Abstract
Background:
Breast cancer is one of the most prevalent types of cancer. Factors such as ionizing radiation and chemotherapeutic agents can trigger apoptosis and cancer cell death. An anticonvulsant drug named Valproic acid is a histone deacetylase inhibitor that shows promising anti-tumor effects in a variety of cancers. Telomerase is a ribonucleoprotein enzyme that activated in cancer cells and lead to telomeres shortening inhibition and triggering the apoptosis.Objectives:
The purpose of this research was to investigate the simultaneously effect of Valproic acid and gamma radiation on telomerase activity and bax and Bcl-2 protein level in MCF-7 breast cancer cell line.Materials and Methods:
MCF-7 cells was treated with different dose of Valproic acid (0, 2, 8 and 16 mM/l) and single dose of gamma radiation (4 Gy/min. (cell toxicity was determined using neutral uptake test. Telomerase activity was determined using TRAP assay (PCR-ELISA) method. Bax and Bcl-2 protein level was determined by ELISA method, as well.Results:
Combination of Valproic acid and gamma radiation increased significantly cell toxicity in a time and dose dependent manner compared with control (P < 0.0001). The ratio of Bax/Bcl-2 was increased in a dose dependent manner at 48 and 72 hour treatment (P < 0.0001). There was a decrease in Telomerase activity after 24, 48 and 72 hours treatment in a dose dependent manner (P < 0.0001).Conclusions:
The increasing cell toxicity, apoptosis-inducing effects and decreasing telomerase activity may play an important role in the Valproic acid and radiation mechanism. The current survey suggested that it is likely beneficial to combine Valproic acid and gamma radiation to treat breast cancer.Keywords
Breast Cancer Telomerase Apoptosis Histone Deacetylase Inhibitor Ionizing Radiation
1. Background
Breast cancer is one of the most prevalent malignancies in women worldwide (1). Programmed cell death (apoptosis) is an important mechanism of cell death. Recent studies have revealed alteration in susceptibility to apoptosis in cancer cells. Deactivation of pre-apoptotic or activation of anti-apoptotic pathways finally causes defect in apoptosis function (2-5). Factors including ionizing radiation (IR) and chemotherapeutic agents can trigger and accelerate the apoptosis process. Some of proteins contributed to apoptosis regulation and responsed to anticancer therapies are Bcl-2 and bax (6). Bcl-2 is a vital antiapoptotic protein induced by various stimuli. A recent study showed a relationship between overexpression of Bcl-2 and reduced response to chemotherapeutic agents (7, 8); moreover, Bax is a member of the Bcl-2 protein family that describes proapoptotic property and γ-radiation and chemotherapeutic agents that induces its expression (6, 9).
Valproic acid (VPA), a kind of histone deacetylase inhibitor (HDACI), is an effective anticonvulsant drug with anti-tumoral properties (10, 11). HDACIs can induce histone hyperacetylation and transcription of a variety of genes. Hyperacetylation of histones also enhance DNA to be damaged (12). Although, HDACIs augment radiosensitivity of cancer cells; however, a study revealed its radioprotective property (13). Radiotherapy is one of the most important method for treatment of cancer (14). There are numerous side effects associated with radiation, so the therapeutic index of radiotherapy might be improved using cancer cell radiosensitizing agents (15). However, IR resulted in apoptosis or growth arrest, its effect vary among the cell lines (12).
Telomerase is a ribonucleoprotein enzyme and has a key role in tumorgenesis (16). Activation of telomerase in tumor cells lead to telomeres shortening inhibition and triggering the poptosis (17, 18), down regulation of telomerase activity (TA) result in sensitizing cancer cells to effects of radiation and chemotherapeutic agents (15). A recent study has showed thatanti telomerase activity caused by VPA (19); although, contradictory results have been reported in terms of radiation and its relationship with TA regulation (20-22).
2. Objectives
The aim of this research was to investigate the simultaneous effect of VPA and gamma radiation on TA and Bcl-2 and bax protein levels in MCF-7 cancer cell line.
3. Materials and Methods
3.1. Cell Culture and Reagents
A MCF-7 cell line was obtained from the Pasteur institute (Tehran, Iran). VPA was purchased from Sigma (Germany) and dissolved in PBS in a stock concentration of 100 mM and stored at -20°C.
The cells were placed into 75-cm3 tissue culture flasks and cultured in tissue culture medium (DMEM) supplemented with 2 mM L-glutamine, 1% non-essential amino acids, 100 U/ml streptomycin, 100 U/ml penicillin and 10% FBS at 37˚C in incubator with 5% CO2 (all reagents were purchased from Gibco, Germany).
Cells were allowed to attach to the surface for 24 hours prior to the treatment; Then, cells cultured with VPA (0, 2, 8 and 16 Mm/l) and irradiation was consequently performed with γ-rays from a Co60 source at a dose rate of 4.0 Gy/min. Controls consisted of unirradiated and untreated cells that were harvested at the same time points as irradiated and treated cells, in brief, MCF-7 cells were harvested with trypsinization and washed with cold PBS, and rapidly frozen and stored at -80°C.
3.2. Cell Toxicity Assay Using Neutral Red Test
Briefly, MCF-7 cells were plated at 50,000 cells/well on a 24-well plates in DMEM medium and After 24 hours, cells were treated with VPA for 24 ,48 and 72 hours at different doses of VPA (0, 2, 8 and 16 mM/l) and single dose of IR. At the end of incubation period, medium was removed and cells were incubated with neutral red solution (33 µg/mL in fresh media, Merck) for 2 hours at 37°C; then, cells were washed with PBS and fixed (acetic acid/methanol, 80%/20%), after 15 min shaking absorbanceat 550 nm was measured.
3.3. Telomerase Activity Assay
Telomerase-PCR-ELISA kit (Roche applied science, Germany) was used to assess the TA. For preparation of cell extracts, frozen samples were washed with cold PBS and resuspended in 200 µL of ice-cold lysis buffer and incubated for 30 min on ice with shaking; thus, centrifuged for 20 min at 16,000 g and after that the supernatant was transferred to a fresh tube. The samples total protein concentration was determined by the method of Bradford et al. (Bio-Rad, USA). Quantification of TA was performed as follows: cell extracts containing 5 g protein was added to 25 µL of reaction mixture and sterile water was added to a final volume of 50 µL. PCR was then performed using (palm cycler) with conditions: primer elongation (25 minutes, 25°C, 1 cycle), telomerase inactivation (15 min, 94°C, 1 cycle), product amplification for 30 cycles (94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 90 seconds) and; then, balanced (10 minutes at 72°C). Then 5 μl of of denatured PCR products was added to a streptavidin-coated plate and hybridized to a digoxigenin (DIG)-labeled telomeric repeat-specific detection probe. Finally, the immobilized PCR products were assessed with a peroxidase-conjugated anti-DIG antibody. After addition of the stop reagent, the plate was assessed with a microplate reader at a wavelength of 450 nm within 30 minutes.
3.4. Determination of the Bcl-2 and Bax Protein Levels
According to the manufacturer's instructions, determining Bcl-2 and bax protein levels was performed by colorimetric procedures by using of human ELISA kit (Biospes, Chongqing, china). Briefly, treated or untreated cells were lysed according to the ELISA kit protocol instruction. Bcl-2 and bax proteins from the cell lysate specifically bound to the primary antibody and detected by Horseradish peroxidase (HRP) conjugated secondary antibody. HRP-conjugated secondary antibody provided sensitive colorimetric; Then, Bcl-2 and bax protein levels were specified by measuring the absorbance at 450 nm using a microplate reader.
3.5. Statistical Analyses
To analyze, one way ANOVA and TUKEY test were used. Significant level was taken P < 0.05. Statistical analyses were conducted using SPSS statistical package (version 16.0).
4. Results
In this study, the MCF-7 cell line was used to investigate the effect of various dose of VPA in combination with single dose of gamma radiation on regulation of bax and Bcl-2 proteins and TA.
4.1. The effect of VPA in Combination With Gamma Radiation on Cell Toxicity of MCF-7 Cell Line
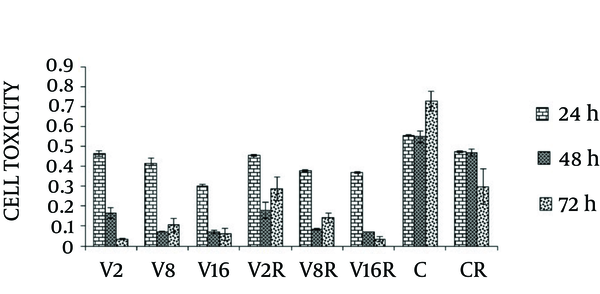
The MCF-7 cell line was treated with different dose of VPA (2, 8 and 16 mM) in combination with single dose of gamma radiation (4 Gy/min) and incubated for 24, 48 and 72 hours. Cell toxicity was determined by neutral red uptake assay after the end of treatment.
Cell toxicity showed a significant increase in a dose and time dependent manner compared with non-treated cells (P < 0.0001). Irradiation of MCF-7 cells at dose of (4 Gy/min) caused less toxicity compared to the cells treated with combination of different dose of VPA and gamma radiation at 24, 48 and 72 hours (Figure 1).
The Effects of VPA and Gamma Radiation on Cell Toxicity of MCF-7 Breast Cancer Cells
4.2. The effect of VPA and Gamma Irradiation on Bcl-2 and Bax Protein
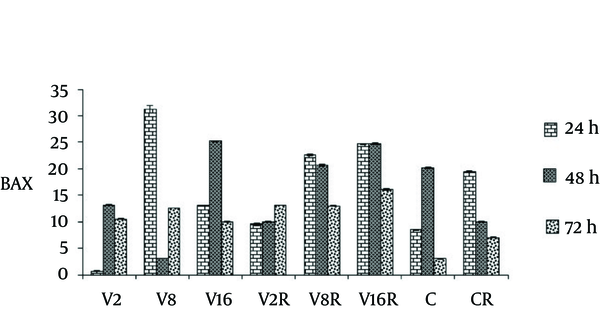
The apoptotic response of MCF-7 cell was assessed after VPA/gamma radiation combined treatment by determining bax and Bcl-2 protein levels.
Treatment of cells with VPA and gamma radiation for 24 and 72 hours demonstrated significant increase in baxprotein level, in a dose and time dependent manner ( P < 0.0001) (Figure 2).
The Effects of VPA and Gamma Radiation on BAX Protein Level
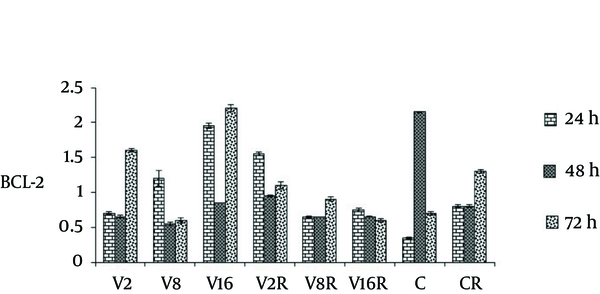
As Figure 3 demonstrates there was a decrease of Bcl-2 protein level in a dose dependent manner after 48 hour treatment of cells with radiation and dose of 2, 8 and 16 Mm/l of VPA.
The Effects of VPA and Gamma Radiation on Bcl-2 Protein Level
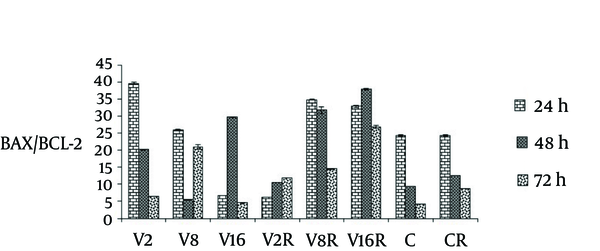
The ratio of bax/Bcl-2 in three different dose of VPA in combination with single dose of gamma radiation was evaluated in different time.
As figure 4 shows, the ratios of bax/Bcl-2 were increased in a dose dependent manner at 48 and 72 hour treatment (P < 0.0001).
The Effects of VPA and Gamma Radiation on BAX/BCL-2 Ratio.
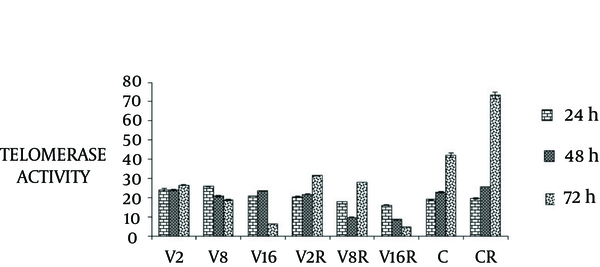
4.3. The Effect of VPA and Radiation on TA
To determine the effect of VPA and irradiation on TA, we used telomerase PCR-ELISA assay.
In the present study, we found dose dependent decrease in TA after 24, 48 and 72 hours combination treatment (P < 0.0001) (Figure 5).
The Effects of VPA and Radiation on Telomerase Activity in MCF-7 Cells
5. Discussion
In the current survey, we investigated the effect of different VPA dose and gamma radiation in different time on TA, bax and Bcl-2 protein level in MCF-7 cancer cell line.
The results of our study showed that treatment of MCF-7 cells with VPA and gamma radiation significantly enhanced cell toxicity in a dose and time dependent manner. The mechanism of synergistic effect of HDACI on radiation seems to be caused by changes in chromatin structure and gene expression induced by HDACIs.
Our result also showed decrease in TA at 48 and 72 hours after treatment of cells with VPA and gamma radiation in a dose dependent manner. These results confirm previous studies that demonstrated the effect of HDACI or radiation on decreasing the TA (23, 24). This effects might be associated with suppression of hTERT transcription induced by HDACI (25).
The cell toxicity increase in treated cells was accompanied by a reduction in TA. It could be due to an inhibitory effect of HDACI on telomerase holoenzyme and consequent cell death (26, 27).
Our results showed increase in bax and decrease in Bcl-2 protein levels, which may triggers the mitochondrial pathway of apoptosis (28).
The present results demonstrated a simultaneous increase in the bax/Bcl-2 ratio and inhibition of the TA by VPA and radiation treatment. These data are in accordance with previous studies (29, 30). Chromatin relaxation using HDACIs resulted in sensitizes DNA to damage agents such as ionizing radiation (31, 32). Therefore, initiate intrinsic apoptosis pathway as well as decrease in TA by shortening the telomeres could trigger apoptosis (33).
Our data indicated that the pathway of apoptosis triggered by VPA and gamma radiation is correlated by an increase in the ratio of bax/Bcl-2 protein or by decrease in TA.
We concluded that the increasing cell toxicity, apoptosis-inducing effects and decreasing telomerase activity may play an important role in the VPA and radiation mechanism. The present investigation suggests the possible therapeutic benefits of combined VPA and gamma radiation for treatment of breast cancer.
Acknowledgements
References
-
1.
Ghoussaini M, Fletcher O, Michailidou K, Turnbull C, Schmidt MK, Dicks E, et al. Genome-wide association analysis identifies three new breast cancer susceptibility loci. Nat Genet. 2012;44(3):312-8. [PubMed ID: 22267197]. https://doi.org/10.1038/ng.1049.
-
2.
Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100(1):57-70. https://doi.org/10.1016/s0092-8674(00)81683-9.
-
3.
Dive C, Hickman JA. Drug-target interactions: only the first step in the commitment to a programmed cell death? Br J Cancer. 1991;64(1):192-6. [PubMed ID: 1854622].
-
4.
O'Connor PM, Wassermann K, Sarang M, Magrath I, Bohr VA, Kohn KW. Relationship between DNA cross-links, cell cycle, and apoptosis in Burkitt's lymphoma cell lines differing in sensitivity to nitrogen mustard. Cancer Res. 1991;51(24):6550-7. [PubMed ID: 1742728].
-
5.
Lowe SW, Ruley H, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74(6):957-67. https://doi.org/10.1016/0092-8674(93)90719-7.
-
6.
Findley HW, Gu L, Yeager AM, Zhou M. Expression and regulation of Bcl-2, Bcl-xl, and Bax correlate with p53 status and sensitivity to apoptosis in childhood acute lymphoblastic leukemia. Blood. 1997;89(8):2986-93. [PubMed ID: 9108419].
-
7.
Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81(11):3091-6. [PubMed ID: 7684624].
-
8.
Porwit-MacDonald A, Ivory K, Wilkinson S, Wheatley K, Wong L, Janossy G. Bcl-2 protein expression in normal human bone marrow precursors and in acute myelogenous leukemia. Leukemia. 1995;9(7):1191-8. [PubMed ID: 7543174].
-
9.
MacNamara BARBARA, Wang W, Chen Z, Hou M, Mazur J, Gruber A, et al. Telomerase activity in relation to pro-and anti-apoptotic protein expression in high grade non-Hodgkin's lymphomas. haematologica. 2001;86(4):386-93.
-
10.
Travaglini L, Vian L, Billi M, Grignani F, Nervi C. Epigenetic reprogramming of breast cancer cells by valproic acid occurs regardless of estrogen receptor status. Int J Biochem Cell Biol. 2009;41(1):225-34. [PubMed ID: 18789398]. https://doi.org/10.1016/j.biocel.2008.08.019.
-
11.
Schulpis KH, Lazaropoulou C, Regoutas S, Karikas GA, Margeli A, Tsakiris S, et al. Valproic acid monotherapy induces DNA oxidative damage. Toxicology. 2006;217(2-3):228-32. [PubMed ID: 16289809]. https://doi.org/10.1016/j.tox.2005.10.004.
-
12.
Zhang Y, Adachi M, Zhao X, Kawamura R, Imai K. Histone deacetylase inhibitors FK228, N-(2-aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)amino- methyl]benzamide and m-carboxycinnamic acid bis-hydroxamide augment radiation-induced cell death in gastrointestinal adenocarcinoma cells. Int J Cancer. 2004;110(2):301-8. [PubMed ID: 15069698]. https://doi.org/10.1002/ijc.20117.
-
13.
Chung YL, Wang AJ, Yao LF. Antitumor histone deacetylase inhibitors suppress cutaneous radiation syndrome: Implications for increasing therapeutic gain in cancer radiotherapy. Mol Cancer Ther. 2004;3(3):317-25. [PubMed ID: 15026552].
-
14.
Saha D, Maity T, Jana M, Mandal S. Cancer treatment strategy: an overview. Asian J Pharm Tech. 2011;1:28-33.
-
15.
Ram R, Uziel O, Eldan O, Fenig E, Beery E, Lichtenberg S, et al. Ionizing radiation up-regulates telomerase activity in cancer cell lines by post-translational mechanism via ras/phosphatidylinositol 3-kinase/Akt pathway. Clin Cancer Res. 2009;15(3):914-23. [PubMed ID: 19188162]. https://doi.org/10.1158/1078-0432.CCR-08-0792.
-
16.
Gupta R, Dong Y, Solomon PD, Wettersten HI, Cheng CJ, Min JN, et al. Synergistic tumor suppression by combined inhibition of telomerase and CDKN1A. Proc Natl Acad Sci U S A. 2014;111(30):E3062-71. [PubMed ID: 25024194]. https://doi.org/10.1073/pnas.1411370111.
-
17.
Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Semin Cancer Biol. 2011;21(6):349-53. [PubMed ID: 22015685]. https://doi.org/10.1016/j.semcancer.2011.10.001.
-
18.
Philippi C, Loretz B, Schaefer UF, Lehr CM. Telomerase as an emerging target to fight cancer--opportunities and challenges for nanomedicine. J Control Release. 2010;146(2):228-40. [PubMed ID: 20381558]. https://doi.org/10.1016/j.jconrel.2010.03.025.
-
19.
Nie D, Huang K, Yin S, Li Y, Xie S, Ma L, et al. Synergistic/additive interaction of valproic acid with bortezomib on proliferation and apoptosis of acute myeloid leukemia cells. Leuk Lymphoma. 2012;53(12):2487-95. [PubMed ID: 22642934]. https://doi.org/10.3109/10428194.2012.698273.
-
20.
Schuck A, Poremba C, Lanvers C, Konemann S, Schleifer T, Wai D, et al. Radiation-induced changes of telomerase activity in a human Ewing xenograft tumor. Strahlenther Onkol. 2002;178(12):701-8. [PubMed ID: 12491058]. https://doi.org/10.1007/s00066-002-0992-x.
-
21.
Turriziani M, Di Giacomo AM, Cardillo A, Torino F, Tentori L, Nasuti P, et al. Residual telomerase activity: a marker of cell survival after exposure to gamma radiation in vitro. Anticancer Res. 2002;23(6C):4561-9.
-
22.
Xing J, Zhu Y, Zhao H, Yang H, Chen M, Spitz MR, et al. Differential induction in telomerase activity among bladder cancer patients and controls on gamma-radiation. Cancer Epidemiol Biomarkers Prev. 2007;16(3):606-9. [PubMed ID: 17372259]. https://doi.org/10.1158/1055-9965.EPI-06-0615.
-
23.
Suenaga M, Soda H, Oka M, Yamaguchi A, Nakatomi K, Shiozawa K, et al. Histone deacetylase inhibitors suppress telomerase reverse transcriptase mrna expression in prostate cancer cells. Int J Cancer. 2002;97(5):621-5. https://doi.org/10.1002/ijc.10082.
-
24.
Khaw AK, Silasudjana M, Banerjee B, Suzuki M, Baskar R, Hande MP. Inhibition of telomerase activity and human telomerase reverse transcriptase gene expression by histone deacetylase inhibitor in human brain cancer cells. Mutat Res. 2007;625(1-2):134-44. [PubMed ID: 17669439]. https://doi.org/10.1016/j.mrfmmm.2007.06.005.
-
25.
Takakura M, Kyo S, Sowa Y, Wang Z, Yatabe N, Maida Y, et al. Telomerase activation by histone deacetylase inhibitor in normal cells. Nucleic Acids Res. 2001;29(14):3006-11. [PubMed ID: 11452025].
-
26.
Rahman R, Osteso-Ibanez T, Hirst RA, Levesley J, Kilday JP, Quinn S, et al. Histone deacetylase inhibition attenuates cell growth with associated telomerase inhibition in high-grade childhood brain tumor cells. Mol Cancer Ther. 2010;9(9):2568-81. [PubMed ID: 20643785]. https://doi.org/10.1158/1535-7163.MCT-10-0272.
-
27.
Sawant SG, Antonacci ROBERTO, Pandita T. Determination of telomerase activity in HeLa cells after treatment with ionizing radiation by telomerase PCR ELISA. Biochemica. 1997;4:22-4.
-
28.
Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22(53):8590-607. [PubMed ID: 14634621]. https://doi.org/10.1038/sj.onc.1207102.
-
29.
Park DI, Lee JH, Moon SK, Kim CH, Lee YT, Cheong J, et al. Induction of apoptosis and inhibition of telomerase activity by aqueous extract from Platycodon grandiflorum in human lung carcinoma cells. Pharmacol Res. 2005;51(5):437-43. [PubMed ID: 15749458]. https://doi.org/10.1016/j.phrs.2004.11.003.
-
30.
Woo HJ, Lee SJ, Choi BT, Park YM, Choi YH. Induction of apoptosis and inhibition of telomerase activity by trichostatin A, a histone deacetylase inhibitor, in human leukemic U937 cells. Exp Mol Pathol. 2007;82(1):77-84. [PubMed ID: 16574101]. https://doi.org/10.1016/j.yexmp.2006.02.004.
-
31.
Karagiannis TC, El-Osta A. The paradox of histone deacetylase inhibitor-mediated modulation of cellular responses to radiation. Cell Cycle. 2006;5(3):288-95. [PubMed ID: 16418577].
-
32.
Chen X, Wong P, Radany E, Wong JY. HDAC inhibitor, valproic acid, induces p53-dependent radiosensitization of colon cancer cells. Cancer Biother Radiopharm. 2009;24(6):689-99. [PubMed ID: 20025549]. https://doi.org/10.1089/cbr.2009.0629.
-
33.
Ramı́rez R, Carracedo J, Jiménez R, Canela A, Herrera E, Aljama P, et al. Massive telomere loss is an early event of DNA damage-induced apoptosis. J Biol Chem. 2003;278(2):836-42.