Abstract
Keywords
1. Background
Diabetes is the most common metabolic disease, which characterized by hyperglycemia. Type 1 diabetes is associated with the destruction of insulin-producing pancreatic β-cells (1). Diabetes, with complex and not fully understood pathophysiology, increases the risk of cardiovascular complications at least 10-fold (2, 3). Diabetes-induced impairments in cardiac function and structure are termed as diabetic cardiomyopathy (DCM) (4, 5). There are several hypotheses about the pathophysiology of diabetes-induced cardiomyopathy. Among them, the role of oxidative stress in DCM is a more experimentally supported mechanism (6). In diabetes, an increase in free radical generation along with a reduction in free radical elimination results in oxidative stress and promotes production of malondialdehyde (MDA), a product of lipid peroxidation, as an oxidative parameter (6-8). Considering the crucial role of oxidative stress in DCM, it seems that approaches for reduction of reactive oxygen species (ROS) or elevation of antioxidant defense mechanisms in the myocardium can improve cardiac function in diabetes (9). In this regard, the beneficial effects of antioxidants on complications of diabetes have been suggested (6). IMODTM is a selenium-based mixture from Rosa canina, Urtica dioica, Tanacetum vulgare extracts, as well as selenium and urea. This herbal compound is used as an antioxidant and anti-inflammatory as well as an immunomodulatory agent (10-12).
It has been shown that physical activity has beneficial influences on diabetic complications, which is partly derived from its antioxidant properties (13, 14). As suggested previously, exercise reduces the cardiovascular risk factors in obese as well as diabetic patients (15, 16). Positive effects of exercise on systemic oxidative stress and reduction of cardiac oxidative stress in diabetes have been demonstrated by human and animal studies, respectively (13, 17, 18). For instance, Lee et al. (13) showed that regular exercise improved coronary vascular function through the reduction of diabetes-induced oxidative stress and inflammation.
2. Objectives
Based on the main role of oxidative stress in DCM and also antioxidant and anti-inflammatory properties of IMODTM and exercise training, the present study investigated the effects of IMODTM in combination with regular exercise on oxidative stress-related cardiac consequences of type 1 diabetes in rats.
3. Methods
3.1. Experimental Animals and Diabetes Induction
In this study, 64 male Wistar rats (250 - 300 g) were housed in standard conditions (at the temperature of 22 ± 2°C and 12-hour darkness and light cycle) with free access to food and water. All experimental protocols were directed according to the regulations for the use and care of Laboratory Animals (1996, published by National Academy Press, 2101 Constitution Ave. NW, Washington, DC 20055, USA).
After the acclimatization period (1 week), animals were randomly divided into 8 groups (n = 8): control (C), treated with exercise (E), treated with IMODTM (I), treated with exercise and IMODTM (E + I), diabetes (D), diabetic rats treated with exercise (D + E), diabetic rats treated with IMODTM (D + I), and diabetic rats that were treated with exercise and IMODTM (D + E + I). Treatments with exercise and/or IMODTM were performed for 8 weeks.
Type 1 diabetes was induced by intraperitoneal injection of 60 mg/kg streptozotocin (dissolved in a citrate buffer) (19, 20). After 72 h of diabetes induction, initial blood glucose levels were measured by a digital glucometer (Gluco Sure, Star, Taiwan), and animals with blood glucose levels higher than 300 mg/dL were classified as diabetic.
In exercise groups, running exercise was performed on a treadmill (5 days/week, 60 min/day at 22 m/min, 0 degree slope), at 10:00 AM, for 8 weeks. Moreover, rats in the non-exercised group were placed on the treadmill for the same duration. An effective dose of IMODTM (Rose Pharmed co., Iran) (20 mg/kg) (11) was injected intraperitoneally, once a day at 8:00 AM for 8 weeks, in IMODTM treated groups.
3.2. Tissue Sampling and Preparation of Heart Homogenate
At the end of treatments, the animals were kept fasted (overnight) for 12 h, and fasting blood glucose (final blood glucose) was measured by a digital glucometer (Gluco Sure, Star, Taiwan). Then, all rats were deeply anesthetized by ketamine (60 mg/kg) and xylazine (10 mg/kg) (21), and afterwards, blood samples were collected and stored at deep freeze (-70°C). Moreover, heart samples were immediately removed, frozen in liquid nitrogen, and stored at -70°C for later measurements. The blood samples were centrifuged (1000 rpm for 15 minutes at 4°C), and the serum was collected and stored at -20°C for total antioxidant capacity (TAC) and MDA assays. The heart samples were homogenized in a KCl solution (1.15%) (Sigma-Aldrich, Steinheim, Germany) and centrifuged (1000 rpm for 1 min at 4°C) (22). Then, the supernatants were collected and stored at -20°C for LDH, MDA, superoxide dismutase (SOD), and glutathione peroxidase (GPx) measurements. Protein content in the serum and heart samples’ homogenates was estimated by the Bradford technique (23).
3.3. Measurement of Oxidative Stress
Levels of LDH, as a cardiac injury marker, were measured in supernatants obtained from the heart samples spectrophotometrically by an automatic biochemistry analyzer using a commercially available kit according to manufacturer protocol.
Furthermore, MDA was assayed, as a lipid peroxidation indicator, by measuring thiobarbituric acid-reactive substances (TBARS) in the serum and the supernatant obtained from the heart samples. In brief, all samples were mixed with 10% trichloroacetic acid (1 mL) and 0.67% thiobarbituric acid (1 mL) and heated in boiling water (for 15 min), and N-butanol (2:1, v:v) was added to this solution. After centrifugation, TBARS were determined by absorbance at 532 nm through a spectrophotometer (24).
3.4. Measurement of Enzymatic Antioxidants Activities
The activity of SOD in the supernatant obtained from the heart was assayed through RANSOD kit (Randox labs. Crumlin, UK), as previously described by Delmas-Beauvieux et al. (25) at 505 nm through a spectrophotometer. This method uses a xanthine-xanthine oxidase system as a superoxide radical generator which reacts with 2 (4 iodophenyl) 3 (4 nitrophenol) 5 phenyl tetrazolium chloride (ITN) to generate a red formazan dye. Substrate concentrations were 0.025 mmol/l for ITN and 0.05 mmol/L for xanthine. The SOD activity was assayed through the rate of inhibition of this reaction. Then, the activity of this antioxidant enzyme was calculated and expressed as ng/mg protein in tissue, as previously described (22).
The activity of GPx in the supernatant obtained from the heart tissue was measured by a RANSEL kit (Randox Crumlin, UK) as previously described by Paglia and Valentine (26) at 340 nm by a spectrophotometer. This enzymatic activity was determined through measuring the rate of oxidized glutathione formation, which is generated by the reduction of organic peroxide ((26, 27), and was expressed as U/mg protein in the tissue.
Measurement of TAC in supernatant from heart tissue homogenate was performed using a Randox commercial kit according to the manufacturer’s protocol (28). Then, final absorbance was detected at 600 nm by a spectrophotometer.
3.5. Statistical Analysis
In the present study, results are expressed as mean ± SEM. The one-way ANOVA, followed by Tukey’s post-hoc test, was used for comparing data between experimental groups, and P < 0.05 was considered statistically significant. Graphs were created using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA) software.
4. Results
4.1. Effects of Exercise and IMODTM on Blood Glucose Level
As shown in Table 1, the initial blood glucose (after 72 h of diabetes induction) and final blood glucose levels were significantly (P < 0.05) increased in the D group compared with the C group. On the contrary, IMODTM, in combination with exercise or either of them alone significantly (P < 0.05), reduced the final blood glucose in D + E, D + I, and D + E + I groups compared with D group.
| Variables | Groups | |
|---|---|---|
| Initial Blood Glucose, mg/dL | Final Blood Glucose, mg/dL | |
| C | 95.12 ± 2.68 | 98.87 ± 6.77 |
| E | 94.87 ± 3.11 | 96.12 ± 5.65 |
| I | 95.37 ± 5.22 | 88.87 ± 8.21 |
| E+I | 103.2 ± 3.04 | 94.75 ± 5.01 |
| D | 447.83 ± 28.98b | 515.50 ± 39.38b |
| D + E | 501.83 ± 11.58b | 385.67 ± 30.51b, c |
| D + I | 498.88 ± 18.37b | 379.1 ± 22.66b, c |
| D + E + I | 453.88 ± 22.92b | 342.50 ± 27.8b, c |
4.2. Effects of Exercise and IMODTM on MDA and LDH
As shown in Figure 1A, serum MDA levels were unchanged in D compared with the C group. However, IMODTM significantly (P < 0.001) decreased this variable in D + I compared with the D and C groups. In analyzing heart tissue data, results showed a significant (P < 0.001) elevation of MDA in the D (3.16 ± 0.19) group compared with the C (2.16 ± 0.12) group (Figure 1B). Moreover, IMODTM and exercise significantly (P < 0.001) decreased cardiac levels of this variable in D + E (1.9 ± 0.07), D + I (2 ± 0.04), and D + E + I (1.84 ± 0.04) groups compared with the D group (Figure 1B).
Effects of exercise and IMODTM on serum (A) and cardiac (B) MDA and cardiac LDH (C) levels. Data are expressed as mean ± SEM. ***, P < 0.001 versus C; ###, P < 0.001 versus D.
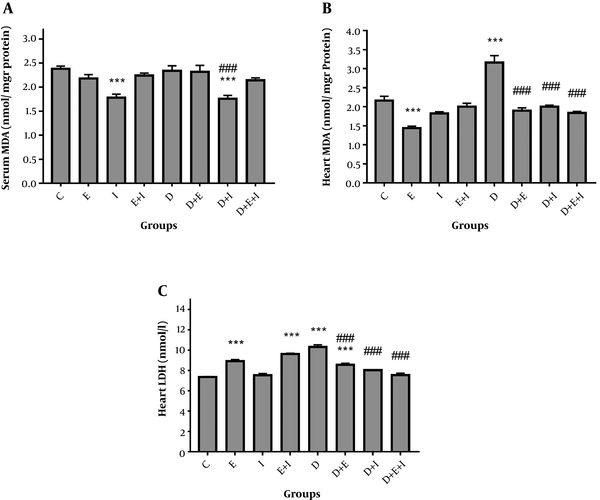
Regarding cardiac levels of LDH, results showed that diabetes significantly (P < 0.001) increased LDH levels in D (10.32 ± 0.2) compared with the C (7.36 ± 0.05) group (Figure 1C). However, IMODTM in combination with exercise or either of them alone significantly (P < 0.001) reduced LDH levels in D + E (8.56 ± 0.15), D + I (8.02 ± 0.05), and D + E + I (7.54 ± 0.19) groups compared with the D group. Treatment of healthy rats with exercise alone or in combination with IMODTM significantly (P < 0.001) increased cardiac LDH levels (Figure 1C).
4.3. Effects of Exercise and IMODTM on Antioxidant Enzymes and TAC
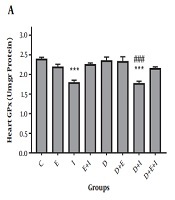
The current study showed a non-significant decrease in GPx activity in the hearts of rats in D compared with the C group (Figure 2A). Moreover, a significant elevation in GPx activity was found in D + E (2.96 ± 0.1) (P < 0.001) and D + E + I (2.56 ± 0.12) (P < 0.05) groups compared with the D (2.1 ± 0.07) group (Figure 2A). As shown in Figure 2B, SOD activity was significantly (P < 0.05) decreased with diabetes induction in D (2.46 ± 0.05) compared with the C (3.38 ± 0.08) group. On the contrary, IMODTM and exercise significantly (P < 0.001) increased SOD activity in the heart of D + E (4.74 ± 0.26), D + I (5.5 ± 0.3), and D + E + I (4.18 ± 0.24) groups compared with the D (2.46 ± 0.05) group (Figure 2B).
Effects of exercise and IMODTM on cardiac GPX (A) and SOD (B) enzymes. Data are expressed as mean ± SEM. *, P < 0.05 versus C; **, P < 0.01 versus C; ***, P < 0.001 versus C; #, P < 0.05 versus D; ###, P < 0.001 versus D.
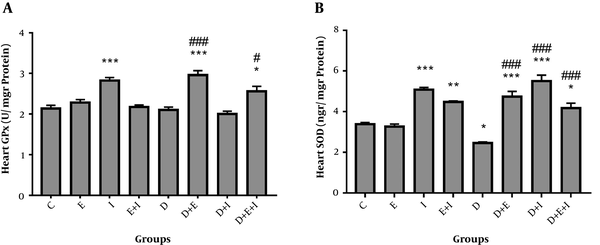
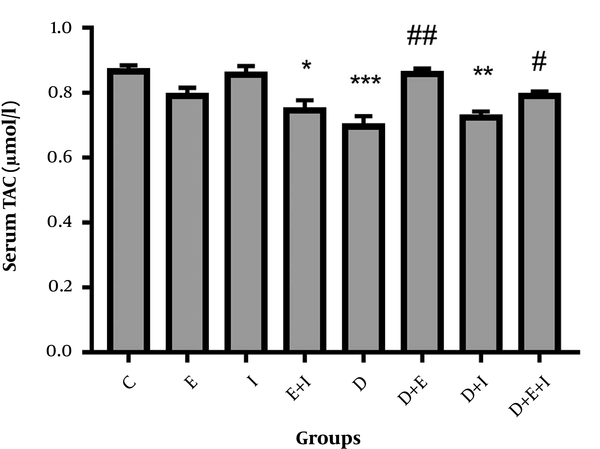
Regarding serum levels of TAC, diabetes significantly (P < 0.001) decreased serum levels of TAC in D compared with the C group. However, exercise alone or in combination with IMODTM significantly increased these levels in D + E (P < 0.01) and D + E + I (P < 0.05) compared with the D group (Figure 3).
Effects of exercise and IMODTM on cardiac TAC. Data are expressed as mean ± SEM. *, P < 0.05 versus C; ***, P < 0.001 versus C; **, P < 0.01 versus C; #, P < 0.05 versus D; ##, P < 0.01 versus D.
5. Discussion
The present study for the first time showed that oral administration of IMODTM alone or in combination with exercise had favorable impacts on alleviating diabetes-induced hyperglycemia as well as systemic and cardiac oxidative stress in type 1 diabetic rats.
This study showed that IMODTM, in combination with exercise or either of them alone, reduced diabetic hyperglycemia. Consistent with these findings, exercise-mediated improvement in hyperglycemia has been shown by several previous studies (29-31). However, a previous study suggested that the treatment of type 1 diabetic mice with IMODTM (for 21 days) had no significant effect on blood glucose levels (32). It seems that the long period (8 weeks) of IMODTM administration in this study is responsible for its blood glucose-lowering effect.
Furthermore, the role of hyperglycemia in the production of oxidative stress has been documented. Oxidative stress plays a principal role in the pathogenesis of vascular diabetic complications (33, 34). In this condition, oxidative stress is caused by an imbalance between the production of the common oxidant (reactive oxygen and nitrogen species) and the antioxidant defense system in favor of pro-oxidants (34).
This study examined the effects of IMODTM and exercise on MDA and LDH levels to determine oxidative stress and cardiac damage, respectively. It has been shown that MDA is a primary marker for oxidative stress, and its elevation in diabetes has a role in diabetic complication development (35, 36). Moreover, previous studies introduced LDH as a cardiac tissue damage marker and also an early predictor of heart damage (37). In the present study, diabetes led to the elevation of cardiac MDA and LDH levels. However, IMODTM alone or, in combination with exercise, reduced heart levels of these variables in diabetic rats. Consistent with the present study, the beneficial effects of regular exercise on decreasing lipid peroxidation and oxidative stress have been shown by some studies (18, 38, 39). Coskun et al. (38) suggested that regular exercise exerted a protective effect against diabetes through the preservation of pancreatic β-cells, reduction of lipid peroxidation, and also elevation of antioxidant enzyme activity. In agreement with the present study, Mohseni-Salehi-Monfared et al. (32) reported that IMODTM reduced oxidative stress in the pancreas of streptozotocin (STZ) injected diabetic mice. Moreover, a recent in vitro study showed that IMODTM reduced oxidative stress in erythrocytes (40). Regarding the effect of exercise on healthy rats, in this study, swimming exercise increased cardiac LDH levels. In agreement with this study, a previous study indicated that the treatment of rats with 4 hours of swimming exercise increased LDH activity in the plasma, which could result from an escape of LDH from the liver, heart, skeletal muscle, and possibly platelets and red cells (41).
In the present study, diabetes decreased serum TAC levels, and exercise alone or in combination with IMODTM increased these levels in diabetic rats. However, IMODTM could not affect this variable. Moreover, this study showed the reduction of SOD activity in diabetic rats, which was reversed by IMODTM in combination with exercise or either alone. Furthermore, our findings suggested the role of exercise alone or in combination with IMODTM in elevation of GPx activity. However, IMODTM could not affect cardiac GPx activity in diabetic rats. In line with this study, Ghattas et al. (42) showed that activities of antioxidant enzymes decreased in diabetes. It has been reviewed that regular physical exercise, as a therapeutic and preventive strategy for diabetes, improved the antioxidant defense system (39). Other study showed antioxidative properties of exercise training in chronic heart failure through increasing antioxidant enzyme activities (43). Regarding the beneficial effects of IMODTM, antioxidant properties of IMODTM were shown by some studies (12, 44). Rezvanfar et al. (12) reported that IMODTM significantly decreased oxidative stress, and also increased antioxidant potential through the elevation of serum and ovary SOD and GPx activities in a rat model of polycystic ovary. Larijani et al. (44) reported that IMODTM at low doses exerted antioxidative effects as well as increased insulin secretion and cell viability in isolated rat pancreatic islets. In addition, antioxidant, anti-inflammatory, and immunomodulatory properties of IMODTM have been confirmed by another previous study in a rat model of immunologic colitis (11). Therefore, regular supplementation of IMODTM in combination with exercise training may protect type 1 diabetic rats against diabetes-induced heart injury through their hypoglycemic and antioxidant properties. Moreover, in this study, the beneficial effect of exercise on diabetic-induced impairment in antioxidant defense system is stronger than IMODTM.
5.1. Conclusions
In summary, it seems that IMODTM, in combination with exercise, decreases systemic and cardiac oxidative stress markers and also improves the cardiac antioxidant defense system, which leads to alleviating cardiac LDH levels as a cardiac injury marker in diabetic rats.
Acknowledgements
References
-
1.
Ozougwu O. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J Physiol Pathophysiol. 2013;4(4):46-57. https://doi.org/10.5897/jpap2013.0001.
-
2.
Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-53. [PubMed ID: 16371630]. [PubMed Central ID: PMC2637991]. https://doi.org/10.1056/NEJMoa052187.
-
3.
Abdollahzade Fard A, Abbasnezhad P, Makhdomi K, Salehi M, Karamdel HR, Saboory E. Association of Serum Prolactin Concentrations with Renal Failure in Diabetic Patients. Rom J Diabetes Nutr Metab Dis. 2017;24(3). https://doi.org/10.1515/rjdnmd-2017-0023.
-
4.
Gilca GE, Stefanescu G, Badulescu O, Tanase DM, Bararu I, Ciocoiu M. Diabetic Cardiomyopathy: Current Approach and Potential Diagnostic and Therapeutic Targets. J Diabetes Res. 2017;2017:1310265. [PubMed ID: 28421204]. [PubMed Central ID: PMC5379137]. https://doi.org/10.1155/2017/1310265.
-
5.
Bugger H, Bode C. The vulnerable myocardium. Diabetic cardiomyopathy. Hamostaseologie. 2015;35(1):17-24. [PubMed ID: 25408270]. https://doi.org/10.5482/HAMO-14-09-0038.
-
6.
Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK. Oxidative stress: a key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol. 2010;88(3):233-40. [PubMed ID: 20393588]. https://doi.org/10.1139/Y10-016.
-
7.
Erciyas F, Taneli F, Arslan B, Uslu Y. Glycemic control, oxidative stress, and lipid profile in children with type 1 diabetes mellitus. Arch Med Res. 2004;35(2):134-40. [PubMed ID: 15010193]. https://doi.org/10.1016/j.arcmed.2003.10.002.
-
8.
Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12(1):5-18. [PubMed ID: 22375253]. [PubMed Central ID: PMC3286717]. https://doi.org/10.12816/0003082.
-
9.
Adebiyi OA, Adebiyi OO, Owira PM. Naringin Reduces Hyperglycemia-Induced Cardiac Fibrosis by Relieving Oxidative Stress. PLoS One. 2016;11(3). e0149890. [PubMed ID: 26967518]. [PubMed Central ID: PMC4788433]. https://doi.org/10.1371/journal.pone.0149890.
-
10.
Paydary K, Emamzadeh-Fard S, Khorram Khorshid HR, Kamali K, SeyedAlinaghi S, Mohraz M. Safety and efficacy of Setarud (IMOD TM ) among people living with HIV/AIDS: a review. Recent Pat Antiinfect Drug Discov. 2012;7(1):66-72. [PubMed ID: 22353002]. https://doi.org/10.2174/157489112799829756.
-
11.
Baghaei A, Esmaily H, Abdolghaffari AH, Baeeri M, Gharibdoost F, Abdollahi M. Efficacy of Setarud (IMod), a novel drug with potent anti-toxic stress potential in rat inflammatory bowel disease and comparison with dexamethasone and infliximab. Indian J Biochem Biophys. 2010;47(4):219-26. [PubMed ID: 21174949].
-
12.
Rezvanfar MA, Rezvanfar MA, Ahmadi A, Shojaei-Saadi HA, Baeeri M, Abdollahi M. Molecular mechanisms of a novel selenium-based complementary medicine which confers protection against hyperandrogenism-induced polycystic ovary. Theriogenology. 2012;78(3):620-31. [PubMed ID: 22541319]. https://doi.org/10.1016/j.theriogenology.2012.03.008.
-
13.
Lee S, Park Y, Zhang C. Exercise Training Prevents Coronary Endothelial Dysfunction in Type 2 Diabetic Mice. Am J Biomed Sci. 2011;3(4):241-52. [PubMed ID: 22384308]. [PubMed Central ID: PMC3289260]. https://doi.org/10.5099/aj110400241.
-
14.
Lakka TA, Venalainen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med. 1994;330(22):1549-54. [PubMed ID: 8177243]. https://doi.org/10.1056/NEJM199406023302201.
-
15.
Ghiasi S, Faridniya S, Maleki Mansourabad S, Abdollahzade Fard A, Abdollahzade Fard N. Response of Leptin and C-reactive Protein Serum Levels to 12 Weeks Moderate Intensity Aerobic Exercise in Obese Men. J Chem Health Risks. 2017;7(1):33-7.
-
16.
Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24(1):275-86. [PubMed ID: 18053320]. https://doi.org/10.1185/030079908x253870.
-
17.
Lew JK, Pearson JT, Schwenke DO, Katare R. Exercise mediated protection of diabetic heart through modulation of microRNA mediated molecular pathways. Cardiovasc Diabetol. 2017;16(1):10. [PubMed ID: 28086863]. [PubMed Central ID: PMC5237289]. https://doi.org/10.1186/s12933-016-0484-4.
-
18.
Naderi R, Mohaddes G, Mohammadi M, Ghaznavi R, Ghyasi R, Vatankhah AM. Voluntary Exercise Protects Heart from Oxidative Stress in Diabetic Rats. Adv Pharm Bull. 2015;5(2):231-6. [PubMed ID: 26236662]. [PubMed Central ID: PMC4517093]. https://doi.org/10.15171/apb.2015.032.
-
19.
Mitton KP, Dean PA, Dzialoszynski T, Xiong H, Sanford SE, Trevithick JR. Modelling cortical cataractogenesis. 13. Early effects on lens ATP/ADP and glutathione in the streptozotocin rat model of the diabetic cataract. Exp Eye Res. 1993;56(2):187-98. [PubMed ID: 8462652]. https://doi.org/10.1006/exer.1993.1026.
-
20.
Ozkaya YG, Agar A, Hacioglu G, Yargicoglu P. Exercise improves visual deficits tested by visual evoked potentials in streptozotocin-induced diabetic rats. Tohoku J Exp Med. 2007;213(4):313-21. [PubMed ID: 18075235]. https://doi.org/10.1620/tjem.213.313.
-
21.
Karimi-Sales E, Jeddi S, Ghaffari-Nasab A, Salimi M, Alipour MR. Effect of trans-chalcone on hepatic IL-8 through the regulation of miR-451 in male rats. Endocr Regul. 2018;52(1):1-5. [PubMed ID: 29453920]. https://doi.org/10.2478/enr-2018-0001.
-
22.
Ghiasi R, Mohammadi M, Ashrafi Helan J, Jafari Jozani SR, Mohammadi S, Ghiasi A, et al. Influence of Two Various Durations of Resistance Exercise on Oxidative Stress in the Male Rat's Hearts. J Cardiovasc Thorac Res. 2015;7(4):149-53. [PubMed ID: 26702343]. [PubMed Central ID: PMC4685280]. https://doi.org/10.15171/jcvtr.2015.32.
-
23.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-54. [PubMed ID: 942051]. https://doi.org/10.1006/abio.1976.9999.
-
24.
Badalzadeh R, Shaghaghi M, Mohammadi M, Dehghan G, Mohammadi Z. The effect of cinnamon extract and long-term aerobic training on heart function, biochemical alterations and lipid profile following exhaustive exercise in male rats. Adv Pharm Bull. 2014;4(Suppl 2):515-20. [PubMed ID: 25671183]. [PubMed Central ID: PMC4312399]. https://doi.org/10.5681/apb.2014.076.
-
25.
Delmas-Beauvieux MC, Peuchant E, Dumon MF, Receveur MC, Le Bras M, Clerc M. Relationship between red blood cell antioxidant enzymatic system status and lipoperoxidation during the acute phase of malaria. Clin Biochem. 1995;28(2):163-9. [PubMed ID: 7628075]. https://doi.org/10.1016/0009-9120(94)00071-3.
-
26.
Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158-69.
-
27.
El-Sayed YS, Lebda MA, Hassinin M, Neoman SA. Chicory (Cichorium intybus L.) root extract regulates the oxidative status and antioxidant gene transcripts in CCl4-induced hepatotoxicity. PLoS One. 2015;10(3). e0121549. [PubMed ID: 25807561]. [PubMed Central ID: PMC4373694]. https://doi.org/10.1371/journal.pone.0121549.
-
28.
Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond). 1993;84(4):407-12. [PubMed ID: 8482045]. https://doi.org/10.1042/cs0840407.
-
29.
Sato K, Fujita S, Yamauchi H, Shiroya Y, Kitamura H, Minato K, et al. The Exercise-Induced Improvement in hyperglycemia is Mediated by DHT Produced in the Skeletal Muscle of Zucker Diabetic Fatty Rats. J Diabetes Metab. 2012;4(1). https://doi.org/10.4172/2155-6156.1000239.
-
30.
Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25(12):2335-41. [PubMed ID: 12453982]. https://doi.org/10.2337/diacare.25.12.2335.
-
31.
Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol (1985). 2011;111(6):1554-60. [PubMed ID: 21868679]. https://doi.org/10.1152/japplphysiol.00921.2011.
-
32.
Mohseni-Salehi-Monfared SS, Habibollahzadeh E, Sadeghi H, Baeeri M, Abdollahi M. Efficacy of Setarud (IMOD), a novel electromagnetically-treated multi-herbal compound, in mouse immunogenic type-1 diabetes. Arch Med Sci. 2010;6(5):663-9. [PubMed ID: 22419922]. [PubMed Central ID: PMC3298332]. https://doi.org/10.5114/aoms.2010.17078.
-
33.
Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48(1):1-9. [PubMed ID: 9892215]. https://doi.org/10.2337/diabetes.48.1.1.
-
34.
Ceriello A, Testa R, Genovese S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr Metab Cardiovasc Dis. 2016;26(4):285-92. [PubMed ID: 27036849]. https://doi.org/10.1016/j.numecd.2016.01.006.
-
35.
Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of Oxidative Stress during Diabetes Mellitus. J Biomark. 2013;2013:378790. [PubMed ID: 26317014]. [PubMed Central ID: PMC4437365]. https://doi.org/10.1155/2013/378790.
-
36.
Gul M, Laaksonen DE, Atalay M, Vider L, Hanninen O. Effects of endurance training on tissue glutathione homeostasis and lipid peroxidation in streptozotocin-induced diabetic rats. Scand J Med Sci Sports. 2002;12(3):163-70. [PubMed ID: 12135449]. https://doi.org/10.1034/j.1600-0838.2002.120307.x.
-
37.
Khan HA, Alhomida AS, Sobki SH, Habib SS, Al Aseri Z, Khan AA, et al. Serum markers of tissue damage and oxidative stress in patients with acute myocardial infarction. Biomed Res. 2013;24(1):15-20.
-
38.
Coskun O, Ocakci A, Bayraktaroglu T, Kanter M. Exercise training prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Tohoku J Exp Med. 2004;203(3):145-54. [PubMed ID: 15240923]. https://doi.org/10.1620/tjem.203.145.
-
39.
Atalay M, Laaksonen DE. Diabetes, oxidative stress and physical exercise. J Sports Sci Med. 2002;1(1):1-14. [PubMed ID: 24672266]. [PubMed Central ID: PMC3957575].
-
40.
Fakhri-Bafghi MS, Ghasemi-Niri SF, Mostafalou S, Navaei-Nigjeh M, Baeeri M, Mohammadirad A, et al. Protective Effect of Selenium-Based Medicines on Toxicity of Three Common Organophosphorus Compounds in Human Erythrocytes In Vitro. Cell J. 2016;17(4):740-7. [PubMed ID: 26862533]. [PubMed Central ID: PMC4746424]. https://doi.org/10.22074/cellj.2016.3846.
-
41.
Papadopoulos NM, Leon AS, Bloor CM. Effects of exercise on plasma and tissue levels of lactate dehydrogenase and isoenzymes in rats. Proc Soc Exp Biol Med. 1967;125(3):999-1002. [PubMed ID: 15938321]. https://doi.org/10.3181/00379727-125-32260.
-
42.
Ghattas MH, Abo-Elmatty DM. Association of polymorphic markers of the catalase and superoxide dismutase genes with type 2 diabetes mellitus. DNA Cell Biol. 2012;31(11):1598-603. [PubMed ID: 22970972]. https://doi.org/10.1089/dna.2012.1739.
-
43.
Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, et al. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111(14):1763-70. [PubMed ID: 15809365]. https://doi.org/10.1161/01.CIR.0000165503.08661.E5.
-
44.
Larijani B, Salimi M, Pourkhalil N, Mohammadir A, Baeeri M, Nili-Ahmad A. Positive Response of Isolated Rat Pancreatic Islets to IMOD; Hopes for Better Transplant Outcome and Graft Function. Asian J Anim Vet Adv. 2011;6(10):1019-25. https://doi.org/10.3923/ajava.2011.1019.1025.