-
29.
Hall ED, Braughler JM. Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med. 1989;6(3):303–13.
-
30.
Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990;87(13):5144-7. [PubMed ID: 1973301].
-
31.
Forouzanfar F, Afkhami Goli A, Asadpour E, Ghorbani A, Sadeghnia HR. Protective Effect of Punica granatum L. against Serum/Glucose Deprivation-Induced PC12 Cells Injury. Evid Based Complement Alternat Med. 2013;2013:716730. [PubMed ID: 23935674]. https://doi.org/10.1155/2013/716730.
-
32.
Bakhtiari E, Hosseini A, Mousavi SH. Protective effect of Hibiscus sabdariffa against serum/glucose deprivation-induced PC12 cells injury. Avicenna J Phytomed. 2015;5(3):231-7. [PubMed ID: 26101756].
