Abstract
Background:
Suppression of the immune responses, using products derived from medicinal plant, as a likely therapeutic measure, has become an important subject of scientific studies, recently. Rosmarinus officinalis (Lamiaceae), known as rosemary, is widely distributed in many parts of the world.Objectives:
The immunomodulatory effect of carnosol, a natural antioxidant derived from rosemary, was investigated using BALB/c mice.Materials and Methods:
Carnosol was administered at doses of 0.04, 0.2, 0.8, 2.4 and 4 mg/kg, for 5 days. Another group of mice was treated with 20 mg cyclophosphamide/kg/day (positive control); a final group received solvent only (solvent control). Delayed-type of hypersensitivity response and hemagglutination titer were studied in these groups of animals.Results:
Results showed that three high doses of carnosol (0.8, 2.4 and 4 mg/kg) could significantly suppress both cellular and humoral activity of the immune system. Interestingly, carnosol, at doses of 2.4 and 4 mg/kg, was able to inhibit acquired immunity, higher than cyclophosphamide, which is a known potent immunosuppressant.Conclusions:
Based on its effects, carnosol might be considered a source of drug, showing effective immunosuppression properties. Further studies should be performed on carnosol to develop an effective immunosuppressive drug or coadjuvant for the treatment of disorders caused by an exaggerated or unwanted immune response.Keywords
1. Background
A broad range of immunosuppressants have now been approved to limit unwanted immune responses, particularly those creating autoimmune disease and graft rejection. Use of immunosuppressive agents has notably increased 1-year survival (up to 90%) in patients with renal transplant. However, patients who use immunosuppressants (cyclosporine and tacrolimus) suffer from a number of serious side effects, such as nephrotoxicity, hepatotoxicity, neurotoxicity, induction of diabetes and hypertension (1). For this reason, there is a high demand for new immunosuppressive agents. The immunosuppressive agents without any side effects have been a challenge to the medical system. Suppressing of the immune responses using products of medicinal plants, as a likely therapeutic measure, has become an important subject of scientific studies, recently (2). Despite the overt reviews about the presence of immunostimulant compounds in plants, there is only little information about immunosuppressive products of plant origin. If these products are well tolerated by the patient, they can be developed into alternative coadjuvants in the treatment of disorders caused by an exaggerated or unwanted immune response, such as autoimmune diseases, allergies, glomerulonephritis (3).
Rosmarinus officinalis (Lamiaceae), known as rosemary, is a herb locally to the Mediterranean region, which is also widely distributed in many parts of the world (4). It is used as a source of antioxidants in food industry (5, 6). Antioxidant activity of rosemary is originated from several polyphenolic compounds, such as carnosol and carnosic acid (6, 7). In a small number of studies, anti-inflammatory and anticancer properties of carnosol have been demonstrated (7-10). In one study, performed by John et al., the anti-inflammatory effects of carnosol were attained via reducing nitric oxide (NO) and pro-inflammatory leukotrienes levels (7).
2. Objectives
According to the mentioned background, the present study was carried out to evaluate the immunomodulatory activity of carnosol. Therefore, in this study, we decided to investigate the effects of subacute exposure to carnosol on the functions of the acquired immune system cells, using hemagglutination (HA) titer and delayed type hypersensitivity tests (DTH). To our knowledge, this is the first time that effects of carnosol on acquired immunity are investigated.
3. Materials and Methods
3.1. Animals
Male BALB/c inbred mice (6–8 weeks old, 19–21 grams body weight) were purchased from Bu-Ali research center, Mashhad university of medical sciences, Mashhad, Iran. Animals were acclimatized for 1 week in the lab, prior to administration. The mice were randomly allocated into seven groups (six mice per group). Seven groups of mice were treated with different doses of carnosol (five doses), positive (cyclophosphamide) and negative (carnosol solvent) controls. Mice were housed in polystyrene cages, with free access to food and water, with an ambient temperature of 20 - 25ºC.
3.2. Chemicals
Carnosol and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (Dorset, UK). To prepare different doses, carnosol was dissolved in the solution of DMSO/phosphate-buffered saline (PBS) (1/20). Sheep red blood cells (SRBCs) were obtained from Razi Institute (Mashhad, Iran). The SRBC suspension was washed three times with pyrogen free sterile PBS. The pellet was diluted with PBS to prepare a suspension of 5 × 109 cells/mL, as stock suspension. Then, SRBCs were used for sensitization and challenge at required time schedule.
3.3. Delayed-Type Hypersensitivity Response
Thirty male mice were randomized into seven groups of five mice per group. On the first day, they were subcutaneously immunized with 100 µL of a solution containing 1 × 108 SRBCs suspended in PBS. Five groups received the serial concentrations (0.04, 0.2, 0.8, 2.4, 4 mg/kg/day) of carnosol (100 µL) via the intraperitoneal (ip) route, during a 5-day period. The negative and positive control groups received the carnosol solvents [a solution of DMSO/PBS (1/20)] or cyclophosphamide (20 mg/kg/day) through the same route and time, respectively. The doses of carnosol used here were based on the study of Beninca et al. (2011), demonstrating the anti-inflammatory effects of carnosol (11). On the 5th day, the sensitized animals were subcutaneously challenged with 1 × 108 SRBCs (50 µL) in the left hind footpad. The right hind footpad was injected with the same volume of PBS to serve as trauma control for nonspecific swelling. The footpad thickness was measured at 24, 48, and 72 hours after the booster injection of SRBCs. The results were calculated according to the following formula (Equation 1).
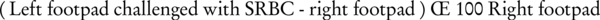
3.4. Hemagglutination Titer Assay
Mice were divided into groups of five animals. On the first day, the mice were immunized with 1 × 108 SRBCs, suspended in PBS via the ip route. The serial concentrations (0.04, 0.2, 0.8, 2.4, 4 mg/kg/day) of carnosol (100 µL) or the solution of DMSO/PBS (1/20) (negative control) were ip administered to each group, for 5 days. Positive control groups were administered cyclophosphamide (20 mg/kg per day, ip). On the 6th day, blood samples were obtained. After preparing sera from peripheral blood samples, aliquots of two-fold diluted sera in PBS were challenged with 1 × 108 SRBCs/tube, in glass tubes. The tubes were incubated at 37ºC for 1 hour and then observed for HA. The highest dilution giving HA was taken as the antibody titer.
3.5. Statistical Analysis
Data were statistically analyzed using Student’s t-test to determine significant differences in the data of various groups. Statistical tests were conducted using INSTAT software (GraphPad, San Diego, CA, USA). Only P values < 0.05 were considered significant. The values are expressed as Means ± standard elevation (SE).
4. Results
4.1. Delayed-Type Hypersensitivity Response
Comparative data of DTH among the seven groups are shown in Table 1. The results showed that the doses of 0.8, 2.4 and 4 mg/kg/day of carnosol significantly contributed to smaller DTH response after 24, 48 and 72 hours, after the footpad challenge, as compared to negative control (P < 0.001). Carnosol, at doses of 0.04 mg/kg, did not show any significant changes in DTH response after 24 and 48 hours, in comparison to negative control (P > 0.05), whereas this dose of carnosol was able to suppress hypersensitivity, 72 hours after challenge (P < 0.01). The positive control group showed significant suppression in DTH response (P < 0.001).
4.2. Serum Antibody Titer: Hemagglutination Titer Assay
Mean ± SE antibody titer are presented in Table 2. Chart illustrates that treatment with carnosol at doses of 0.8, 2.4 and 4 mg/kg/day reduced the concentration of SRBC-specific antibodies, whereas no significant difference in antibody titer was observed in the groups given lower doses of carnosol (0.04 and 0.2 mg/kg/day). The positive control group showed significant suppression in HA titer (P < 0.001).
| Groups | 24 h | 48 h | 72 h |
|---|---|---|---|
| Solvent | 14.9 ± 0.42 | 18.6 ± 1.2 | 16.46 ± 0.47 |
| Cyclophosphamide 20 mg/kg | 11.59 ± 0.48 c | 10.4 ± 0.48 c | 8.88 ± 0.36 c |
| Carnosol 0.04 mg/kg | 14.39 ± 0.6 | 15.65 ± 0.36 | 13.36 ± 0.85 d |
| Carnosol, 0.2 mg/kg | 13.03 ± 0.4 | 14.49 ± 0.62 c | 11.57 ± 0.33 c |
| Carnosol, 0.8 mg/kg | 10.87 ± 0.48 c | 12.32 ± 0.5 c | 9.27 ± 0.36 c |
| Carnosol, 2.4 mg/kg | 9.26 ± 1.18 c | 9.53 ± 0.21 c | 7.18 ± 0.31 c |
| Carnosol, 4 mg/kg | 8.91 ± 0.77 c | 7.20 ± 0.25 c | 5.55 ± 0.39 c |
5. Discussion
Carnosol is a dietary diterpene isolated from rosemary (≈ 5% of the weight of rosemary leaves are carnosol/carnosic acid) that has anti-inflammatory and anti-cancer properties (7, 12, 13). In this study, we focused on effect of carnosol on the acquired immune response. The results revealed that carnosol, at higher doses, could diminish the function of humoral and cellular immunity. It exhibited significant immunosuppression effect in a dose dependent manner. Interestingly, carnosol, at doses of 2.4 and 4 mg/kg/day, was able to inhibit acquired immunity higher than cyclophosphamide, which is a known potent immunosuppressant.
A significant decrease in DTH and HA may suggest a direct effect on the activation and differentiation of the T and B lymphocytes, respectively (14). Acquired immunity includes two arms: the effector B-cell arm and effector T-cell arm, which act together to destroy non-self. The equilibrium between oxidizing and reducing agents within these cells controls their redox state. Transient controlled changes in the redox state, such as elevated production of reactive oxygen species, are critical for signaling and induction of various biological processes. Low levels of reactive oxygen species (ROS) have been shown to be vital for T-cell function (15). One study has reported that small amounts of ROS are pivotal for inducing transcription of nuclear factor kB (NF-kB) and gene expression of cytokines and receptors required for T-cell proliferation, highlighting an important role for cellular redox environment on T-cell function (16). On the other hand, suppression of humoral immunity response to SRBC (a T-dependent antigen) may be a side effect of T cells. Therefore, carnosol, at high doses, may act as a potent antioxidant (a polyphenolic compound) to remove needed ROS for activation and proliferation of T-cells.
Inhibitory effects of carnosol may also be due to the likely interaction of carnosol with other processes involved in the stimulation of acquired immunity, such as antigen presentation or co-stimulatory factor production. Specifically, Lo et al. (2002) reported that treatment of RAW 264.7 cells (mouse macrophages) with carnosol caused reduced lipopolysaccharide-stimulated nitric oxide (NO) production (suppression of NO production and inducible nitric oxide synthase iNOS gene expression was due, in part, to inhibition of NF-κB activation) (8). As a result, carnosol may disrupt the function of macrophage to present antigens to T-cells and, consequently, diminish adaptive immunity. Further studies are needed to investigate mechanistically suppressive effects of carnosol on the immune system.
However, in our work, as a primary/screening study, carnosol was found to be a potent immunosuppressant, as compared to cyclophosphamide. Carnosol, at three high doses, was able to decreased delayed type hypersensitivity and also significantly inhibited the production of antibody against SRBCs. As mentioned in introduction, patients who use immunosuppressants, such as cyclophosphamide, cyclosporine and tacrolimus, suffer from serious side effects, whereas carnosol, with regards to its antioxidant properties, might have less adverse effect, as compared to above drugs. Therefore, we can say that it could be considered as a source of drug, having effective immunosuppression properties. Therefore, further studies can be performed on carnosol to develop an immunosuppressive drug, which is effective with no side effect.
Acknowledgements
References
-
1.
Waldmann H. The new immunosuppression. Curr Opin Chem Biol. 2003;7(4):476-80. [PubMed ID: 12941422].
-
2.
Serkova N, Brand A, Christians U, Leibfritz D. Evaluation of the effects of immunosuppressants on neuronal and glial cells in vitro by multinuclear magnetic resonance spectroscopy. Biochim Biophys Acta. 1996;1314(1-2):93-104. [PubMed ID: 8972722].
-
3.
Cruz EA, Da-Silva SA, Muzitano MF, Silva PM, Costa SS, Rossi-Bergmann B. Immunomodulatory pretreatment with Kalanchoe pinnata extract and its quercitrin flavonoid effectively protects mice against fatal anaphylactic shock. Int Immunopharmacol. 2008;8(12):1616-21. [PubMed ID: 18675940]. https://doi.org/10.1016/j.intimp.2008.07.006.
-
4.
de Albuquerque UP, Muniz de Medeiros P, de Almeida AL, Monteiro JM, Machado de Freitas Lins Neto E, Gomes de Melo J, et al. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: a quantitative approach. J Ethnopharmacol. 2007;114(3):325-54. [PubMed ID: 17900836]. https://doi.org/10.1016/j.jep.2007.08.017.
-
5.
Nissen LR, Mansson L, Bertelsen G, Huynh-Ba T, Skibsted LH. Protection of dehydrated chicken meat by natural antioxidants as evaluated by electron spin resonance spectrometry. J Agric Food Chem. 2000;48(11):5548-56. [PubMed ID: 11087517].
-
6.
Sotelo-Felix JI, Martinez-Fong D, Muriel P, Santillan RL, Castillo D, Yahuaca P. Evaluation of the effectiveness of Rosmarinus officinalis (Lamiaceae) in the alleviation of carbon tetrachloride-induced acute hepatotoxicity in the rat. J Ethnopharmacol. 2002;81(2):145-54. [PubMed ID: 12065145].
-
7.
Johnson JJ, Syed DN, Suh Y, Heren CR, Saleem M, Siddiqui IA, et al. Disruption of androgen and estrogen receptor activity in prostate cancer by a novel dietary diterpene carnosol: implications for chemoprevention. Cancer Prev Res (Phila). 2010;3(9):1112-23. [PubMed ID: 20736335]. https://doi.org/10.1158/1940-6207.CAPR-10-0168.
-
8.
Lo AH, Liang YC, Lin-Shiau SY, Ho CT, Lin JK. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis. 2002;23(6):983-91. [PubMed ID: 12082020].
-
9.
Lai CS, Lee JH, Ho CT, Liu CB, Wang JM, Wang YJ, et al. Rosmanol potently inhibits lipopolysaccharide-induced iNOS and COX-2 expression through downregulating MAPK, NF-kappaB, STAT3 and C/EBP signaling pathways. J Agric Food Chem. 2009;57(22):10990-8. [PubMed ID: 19856917]. https://doi.org/10.1021/jf9025713.
-
10.
Takaki I, Bersani-Amado LE, Vendruscolo A, Sartoretto SM, Diniz SP, Bersani-Amado CA, et al. Anti-inflammatory and antinociceptive effects of Rosmarinus officinalis L. essential oil in experimental animal models. J Med Food. 2008;11(4):741-6. [PubMed ID: 19053868]. https://doi.org/10.1089/jmf.2007.0524.
-
11.
Benincá JP, Dalmarco JB, Pizzolatti MG, Fröde TS. Analysis of the anti-inflammatory properties of Rosmarinus officinalis L. in mice. Food Chem. 2011;124(2):468-75.
-
12.
Moss M, Cook J, Wesnes K, Duckett P. Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. Int J Neurosci. 2003;113(1):15-38. [PubMed ID: 12690999].
-
13.
Lopez-Jimenez A, Garcia-Caballero M, Medina MA, Quesada AR. Anti-angiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. Eur J Nutr. 2013;52(1):85-95. [PubMed ID: 22173778]. https://doi.org/10.1007/s00394-011-0289-x.
-
14.
Riahi B, Rafatpanah H, Mahmoudi M, Memar B, Brook A, Tabasi N, et al. Immunotoxicity of paraquat after subacute exposure to mice. Food Chem Toxicol. 2010;48(6):1627-31. [PubMed ID: 20347915]. https://doi.org/10.1016/j.fct.2010.03.036.
-
15.
Griffiths HR. ROS as signalling molecules in T cells--evidence for abnormal redox signalling in the autoimmune disease, rheumatoid arthritis. Redox Rep. 2005;10(6):273-80. [PubMed ID: 16438798]. https://doi.org/10.1179/135100005X83680.
-
16.
Los M, Droge W, Stricker K, Baeuerle PA, Schulze-Osthoff K. Hydrogen peroxide as a potent activator of T lymphocyte functions. Eur J Immunol. 1995;25(1):159-65. [PubMed ID: 7843227]. https://doi.org/10.1002/eji.1830250127.