Abstract
Background:
In traditional medicine, asafoetida obtained from the roots of different Ferula assafoetida (F. asafoetida) is used for various therapeutic purposes such as whooping cough, pneumonia and bronchitis in children, as well as asthma.Objectives:
In this study, the effect of asafoetida on muscarinic receptors of tracheal smooth muscle, as one possible mechanism responsible for the relaxant effect seen for the plant, was evaluated.Materials and Methods:
The effects of three cumulative concentrations of aqueous extract of F. asafoetida (2.5, 5 and 10 mg/mL), 10 nM atropine, and saline on muscarinic receptors were tested in tracheal smooth muscle samples by performing cumulative concentration response curve to methacholine (a muscarinic receptor agonist) and assessing the shift in concentration response curves due to different concentrations of the extract and atropine.Results:
The EC50 obtained in the presence of higher concentration of the extract muscle was significantly higher compared to saline (P < 0.01). The maximum responses to methacholine in the presence of higher concentration of the extract (10 mg/mL) was significantly lower than that of saline (P < 0.05). The values of CR-1, obtained in the presence of all concentrations of the extract, were significantly lower compared to atropine in the experimental group (P < 0.01 to P < 0.001).Conclusions:
These results showed an inhibitory effect for the extract of asafetida on muscarinic receptors of tracheal smooth muscle.Keywords
Guinea Pigs Aqueous Gum Extract Relaxant Effects Tracheal Smooth Muscle Ferula assafoetida
1. Background
The inhibitory effect of several herbal medicines was shown on muscarinic receptors of tracheal smooth muscle. Carum copticum (1) and Crocus sativus (2) showed non-parallel rightward shift in methacholine concentration response curves, and maximum effect to methacholine in the presence of the plant was not achieved, which caused non-competitive antagonistic effect of this plant on muscarinic receptors. However, Nigella sativa (3), Zataria multiflora (4) and Achillea millefolium (5) showed competitive antagonistic effects on muscarinic receptors by parallel rightward shift in methacholine concentration curves, and achievement of maximum repose to methacholine in the presence of the extracts of the above mentioned plants.
Asafoetida (Ferula assafoetida) is an oleo-gum-resin obtained from the exudates of the living underground rhizome or taproots of the plant. Asafoetida belongs to the family Apiaceae and is often considered to be the main source of asafoetida, although other Ferula species also contain asafetida. The oleo-gum-resin asafoetida is called “Anghouzeh”, “Khorakoma” and “Anguzakoma” in Iran (6). Asafoetida has been used as a spice and a traditional medicine for centuries. It is used as a flavoring spice in a variety of foods in some countries, particularly in India and Nepal. In addition, it is believed that asafoetida has aphrodisiac, diuretic and sedative properties (7). It is also used in folk medicine for the treatment of different diseases, such as asthma, epilepsy, stomachache, flatulence, intestinal parasites, and influenza (8-10). Pharmacological studies have also shown the antioxidant (11), antiviral (11), antifungal (12), cancer chemopreventive (13), anti-diabetic (14), antispasmodic (15) and hypotensive (15) activities of this oleo-gum-resin.
One of the most important traditional uses of asafoetida is for the treatment of asthma (respiratory diseases) (7). In Indian folk medicine, asafoetida is traditionally used as a useful symptomatic treatment for angina pectoris and asthma (16). In Ayurveda, asafoetida is used for the treatment of whooping cough, pneumonia and bronchitis in children (17). The dried gum is taken for whooping cough in Afghanistan and Fiji (18). It was used as a culinary spice and as a replacement for silphion cyrenaicum in ancient Rome. Dried asafoetida is also medicinally used for whooping cough, asthma, bronchitis and tuberculosis (19).
Different parts of the plant include; gum fraction (25%, including glucose, galactose, l-arabinose, rhamnose and glucuronic acid), resin (40 - 64%, which contains ferulic acid esters), free ferulic acid (1.3%), coumarin derivatives (e.g. umbelliferone), volatile oils (3 - 17%) including sulfur-containing compounds, and various monoterpenes (20). Terpenyl oxy coumarins are synthesized by some Ferula species, including F. asafoetida. With regards to the effect of asafetida on asthma, it could be effective in asthma treatment as preventive or relieving mechanisms or both. In fact the relaxant effect of F. asafoetida was shown previously on tracheal smooth muscle, which may indicate a bronchodilatory effect (21).
Potential relaxant effects of asafoetida and F. asafoetida seed’s essential oil (FAFSEO) on isolated ileum was also demonstrated (10, 22), which could be due to the inhibitory effect of the plant on muscarinic receptors.
2. Objectives
The present study aimed to investigate the effects of the asafoetida on muscarinic receptors of tracheal smooth muscle, as one possible mechanism of its relaxant effect.
3. Materials and Methods
3.1. Plant and Extraction
Ferula asafoetida was purchase from the local market of Mashhad. A voucher specimen number was kept in record (Herbarium number; 293-0606-2) at the Department of Pharmacognosy, Faculty of Pharmacy, Mashhad University of Medical Sciences. The aqueous extract of the oleo-gum-resin was prepared as follows: the powdered dried gum (10 g) was soaked in distilled water (50 mL) and boiled for five minutes, allowing the decoction to stand for 30 minutes, followed by filtering through paper filter. The concentration of asafoetida in the stock solution was 250 mg/mL. Concentrations of the aqueous extract are expressed as total amount of dried gum used in preparing the extract.
3.2. Animals
Guinea pigs of both sexes (600 - 800 g) were purchased from Razi Institute, Mashhad, Iran, and were used in this study. Animals were kept in a temperature-controlled room with access to standard food and water ad libitum and were maintained at 22 ± 2°C on a 12-hour light/dark cycle during the study period. Experiments were performed in compliance with the guidelines of the Institute of Laboratory Animal Resources, Commission of Life Sciences (23). This study was approved by the Ethical Committee of Mashhad University of Medical Sciences.
3.3. Tissue Preparation
Tracheas were removed after animals were sacrificed by a blow on their necks. Each trachea was cut into 8 - 10 ring shaped pieces. Each piece containing two to three cartilaginous rings, which was cut open opposite the tracheal muscle, and sutured together to form a tracheal chain (24). The tissue was suspended in a 10 mL organ bath (organ bath 61300, Bio Science Palmer-Washington, Sheerness, Kent U.K.) containing Krebs-Henseleit solution (25).
The Krebs solution was maintained at 37°C and gassed with 95% O2 and 5% CO2. The tissue was suspended under isometric tension of 1 g and allowed to equilibrate for at least one hour, while it was washed with Krebs solution every 15 minutes.
Tracheal smooth muscle contractions were measured using an isotonic transducer (MLT0202, AD instruments, Australia), which was connected to a power lab system (power lab 8/30, ML870, AD instruments, Australia).
3.4. Protocols
Tissues were exposed to a test solution including; 10 nM atropine maleate (Sigma Chemical Ltd UK, Catalogue No. C4915) as the positive and saline as the negative control, as well as three concentrations of aqueous-ethanolic extract of F. asafoetida (2.5, 5 and 10 mg/mL) for 10 minutes (n = 6 for each group). Cumulative concentrations of methacholine hydrochloride (Sigma chemical Ltd, UK) were added to an organ bath to obtain a concentration-response curve in each experiment (26). The consecutive concentrations of methacholine were added every two minutes (range 0.1 - 1000 µM), and the percentage of contraction due to each concentration in proportion to the maximum contraction of methacholine was plotted against log concentration of methacholine.
The effective concentration of methacholine causing 50% of maximum response (EC50) in each experiment was measured using the log concentration-response curve of the corresponding experiment (26). The maximum responses to methacholine and slope of the methacholine-response curve of each experiment were also measured. In experiments with parallel shift in methacholine-response curve, the concentration-ratio minus one (CR-1) as an index of the competitive antagonism effect was calculated by the following equation:
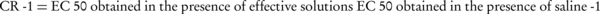
The tissue had one hour of rest between each of the two experiments while being washed every 15 minutes with Krebs solution.
3.5. Statistical Analysis
All data were expressed as mean ± standard error of the mean (SEM) (n = 6 for each group). The data obtained in the presence of the extract and atropine were compared with those of saline. The results of CR-1 values obtained in the presence of the extract were also compared to those of atropine using paired t-test. The data of three concentrations of the extract were compared using One-way Analysis of Variance (ANOVA) with Tukey-Kramer multiple pot test. Significance was accepted at P < 0.05.
4. Results
4.1. Comparison of Methacholine Concentration-Response Curves between Saline, Atropine and the Extract at Different Concentrations
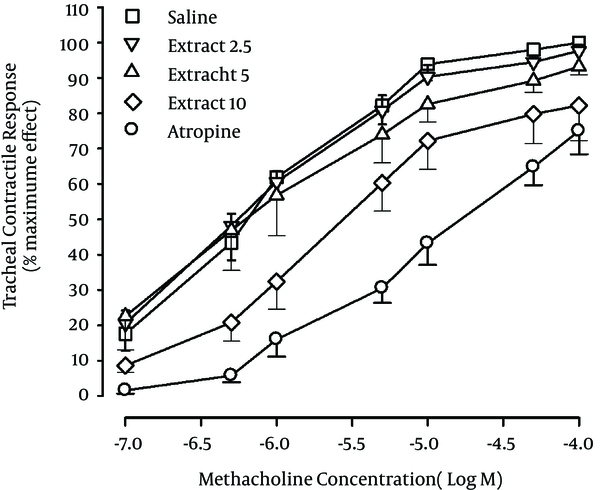
The methacholine concentration response curves obtained in the presence of two higher concentrations of the extract (5 and 10 mg/mL) and in the presence of atropine showed a right ward shift compared to saline (Figure 1).
Cumulative Log Concentration-Response Curves (CCRC) of Methacholine
4.2. Comparison of EC50 Values of Saline, Atropine and Extract
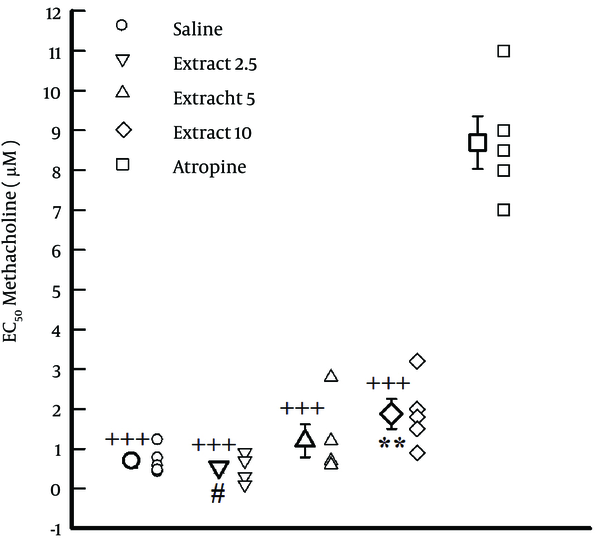
The values of EC50 obtained in the presence of atropine and highest concentration of the extract (10 mg/mL) were significantly higher than that of saline (P < 0.01). In addition, the value of EC50 obtained in the presence of the highest concentration of the extract (10 mg/mL) was significantly higher than that at low concentrations (2.5 mg/mL), (P < 0.01, Figure 2).
The Values of EC50 Obtained in the Presence of Atropine and Concentrations of the Extract
4.3. Comparison of Maximum Response to Methacholine of Saline, Atropine and the Extract
Maximum response to methacholine obtained in the presence of the highest concentration of the extract was significantly lower than that of saline (P < 0.05, Table 1).
Maximum Response to Methacholine Obtained in the Presence of Different Concentrations of the Extract of Ferula asafoetida and 10 nM Atropine Relative to That of Saline (Percentage)
4.4. Comparison of the Slope of Methacholine Concentration Response Curves between Saline, Atropine and the Extract at Different Concentrations
There was no significant difference in the slopes of methacholine concentration response curves between different experimental groups (Table 2).
Slope of Methacholine Response Curves Obtained in the Presence of Different Concentrations of Ferula asafoetida Extract, 10 nM Atropine and Saline
4.5. Comparison of CR-1Values Between Saline, Atropine and Different Concentrations of the Extract
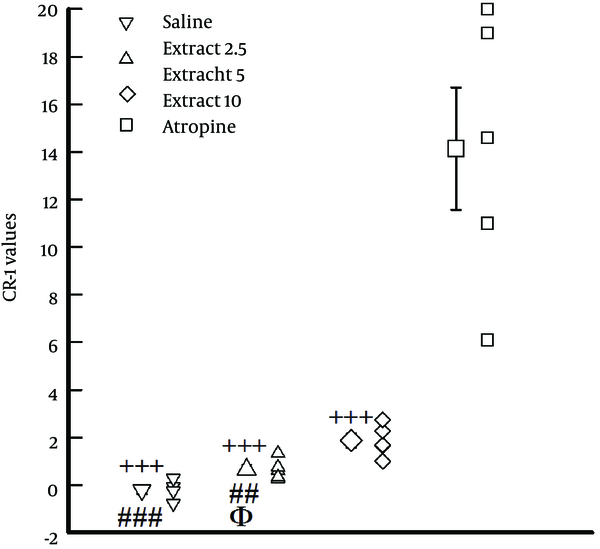
The values of CR-1 obtained in the presence of different concentrations of the extract were significantly lower than that of atropine (P < 0.01 to P < 0.001, Figure 3). In addition, the values of CR-1 obtained in the presence of the lowest concentration of the extract (2.5 mg/mL) and the medium concentration of the extract (5 mg/mL) were significantly lower than that of the highest concentration (10 mg/mL), (P < 0.01 for medium and P < 0.001 for the lowest concentration). The value of CR-1 obtained in the presence of the lowest concentration of the extract was also significantly lower than that of the medium concentration in the experimental group (P < 0.05, Figure 3).
The Values of CR-1 Obtained in the Presence of Different Concentrations of the Extract
4.6. The Correlation Between Different Concentrations of the Extract and EC50 Values
There were significant positive correlations between concentrations of the extract and EC50 values in the experimental group (P < 0.01).
5. Discussion
In previous studies, the relaxant effect of F. asafoetida on tracheal smooth muscle were indicated (21, 27). The possible mechanisms responsible for the relaxant effect on smooth muscle that have been reported for F. asafoetida include an inhibitory effect on muscarinic receptors (28). Therefore, in the present study, the inhibitory effect of the extract of this plant on muscarinic receptors of guinea pig tracheal smooth muscle was examined to elucidate the contribution of muscarinic blocking effect in this muscle relaxant mechanism. The classical and pharmacological method to test the competitive antagonistic effect of an agent such as Ferula assafoetida on a receptor type (e.g. muscarinic receptors) is performing a concentration response curve of that receptor agonis in the presence of the studied agent and a known competitive antagonist (e.g. atropine), and evaluate the right ward shift of the concentration response curve in the presence of the agonist compared to the curve obtained in the presence of a neuter agent (e.g. saline) (21). To the best of our knowledge the classical and pharmacological inhibitory effect as described above has not been studied for Ferula assafoetida. Therefore, in the present study the inhibitory effect of Ferula assafoetida on muscarinic receptor of tracheal smooth muscle was examined.
A small parallel rightward shift occurred in the methacholine log concentration-response curve in the presence of the medium concentration of F. asafoetida, yet a considerable rightward shift in the presence of the highest concentration and a larger shift in the presence of atropine were obtained. These findings may suggest the possible competitive antagonistic effect of the plant at muscarinic receptors of guinea pig trachea smooth muscle (28). The EC50 obtained in the presence of the highest concentration of the plant and atropine were significantly higher than that of saline, The similar values of EC50 obtained in the presence of the highest concentration of the plant and atropine, may confirm the inhibitory effects of higher concentration of the extract at muscarinic receptors. However, the values of CR-1 obtained in the presence of different concentrations of the plant was smaller than atropine, indicating lower competitive antagonism for the plant compared to atropine at the examined concentrations. In addition, maximum contraction effect to methacholine obtained in the presence of high plant concentration was lower than that of saline. These findings suggest a competitive antagonistic effect for the plant at muscarinic receptors of tracheal smooth muscle. However, the absence of maximum responses to methacholine in the presence of high concentration of the extract indicated a functional antagonistic effect for the extract in this study (28).
The effect of F. asafoetida was concentration dependent and there were significant positive correlations between the values of EC50 and plant concentrations. These findings also confirm the concentration dependent effect of the plant and therefore a specific competitive antagonism on muscarinic receptors.
The results of our previous studies also showed a potent relaxant effect for the extract of F. asafoetida on tracheal smooth contracted by methacholine yet a very weak relaxant effect from KCl-induced contraction, which suggests the muscarinic inhibitory effect of the plant and supports the current findings (27). The constituents of F. asafoetida include thymol (10%) (29), which did not show any relaxant effect on tracheal smooth muscle (1), ambelliprenin (less than 1%), which showed significantly lower relaxant effect compared to the plant extract (27) and carvacrol, which showed a potent relaxant effect on tracheal smooth muscle (30). The inhibitory effect of carvacrol on muscarinic receptors of tracheal smooth muscle was also observed previously (31). Therefore, carvacrol could contribute to the observed relaxant and inhibitory effect on muscarinic receptors of F. asafoetida gum extract. However, the effect of ambelliprenin on muscarinic receptors should be examined by further studies.
Acetylcholine and methacholine cause tonic contractions of smooth muscles. The activation of muscarinic receptors of tracheal smooth muscle leads to an increased frequency of action potential and depolarization, which results in muscle contraction (32). The acetylcholine (or methacholine) induces contraction, mediated via M3 subtype of muscarinic receptor. However, a muscarinic receptor competitive agent binds to the receptor and prevents the agonist from binding and causes post-receptor activity leading to response (21). Therefore, the results of the present study suggest that F. asafoetida or its constituents my bind to muscarinic receptor of tracheal smooth muscle and prevent the binding of methacholine to this receptor, which leads to rightward shift of the methacholine concentration curve.
In conclusion, these results suggested the competitive antagonistic effect of F. asafoetida at muscarinic receptors.
References
-
1.
Boskabady MH, Rakhshandah H, Moetamed Shariati V. Bronchodilatory And An Ticholinergic Effects Of Carum Copticum On Isolated Guinea Pig Tracheal Chains. Med J Islam Repub Iran. 1998;11(4):329-34.
-
2.
Neamati N, Boskabady MH. Effect of Crocus sativus (saffron) on muscarinic receptors of guinea pig tracheal chains. Func Plant Sci Biotec. 2010;4:128-31.
-
3.
Boskabady MH, Shahabi M. Bronchodilatory and anticholinergic effects of Nigella sativa on isolated guinea-pig tracheal chains. Iran J Med Sci. 1997;22(3):127-33.
-
4.
Boskabady MH, Jafari Z, Pouraboli I, Babazade B, Rahbardar MG. Anti-cholinergic effect of Zataria multiflora Boiss on guinea pig tracheal chains. Nat Prod Res. 2012;26(16):1523-8. [PubMed ID: 21978254]. https://doi.org/10.1080/14786419.2011.565007.
-
5.
Feizpour A, Boskabady MH, Byrami G, Golamnezhad Z, Shafei MN. The effect of hydro-ethanolic extract of Achillea millefolium on muscarinic receptors of guinea pig tracheal smooth muscle. Indian J Pharmacol. 2013;45(1):13-7. [PubMed ID: 23543621]. https://doi.org/10.4103/0253-7613.106428.
-
6.
Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)-a review. J Ethnopharmacol. 2011;134(1):1-10. [PubMed ID: 21130854]. https://doi.org/10.1016/j.jep.2010.11.067.
-
7.
Zargari A. Medicinal Plants [in Persian]. 6 ed. Tehran: Tehran University Publications; 1997.
-
8.
Takeoka G. Volatile Constituents of Asafoetida, Aroma Active Compounds in Foods. America Chem Soc. 2001;794:33-44. https://doi.org/10.1021/bk-2001-0794.ch004.
-
9.
Lee CL, Chiang LC, Cheng LH, Liaw CC, Abd El-Razek MH, Chang FR, et al. Influenza A (H(1)N(1)) Antiviral and Cytotoxic Agents from Ferula assa-foetida. J Nat Prod. 2009;72(9):1568-72. [PubMed ID: 19691312]. https://doi.org/10.1021/np900158f.
-
10.
Evans WC. Trease and Evans Pharmacognosy. London: Saunders; 2002. 286 p.
-
11.
Nabavi SM, Dehpour AA, Ebrahimzadeh MA, Nabavi SF. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas y Aceites. 2009;60(4):405-12. https://doi.org/10.3989/gya.010109.
-
12.
Angelini P, Pagiotti R, Venanzoni R, Granetti B. Antifungal and allelopathic effects of Asafoetida against Trichoderma harzianum and Pleurotus spp. Allelopathy J. 2009;(23):357-68.
-
13.
Saleem M, Alam A, Sultana S. Asafoetida inhibits early events of carcinogenesis: a chemopreventive study. Life Sci. 2001;68(16):1913-21. [PubMed ID: 11292069].
-
14.
Abu-Zaiton AS. Anti-diabetic activity of Ferula assafoetida extract in normal and alloxan-induced diabetic rats. Pak J Biol Sci. 2010;13(2):97-100. [PubMed ID: 20415145].
-
15.
Fatehi M, Farifteh F, Fatehi-Hassanabad Z. Antispasmodic and hypotensive effects of Ferula asafoetida gum extract. J Ethnopharmacol. 2004;91(2-3):321-4. [PubMed ID: 15120456]. https://doi.org/10.1016/j.jep.2004.01.002.
-
16.
Srinivasan K. Spices as influencers of body metabolism: an overview of three decades of research. Food Res Int. 2005;38(1):77-86. https://doi.org/10.1016/j.foodres.2004.09.001.
-
17.
Kapoor LD. Handbook of Ayurvedic Medicinal Plants. 1 ed. Washington, D.C: CRC Press; 2001.
-
18.
Ross IA. Medicinal plants of the world: chemical constituents, traditional, and modern medicinal uses. Totowa, N.J: Humana Press; 2005.
-
19.
Appendino G, Maxia L, Bascope M, Houghton PJ, Sanchez-Duffhues G, Munoz E, et al. A meroterpenoid NF-kappaB inhibitor and drimane sesquiterpenoids from Asafetida. J Nat Prod. 2006;69(7):1101-4. [PubMed ID: 16872156]. https://doi.org/10.1021/np0600954.
-
20.
Kajimoto T, Yahiro K, Nohara T. Sesquiterpenoid and disulphide derivatives from ferula assa-foetida. Phytochemistry. 1989;28(6):1761-3. https://doi.org/10.1016/s0031-9422(00)97841-5.
-
21.
Gholamnezhad Z, Byrami G, Boskabady MH, Iranshahi M. Possible mechanism (s) of the relaxant effect of asafoetida (Ferula assa-foetida) oleo-gum-resin extract on guinea-pig tracheal smooth muscle. Avicenna J Phytomed. 2011;2(1):10-6.
-
22.
Bagheri S, Hejazian S, Dashti RM. The Relaxant Effect of Seed's Essential Oil and Oleo-Gum-Resin of Ferula Assa-Foetida on Isolated Rat's Ileum. Ann Med Health Sci Res. 2014;4(2):238-41. [PubMed ID: 24761245]. https://doi.org/10.4103/2141-9248.129050.
-
23.
Clark J, Baldwin R, Bayne K. Guide for the care and use of laboratory animals. Washington, DC: Institute of Laboratory Animal Resources, Nat Res Council; 1996.
-
24.
Boskabady MH, Ramazani M, Tabei T. Relaxant effects of different fractions of essential oil from Carum copticum on guinea pig tracheal chains. Phytother Res. 2003;17(10):1145-9. [PubMed ID: 14669246]. https://doi.org/10.1002/ptr.1238.
-
25.
Boskabady MH, Keyhanmanesh R, Khameneh S, Doostdar Y, Khakzad MR. Potential immunomodulation effect of the extract of Nigella sativa on ovalbumin sensitized guinea pigs. J Zhejiang Univ Sci B. 2011;12(3):201-9. [PubMed ID: 21370505]. https://doi.org/10.1631/jzus.B1000163.
-
26.
Jafari Z, Boskabady MH, Pouraboli I, Babazade B. Zataria multiflora Boiss inhibits muscarinic receptors of incubated tracheal smooth muscle with propranolol. Avicenna J Phytomed. 2011;1(1):7-13.
-
27.
Bayrami G, Boskabady MH, Iranshahi M, Gholamnezhad Z. Relaxant effects of asafoetida extract and its constituent umbelliprenin on guinea-pig tracheal smooth muscle. Chin J Integr Med. 2013. [PubMed ID: 24338082]. https://doi.org/10.1007/s11655-013-1550-3.
-
28.
Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959;14(1):48-58. [PubMed ID: 13651579].
-
29.
Bandyopadhyay D, Basak B, Chatterjee A, Lai TK, Banerji A, Banerji J, et al. Saradaferin, a new sesquiterpenoid coumarin from Ferula assafoetida. Nat Prod Res. 2006;20(10):961-5. [PubMed ID: 16854726]. https://doi.org/10.1080/14786410600823431.
-
30.
Boskabady MH, Jandaghi P. Relaxant effects of carvacrol on guinea pig tracheal chains and its possible mechanisms. Pharmazie. 2003;58(9):661-3. [PubMed ID: 14531466].
-
31.
Boskabady MH, Jafari Z, Pouraboli I. The effect of carvacrol on muscarinic receptors of guinea-pig tracheal chains. Phytother Res. 2011;25(4):530-5. [PubMed ID: 20839212]. https://doi.org/10.1002/ptr.3290.
-
32.
Reddy H, Watson N, Ford AP, Eglen RM. Characterization of the interaction between muscarinic M2 receptors and beta-adrenoceptor subtypes in guinea-pig isolated ileum. Br J Pharmacol. 1995;114(1):49-56. [PubMed ID: 7712028].