Abstract
Background:
Persicaria sagittata (L.) H.Gross (Polygonaceae) has long been used in Indonesian traditional medicine to treat diarrhea, skin diseases, and internal problems. To date, the chemistry and biological activity of P. sagittata have not investigated, requiring more research.Objectives:
This study aimed to investigate the chemical constituents of P. sagittata stems, evaluate their antioxidant activity, and discuss their chemotaxonomy values in genus Persicaria.Methods:
Compounds isolated from the plant stems were identified using physicochemical and spectroscopic measurements (1D and 2D NMR) and by comparison with literature data. The antioxidant activity was evaluated based on the qualitative and quantitative DPPH assays. The chemotaxonomy value was assessed based on extensive studies of the phytochemically investigated Persicaria species.Results:
We successfully isolated 10 compounds for the first time from P. sagittata, including arborinone (1), 25-hydroxycholest-5-en-3β-yl acetate (2), β-sitosterol (3), methyl-4-hydroxy cinnamate (4), protocatechuic acid (5), gallic acid (6), methyl gallate (7), quercetin (8), vanicoside A (9), and vanicoside B (10). Of these, compound 6 (IC50: 8.88 μM) exhibited the most significant antioxidant activity relative to ascorbic acid (IC50: 30.49 μM), followed by compounds 7, 9, 8, 5, and 10 (IC50: 15.37, 26.82, 29.18, 32.38, and 35.06 μM, respectively). Meanwhile, compounds 1 - 4 were inactive toward DPPH radicals (IC50 > 400 μM). The chemistry of P. sagittata showed a relatively similar profile to other species profiles, implying a close chemotaxonomy relationship of P. sagittata with other species of genus Persicaria. Compounds 2 and 4 were first reported in genus Persicaria, which may serve as chemical markers for P. sagittata.Conclusions:
The chemical constituents of P. sagittata have potent antioxidant activities, in particular, phenolics, flavonoids, and sucrose esters. The presence of compounds 1 - 10 could enrich the chemotaxonomy value of P. sagittata in genus Persicaria.Keywords
Persicaria sagittata Polygonaceae Chemical Constituent Antioxidant Chemotaxonomy Significance
1. Background
Genus Persicaria belongs to the Polygonaceae family and comprises 150 species of annual or perennial herbs of worldwide distribution in the northern temperate to tropical and subtropical regions (1). Persicaria sagittata (L.) H.Gross (synonym: Polygonum sagittatum L., Polygonum sieboldii Meisn.), known as tearthumb, is a herb native to Asia, India, and North America that has been naturalized elsewhere (2). This species has been reported in Chinese traditional medicine (3) and has been used to treat diarrhea, skin diseases, and internal problems in Central Java, Indonesia.
Based on our extensive search, the phytochemistry and biological activity of P. sagittata have not been studied thus far, requiring more research. Hence, the present study first reported the phytochemistry of P. sagittata and discussed the antioxidant activity and chemotaxonomy value of isolated compounds from genus Persicaria.
2. Objectives
This study aimed to investigate the chemical constituents of stems of P. sagittata, evaluate their antioxidant activity based on the DPPH assay, and discuss their chemotaxonomy values in genus Persicaria.
3. Methods
3.1. General
Kieselgel 60 PF254 (aluminum plate), silica gel 60 G, and 60 PF254 containing gypsum were purchased from Merck (Darmstadt, Germany). Diphenyl-picrylhydrazyl (DPPH) and ascorbic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). Absorbance was measured using a Cary 100 Conc UV-Vis spectrophotometer (Varian, Palo Alto, CA, USA). Melting points were measured using a Melting Point M-565 apparatus (Buchi, Flawil, Switzerland). The NMR spectra (1D and 2D) were recorded using an ECP 500 MHz spectrometer (JEOL Ltd., Tokyo, Japan).
3.2. Plant Material
Stems of P. sagittata were collected from the Medicinal Plants and Traditional Medicine Research and Development Centre of the National Institute of Health Research and Development (NIHRD), Tawangmangu, Central Java, Indonesia, in July 2015 where the specimen was identified and deposited.
3.3. Extraction and Isolation of Compounds
The dried powder of P. sagittata stems (230 - 270 mesh, 7 kg) underwent extraction with acetone (3 × 20 L, 24 h each time) at room temperature to yield a dried acetone extract (270 g). This extract was subjected to silica gel Vacuum Liquid Chromatography (VLC, 10 × 5 cm), eluted with a gradient mixture of n-hexane-EtOAc (from 8:2 to 0:10) and subsequently with pure MeOH to yield five main fractions (F1 - F5). Fractions F2 - F5 were subjected to further purification. Fraction F2 (14 g) was separated over silica gel VLC (10 × 5 cm), eluted with n-hexane-EtOAc (from 70:30 to 0:100) and MeOH to yield five subfractions (F21 - F25). Subfraction F23 (1.0 g) was further purified over silica gel Radial Chromatography (RC), eluted with CHCl3-MeOH (95:5) to give pure compound 1 (60 mg). Similarly, subfraction F24 (2.8 g) was chromatographed over silica gel RC, with CHCl3-MeOH (90:10) as a solvent to yield pure compound 2 (80 mg). Furthermore, the main fraction F3 (11.3 g) was separated over silica gel VLC (10 × 5 cm), eluted with a gradient mixture of n-hexane-EtOAc (from 70:30 to 0:100) and subsequently with pure MeOH to yield four subfractions (F31 - F34). Subfraction F32 (1.0 g) was later purified over silica gel RC, with n-hexane-EtOAc (85:15) as a solvent to yield pure compound 3 (100 mg). Subfraction F33 (1.0 g) was also separated over silica gel RC with n-hexane-EtOAc (75:25) to yield pure compound 4 (100 mg). The same method was applied to subfraction F34 and successfully purified compound 5 (200 mg). The main fraction F4 (12.6 g) was further separated over silica gel VLC (10 × 5 cm), eluted with n-hexane-EtOAc (from 6:4 to 0:10) and pure MeOH, to yield five subfractions (F41 - F45). Subfractions F42 (1.3 g), F43 (1.2 g), and F44 (2.2 g) were purified separately over silica gel RC with n-hexane-CHCl3-MeOH (5:4:1) as eluent to yield pure compounds 6 (20 mg), 7 (40 mg), and 8 (30 mg), respectively. Moreover, fraction F5 (118.4 g) was chromatographed over silica gel VLC (10 × 5 cm) to give five subfractions (F51 - F55). The purification of subfraction F53 over silica gel RC with the elution of CHCl3-MeOH (9:1) yielded pure compound 9 (40 mg). Subfraction F54 was also purified using the same method to yield pure compound 10 (30 mg). All isolated compounds were measured for their melting point values and the chemical structures were elucidated using NMR spectra (500 MHz for 1H and 125 MHz for 13C). The physicochemical properties and NMR data, as well as 1H and 13C NMR spectra of the isolated compounds (1-10), can be seen in Appendix 1 in Supplementary File.
3.4. Antioxidant Assay
The DPPH scavenging activity of the isolated compounds was assessed qualitatively using the TLC autographic spray method (4) and quantitatively using the Bios method (5) with slight modifications. For qualitative analysis, the isolated compounds (0.1 - 100 μg) were applied separately on the TLC plate, developed, and dried at room temperature. The plate was further sprayed with DPPH solution (0.2% in MeOH) and incubated for 30 min in dark at room temperature. The activity was determined by the observation of a yellow spot of the compound against the purple background on the plate. Meanwhile, for quantitative analysis, triplicate 1 mL aliquots of samples were mixed with 1 mL of DPPH solution (500 μM) and incubated for 30 min in dark at room temperature. Absorbances were then measured at 517 nm against methanol as a blank. Ascorbic acid was used as a positive control. The concentration of the sample (in mg/mL) at which the absorbance (at 517 nm) reached half of the initial value was determined as the IC50 value. The experiment was repeated three times.
3.5. Chemotaxonomy Study
The chemotaxonomy value was assessed by an extensive systematic study of chemical compounds among species of genus Persicaria. The study included chemical compounds both from preliminary identification using analytical methods and from isolation and identification of pure compounds.
3.6. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5 software (La Jola, CA, USA). Data were analyzed by one-way ANOVA, followed by Tukey post hoc test. A probability of P < 0.05 represented a significance difference. The IC50 values were presented as means ± standard deviation (SD) obtained from three repetitions.
4. Results and Discussion
4.1. Characterization of Isolated Compounds
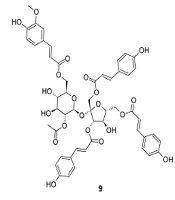
Separation and purification of the acetone extract of dried powder of P. sagittata stems using silica gel vacuum liquid and radial chromatography techniques led to the identification of 10 compounds, including one triterpenoid (1), two steroids (2-3), one phenylpropanoid (4), three simple phenolics (5-7), one flavonoid (8), and two sucrose esters (9-10). The structures of the isolated compounds were determined based on their physicochemical properties and spectroscopic data (1D and 2D NMR), as well as by comparison with literature data (Appendix 1 in Supplementary File). These compounds were identified as arborinone (1) (6), 25-hydroxycholest-5-en-3β-yl acetate (2) (7), β-sitosterol (3) (8), methyl-4-hydroxycinnamate (4) (9), protocatechuic acid (5) (10), gallic acid (6) (11), methyl gallate (7) (12), quercetin (8) (13), vanicoside A (9), and vanicoside B (10) (14). The compound structures are displayed in Figure 1.
Chemical structures of compounds 1-10

4.2. Antioxidant Activity
The acetone extract of P. sagittata stems showed qualitatively weak (++) free radical scavenging activity with an IC50 value of 94.21 μg/mL as compared to ascorbic acid as a positive control (IC50 5.37 μg/mL). The isolated compounds (1-10) from the acetone extract of P. sagittata stems were found to exhibit dose-dependent free radical scavenging activity when assayed using qualitative and quantitative DPPH methods (Table 1). The results of the qualitative analysis revealed that compounds 5-8 performed pronounced free radical scavenging activity as indicated by dominant light yellow spots on the TLC plate with intensities comparable to ascorbic acid. Strong scavenging activity was also exhibited by compounds 9-10. On the other hand, compound 4 showed weak scavenging activity, while compounds 1-3 were unable to scavenge DPPH free radicals. The lowest IC50 value was achieved by compound 6 (IC50 8.88 μM), followed by its derivative, compound 7 (IC50 15.37 μM), suggesting that the replacement of the hydroxyl group (OH) with the methoxyl group (OCH3) may reduce the scavenging activity. In addition, the absence of the OH group on the benzene ring of the hydroxylated benzoic acid derivative affected the scavenging activity of compound 5 with a high IC50 value of 32.38 μM as compared to compound 6. Interestingly, compounds 9-10, classified as cinnamic acid sucrose esters, performed significant free radical scavenging activity (IC50 26.82 and 35.06 μM, respectively) when compared to the cinnamic acid derivative, compound 4 (IC50 456.83 μM), suggesting the important role of the sucrose core in the structures of compounds 9-10 to enhance the scavenging activity. Moreover, the presence of an acetoxy group (OCOCH3) in compound 9 seemed to increase its scavenging activity compared to compound 10. Meanwhile, compound 8, a common flavonol found in genus Persicaria according to our search, was found to perform a consistent free radical scavenging activity in both qualitative and quantitative analyses. It is known that phenolics and flavonoids are major determinants of antioxidant activity in plants (15). The present study suggested that simple phenolics (5-7), a flavonoid (8), and sucrose esters (9-10) played significant roles in performing the free radical scavenging activity of the acetone extract of the investigated plant.
| Structure Number | Compound Name | DPPH Radical Scavenging Activity | |
|---|---|---|---|
| TLC | IC50 (μM) | ||
| 1 | Arborinone | + | 609.46 ± 3.22 |
| 2 | 25-Hydroxycholest-5-en-3β-yl acetate | + | 587.22 ± 3.57 |
| 3 | β-Sitosterol | + | 690.90 ± 4.53 |
| 4 | Methyl-4-hydroxy cinnamate | ++ | 456.83 ± 6.59 |
| 5 | Protocatechuic acid | ++++ | 32.38 ± 1.35 |
| 6 | Gallic acid | ++++ | 8.88 ± 0.43 |
| 7 | Methyl gallate | ++++ | 15.37 ± 0.44 |
| 8 | Quercetin | ++++ | 29.18 ± 0.77 |
| 9 | Vanicoside A | +++ | 26.82 ± 0.93 |
| 10 | Vanicoside B | +++ | 35.06 ± 0.68 |
| Ascorbic acid (positive control) | ++++ | 30.49 ± 1.40 | |
4.3. Chemotaxonomic Significance
Several species of genus Persicaria have been originally classified in genus Polygonum. The Plant List (2013) recorded 66 accepted species in genus Persicaria with low and medium confidence levels (16). Of these, about 25 species have been phytochemically investigated according to our literature search, covering more than 250 compounds. The dominant classes of compounds including phenolics, flavonoids, phenylpropanoids, and sesquiterpenoids have been highlighted among the species of genus Persicaria (17-19). Here, we discuss the isolation and identification of 10 compounds from the stems of P. sagittata, including one triterpenoid (1), two steroids (2-3), three simple phenolics (5-7), one phenylpropanoid (4), one flavonoid (8), and two sucrose esters (9-10) (Figure 1). These compounds were isolated from P. sagittata for the first time.
Species in genus Persicaria have been reported to produce oleanane- and arborane-type triterpenoids. These include the rhizomes of P. bistorta (20) and the whole plants of P. capitata (18) and P. nepalensis (21). Arborinone (1) together with other arborane-type triterpenoids was found in the roots of P. bistorta (22), suggesting a close relationship between P. bistorta and P. sagittata. Meanwhile, steroids with stigmastane skeleton seem to accumulate in the aerial parts of P. barbata (23), P. ferruginea (24), and P. glabra (25), stems of P. chinensis (26) and P. pulchra (27), rhizomes of P. bistorta (20, 22), and the whole plants of P. capitata (28), P. nepalensis (21), and P. stagnina (29). β-sitosterol (3) is a common steroid found in plants and it has been reported in the rhizome of P. bistorta (20) and the whole plants of P. nepalensis (21) and P. stagnina (29). On the other hand, 25-hydroxycholest-5-en-3β-yl acetate (2) and methyl-4-hydroxy cinnamate (4) were described for the first time in genus Persicaria that herein may enrich the chemotaxonomy aspect of this genus (Table 2). These compounds may also be chemotaxonomically significant to differentiate P. sagittata from other species in the genus. The presence of cholestene-type steroids was previously reported in P. amphibia, P. pensylvanica, and P. punctata detected using gas-liquid chromatography and mass spectral analysis (30). Meanwhile, cinnamic acid derivatives are distributed in the aerial parts of P. amphibia, P. bistorta, P. capitata, P. chinensis, P. glabra, P. lapathifolia, P. maculosa, P. mitis, P. stagnina, P. tinctoria (25, 31-35) and rhizomes of P. amplexicaulis (36, 37). Sugar esters containing cinnamic acid have been distributed in several families of plants, covering more than 330 compounds (38). Vanicosides A (9) and B (10) were first reported from the leaves, stems, and roots of P. pensylvanica (14) and later were found in the whole plant of P. hydropiper (39). Compound 10 was also found in the aerial part of P. lapathifolia (40). The presence of sucrose esters in P. capitata, P. hydropiper, P. lapathifolia, and P. pensylvanica is rare and they have not been found so far in other families with a history of sugar esters such as Polygalaceae and Smilacaceae (38). Hence, these compounds could be used as chemical markers for the species of genus Persicaria. Furthermore, protocatechuic acid (5) and gallic acid (6) were distributed in P. amphibia (35, 41), P. bistorta (42), P. capitata (28, 34), and P. lapathifolia (43). Meanwhile, methyl gallate (7) was found in the aerial part of P. lapathifolia (44). Quercetin (8) is a flavonol accumulating remarkably in different parts of 12 species of genus Persicaria (Table 2).
Distribution of Compounds 1-10 in Other Species of Genus Persicaria
| Compound Name | Species (Part) | Reference |
|---|---|---|
| Arborinone (1) | P. bistorta (L.) Samp. (root) | (22) |
| 25-Hydroxycholest-5-en-3β-yl acetate (2) | - | - |
| β-sitosterol (3) | P. bistorta (root) | (20) |
| P. nepalensis (Meisn.) Miyabe (whole plant) | (21) | |
| P. stagnina (Buch.-Ham.Ex Meisn.) Qaiser (whole plant) | (29) | |
| Methyl-4-hydroxy cinnamate (4) | - | - |
| Protocatechuic acid (5) | P. amphibia (L.) Delarbre (aerial part) | (35, 43) |
| P. bistorta (rhizome) | (45) | |
| P. capitata (Buch.-Ham.Ex D.Don) H.Gross (whole plant, aerial part) | (28, 34) | |
| P. lapathifolia (L.) Delarbre, P. maculosa Gray, P. mitis (Schrank) Holub (aerial part) | (35, 43) | |
| Gallic acid (6) | P. amphibia (whole plant, aerial part, leaf) | (41, 43, 46) |
| P. capitata (whole plant, aerial part) | (28, 34) | |
| P. lapathifolia (aerial part) | (43) | |
| P. chinensis (L.) H. Gross, P. maculosa, P. nepalensis (not mentioned) | (42) | |
| Methyl gallate (7) | P. lapathifolia (aerial part) | (44) |
| Quercetin (8) | P. amphibia (whole plant, flower, leaf, stem) | (41) |
| P. amplexicaulis (D.Don) Ronse Decr. (rhizome) | (36, 37) | |
| P. bistorta (aerial part) | (47) | |
| P. capitata (whole plant, aerial part) | (18, 34) | |
| P. chinensis (not mentioned) | (42) | |
| P. decipiens (R.Br.) K.L. Wilson (aerial part) | (48) | |
| P. glabra (Willd.) M.Gómez (leaf) | (49) | |
| P. lapathifolia (whole plant, aerial part, leaf, stem) | (47, 50, 51) | |
| P. maculosa (leaf) | (52) | |
| P. mitis (aerial part) | (17) | |
| P. senegalensis (Meisn.) Soják (aerial part) | (48) | |
| P. tinctoria (Aiton)H.Gross (leaf, stem) | (32) | |
| Vanicoside A (9) | P. pensylvanica (L.) M. Gómez (leaf, stem, root) | (14) |
| P. hydropiper (L.) Delarbre (whole plant) | (39) | |
| Vanicoside B (10) | P. pensylvanica (leaf, stem, root) | (14) |
| P. hydropiper (whole plant) | (39) | |
| P. lapathifolia (aerial part) | (40) |
The chemistry of P. sagittata reported in the present study showed a relatively similar profile to other species of genus Persicaria, which implied a close chemotaxonomy relationship of P. sagittata with other species of this genus. Compounds 2 and 4 are first reported in genus Persicaria, which may serve as chemical markers for P. sagittata. In addition, the presence of cinnamic acid sucrose esters is considered significant for the chemotaxonomy of P. sagittata in this genus.
4. Conclusions
We isolated 10 known compounds (1-10) from P. sagittata stems for the first time in this study. Of these, compounds from the classes of simple phenolics, flavonoids, and sucrose esters were potent natural antioxidant agents. The chemotaxonomy study revealed a close relationship of P. sagittata with other species of genus Persicaria. Compounds 2 and 4 may serve as chemical markers for P. sagittata to probably differentiate this plant from other species in the genus. Since we successfully isolated only 10 compounds from the plant, further research is highly required to explore more about the chemistry, biological activity, and chemotaxonomy value of genus Persicaria.
Acknowledgements
References
-
1.
Brandbyge J. Polygonaceae. In: Kubitzki K, Rohwer JG, Bittrich V, editors. Flowering plants · dicotyledons. The families and genera of vascular plants. Berlin, Heidelberg: Springer; 1993. p. 531-44. https://doi.org/10.1007/978-3-662-02899-5_63.
-
2.
Wiersema JH, León B. World economic plants: A standard reference. Boca Raton: CRC Press; 2016. https://doi.org/10.1201/b13945.
-
3.
Zhang Y, Xu H, Chen H, Wang F, Huai H. Diversity of wetland plants used traditionally in China: A literature review. J Ethnobiol Ethnomed. 2014;10:72. [PubMed ID: 25318542]. [PubMed Central ID: PMC4210556]. https://doi.org/10.1186/1746-4269-10-72.
-
4.
Chacha M, Bojase-Moleta G, Majinda RR. Antimicrobial and radical scavenging flavonoids from the stem wood of Erythrina latissima. Phytochemistry. 2005;66(1):99-104. [PubMed ID: 15649516]. https://doi.org/10.1016/j.phytochem.2004.10.013.
-
5.
Fidrianny I, Utari P, Ruslan KW. Evaluation of antioxidant capacities, flavonoid, phenolic, carotenoid content from various extracts of four kinds of Brassica herbs. Int J Pharm Pharm Sci. 2014;6(2):268-72.
-
6.
Akihisa T, Yamamoto K, Tamura T, Kimura Y, Iida T, Nambara T, et al. Triterpenoid ketones from Lingnania chungii MCCLURE: Arborinone, friedelin and glutinone. Chem Pharm Bull. 1992;40(3):789-91. https://doi.org/10.1248/cpb.40.789.
-
7.
Schmidt AW, Doert T, Goutal S, Gruner M, Mende F, Kurzchalia TV, et al. Regio- and stereospecific synthesis of cholesterol derivatives and their hormonal activity in Caenorhabditis elegans. Euro J Org Chem. 2006;2006(16):3687-706. https://doi.org/10.1002/ejoc.200600394.
-
8.
Nes WD, Norton RA, Benson M. Carbon-13 nmr studies on sitosterol biosynthesized from [13C]mevalonates. Phytochemistry. 1992;31(3):805-11. https://doi.org/10.1016/0031-9422(92)80019-b.
-
9.
Hooper SN, Jurgens T, Chandler RF, Stevens MFG. Methyl p-coumarate: A cytotoxic constituent from Comptonia peregrina. Phytochemistry. 1984;23(9):2096-7. https://doi.org/10.1016/s0031-9422(00)84992-4.
-
10.
He D, Gu D, Huang Y, Ayupbek A, Yang Y, Aisa HA, et al. Separation and purification of phenolic acids and myricetin from black currant by high speed countercurrent chromatography. J Liq Chromatogr Relat Technol. 2009;32(20):3077-88. [PubMed ID: 20161415]. [PubMed Central ID: PMC2786173]. https://doi.org/10.1080/10826070903320756.
-
11.
Kamatham S, Kumar N, Gudipalli P. Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol Rep. 2015;2:520-9. [PubMed ID: 28962387]. [PubMed Central ID: PMC5598244]. https://doi.org/10.1016/j.toxrep.2015.03.001.
-
12.
Ma X, Wu L, Ito Y, Tian W. Application of preparative high-speed counter-current chromatography for separation of methyl gallate from Acer truncatum Bunge. J Chromatogr A. 2005;1076(1-2):212-5. [PubMed ID: 15974092]. https://doi.org/10.1016/j.chroma.2005.04.077.
-
13.
Napolitano JG, Lankin DC, Chen SN, Pauli GF. Complete 1H NMR spectral analysis of ten chemical markers of Ginkgo biloba. Magn Reson Chem. 2012;50(8):569-75. [PubMed ID: 22730238]. [PubMed Central ID: PMC3590027]. https://doi.org/10.1002/mrc.3829.
-
14.
Zimmermann ML, Sneden AT. Vanicosides A and B, protein kinase C inhibitors from Polygonum pensylvanicum. J Nat Prod. 1994;57(2):236-42. [PubMed ID: 8176400]. https://doi.org/10.1021/np50104a007.
-
15.
Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13(10):572-84. [PubMed ID: 12550068]. https://doi.org/10.1016/s0955-2863(02)00208-5.
-
16.
The Plant List. The plant list version 1.1. 2013, [cited 2014 July 7]. Available from: http://www.theplantlist.org/.
-
17.
Granica S, Hinc K. Flavonoids in aerial parts of Persicaria mitis (Schrank) Holub. Biochem Sys Ecol. 2015;61:372-5. https://doi.org/10.1016/j.bse.2015.07.004.
-
18.
Huang G, Gao Y, Wu ZJ, Yang Y, Huang DD, Chen WS, et al. Chemical constituents from Polygonum capitatum Buch-Ham. ex D. Don. Biochem Sys Ecol. 2015;59:8-11. https://doi.org/10.1016/j.bse.2014.12.012.
-
19.
Huq AK, Jamal JA, Stanslas J. Ethnobotanical, phytochemical, pharmacological, and toxicological aspects of Persicaria hydropiper (L.) Delarbre. Evid Based Complement Alternat Med. 2014;2014:782830. [PubMed ID: 24834098]. [PubMed Central ID: PMC4009190]. https://doi.org/10.1155/2014/782830.
-
20.
Manoharan KP, Benny TK, Yang D. Cycloartane type triterpenoids from the rhizomes of Polygonum bistorta. Phytochemistry. 2005;66(19):2304-8. [PubMed ID: 16112695]. https://doi.org/10.1016/j.phytochem.2005.07.008.
-
21.
Rathore A, Sharma SC, Tandon JS. Flavanones from Polygonum nepalense. Phytochemistry. 1986;25(9):2223-5. https://doi.org/10.1016/0031-9422(86)80096-6.
-
22.
Sun XB, Zhao PH, Xu YJ, Sun LM, Cao MA, Yuan CS. Chemical constituents from the roots of Polygonum bistorta. Chem Nat Compd. 2007;43(5):563-6. https://doi.org/10.1007/s10600-007-0193-z.
-
23.
Mazid MA, Datta BK, Nahar L, Bashar SK, Bachar SC, Sarker SD. Phytochemical Studies on Polygonum barbatum (L.) Hara var. barbata (Polygonaceae). Rec Nat Prod. 2011;5(2):143.
-
24.
Brandao GC, Kroon EG, Duarte MG, Braga FC, de Souza Filho JD, de Oliveira AB. Antimicrobial, antiviral and cytotoxic activity of extracts and constituents from Polygonum spectabile Mart. Phytomedicine. 2010;17(12):926-9. [PubMed ID: 20382006]. https://doi.org/10.1016/j.phymed.2010.03.004.
-
25.
Said MS, Chinchansure AA, Nawale L, Durge A, Wadhwani A, Kulkarni SS, et al. A new butenolide cinnamate and other biological active chemical constituents from Polygonum glabrum. Nat Prod Res. 2015;29(22):2080-6. [PubMed ID: 25643642]. https://doi.org/10.1080/14786419.2015.1004674.
-
26.
Tsai PL, Wang JP, Chang CW, Kuo SC, Chao PD. Constituents and bioactive principles of Polygonum chinensis. Phytochemistry. 1998;49(6):1663-6. [PubMed ID: 9862141]. https://doi.org/10.1016/s0031-9422(98)00322-7.
-
27.
Sani A, Anggrenimanggau M, Sukohar A, Widodo H, Baharum SN. Radical scavenging activity of triterpene steroids from stem of Polygonum pulchrum Bl. Int J Pharm Pharm Sci. 2014;6(8):350-4.
-
28.
Yang Y, Wu ZJ, Chen WS. Chemical constituents of Polygonum capitatum. Chem Nat Compd. 2015;51(2):332-5. https://doi.org/10.1007/s10600-015-1274-z.
-
29.
Ahmed M, Datta BK, Rouf AS, Hassan MA. Constituents of Persicaria stagnina. Planta Med. 1991;57(5):503-4. [PubMed ID: 17226193]. https://doi.org/10.1055/s-2006-960187.
-
30.
Wolfe GR, Xu S, Patterson GW, Salt TA. Polygonales and plumbaginales: Sterol composition in relation to the caryophyllidae. Phytochemistry. 1989;28(1):143-5. https://doi.org/10.1016/0031-9422(89)85026-5.
-
31.
Datta BK, Datta SK, Rashid MA, Sarker SD. Flavonoids from Polygonum stagninum (Polygonaceae). Biochem Sys Ecol. 2002;30(7):693-6. https://doi.org/10.1016/s0305-1978(01)00136-3.
-
32.
Kimura H, Ishihara T, Michida M, Ogawa S, Akihiro T, Yokota K. Identification and quantitative analysis of polyphenolic compounds from the indigo plant (Polygonum tinctorium Lour). Nat Prod Res. 2014;28(7):492-5. [PubMed ID: 24437998]. https://doi.org/10.1080/14786419.2013.871719.
-
33.
Klimczak U, Wozniak M, Tomczyk M, Granica S. Chemical composition of edible aerial parts of meadow bistort (Persicaria bistorta (L.) Samp.). Food Chem. 2017;230:281-90. [PubMed ID: 28407912]. https://doi.org/10.1016/j.foodchem.2017.02.128.
-
34.
Liao SG, Zhang LJ, Sun F, Wang Z, He X, Wang AM, et al. Identification and characterisation of phenolics in Polygonum capitatum by ultrahigh-performance liquid chromatography with photodiode array detection and tandem mass spectrometry. Phytochem Anal. 2013;24(6):556-68. [PubMed ID: 24154994]. https://doi.org/10.1002/pca.2432.
-
35.
Smolarz HD. P22 Chromatographical analysis of phenolic acids in some species genus polygonum L. Euro J Pharm Sci. 1994;2(1-2):124. https://doi.org/10.1016/0928-0987(94)90195-3.
-
36.
Xiang M, Su H, Hu J, Yan Y. Isolation, identification and determination of methyl caffeate, ethyl caffeate and other phenolic compounds from Polygonum amplexicaule var. sinense. J Med Plants Res. 2011;5(9):1685-91.
-
37.
Xiang MX, Hu YJ, He F, Liu XQ. [Chemical Composition of n-Butanol Fraction from Polygonum amplexicaule var. sinense]. Zhong Yao Cai. 2015;38(9):1872-4. Chinese. [PubMed ID: 26930981].
-
38.
Tian Y, Liu W, Lu Y, Wang Y, Chen X, Bai S, et al. Naturally occurring cinnamic acid sugar ester derivatives. Molecules. 2016;21(10). [PubMed ID: 27783048]. [PubMed Central ID: PMC6273327]. https://doi.org/10.3390/molecules21101402.
-
39.
Kiem PV, Nhiem NX, Cuong NX, Hoa TQ, Huong HT, Huong le M, et al. New phenylpropanoid esters of sucrose from Polygonum hydropiper and their antioxidant activity. Arch Pharm Res. 2008;31(11):1477-82. [PubMed ID: 19023545]. https://doi.org/10.1007/s12272-001-2133-y.
-
40.
Takasaki M, Kuroki S, Kozuka M, Konoshima T. New phenylpropanoid esters of sucrose from Polygonum lapathifolium. J Nat Prod. 2001;64(10):1305-8. [PubMed ID: 11678656]. https://doi.org/10.1021/np010222q.
-
41.
Svirčev E, Balog K, Lesjak M, Mimica-Dukic N, Orcic D, Francišković M, et al. Antioxidant activity and polyphenolic composition of water knotweed (Polygonum amphibium L.) ethanolic extracts. Planta Med. 2012;78(11). https://doi.org/10.1055/s-0032-1321357.
-
42.
Isobe T, Noda Y. A chemotaxonomic study of flavonoids from Japanese Polygonum species. Yakugaku Zasshi. 1987;107(12):1001-4. https://doi.org/10.1248/yakushi1947.107.12_1001.
-
43.
Smolarz HD. Chromatographical analysis of phenolic acids in some species of Polygonum L. genus. Part 1 Qualitative analysis by two-dimensional thin layer chromatography (TLC). Acta Soc Bot Pol. 2014;68(4):287-90. https://doi.org/10.5586/asbp.1999.040.
-
44.
Kuroyanagi M, Yamamoto Y, Fukushima S, Ueno A, Noro T, Miyase T. Chemical studies on the constituents of Polygonum nodosum. Chem Pharm Bull. 1982;30(5):1602-8. https://doi.org/10.1248/cpb.30.1602.
-
45.
Smolarz HD. Chromatographical analysis of phenolic acids in some species of Polygonum L. genus. Quantitative determination of the major components by high performance liquid chromatography (HPLC). Acta Soc Bot Pol. 2014;69(1):21-3. https://doi.org/10.5586/asbp.2000.003.
-
46.
Nikolaeva GG, Lavrent’eva MV, Nikolaeva IG. Phenolic compounds from several Polygonum species. Chem Nat Compd. 2009;45(5):735-6. https://doi.org/10.1007/s10600-009-9414-y.
-
47.
Smolarz HD. Comparative study on the free flavonoid aglycones in herbs of different species of Polygonum L. Acta Pol Pharm. 2002;59(2):145-8. [PubMed ID: 12365607].
-
48.
Hussein S, El-Magly U, Tantawy M, Kawashty S, Saleh N. Phenolics of selected species of Persicaria and Polygonum (Polygonaceae) in Egypt. Arab J Chem. 2017;10(1):76-81. https://doi.org/10.1016/j.arabjc.2012.06.002.
-
49.
Tiwari KP, Masood M, Tripathi RD. Flavonoid constituents of the leaves of Polygonum glabrum. J India Chem Soc. 1979;56(10):1042-3.
-
50.
Isobe T, Fukushige T, Noda Y. A new flavonoid glycoside from Polygonum nodosum. Chem Lett. 1979;8(1):27-30. https://doi.org/10.1246/cl.1979.27.
-
51.
Makhkamova KF, Bandyukova VA, Khalmatov KK. A study of the polyphenolic compounds of Polygonum amphibium and P. scabrum. Chem Nat Compd. 1970;6(5):672. https://doi.org/10.1007/bf00563488.
-
52.
Mukhamed'yarova MM. Flavonoids of Polygonum persicaria. Chem Nat Compd. 1968;4(2):112-3. https://doi.org/10.1007/bf00568027.