Abstract
Background:
Acne vulgaris is the most common skin disorder, characterized by the inflammation of the pilosebaceous glands. In addition to physical disfigurement, acne vulgaris may be responsible for significant emotional and physiological distress. Many medications involve in prevention and treatment of acne. Benzoyl peroxide could be a potent candidate with special formulation to treat acne.Objectives:
Benzoyl peroxide foamable emulsion was prepared with the aim of improving the permeation of benzoyl peroxide into skin, reducing skin irritation, and increasing consumer compliance towards treatment of acne by using emu oil as an excellent transdermal carrier.Materials and Methods:
The formulations were prepared by using different selected emulsifiers, foam stabilizers, and emu oil owing to their emollient with skin sebum compatible properties towards acne treatment.Results:
Micronized benzoyl peroxide with an average particle size of 4.56 ± 0.61 μm was prepared by using solvent evaporation method and dispersed in foamable emulsion. The most stable foamable emulsion (containing 2% emu oil, 2.5% cocamidopropyl betaine, 10% coconut fatty acid diethanolamine (Lauramid), 1% glycerin mono stearate (GMS), and water up to 100%, according to organoleptic appearance was selected and evaluated by various pharmaceutical parameters such as pH, stability to centrifugal stress, freeze-thaw, drug content, and foam stability. Permeation studies were performed in vitro to check the drug permeation through cellulose acetate membrane by diffusion cell with ethanol 70% as the receptor medium at sink condition. In vitro drug release study of the formulation exhibited a controlled drug release for a period of 4.5 hours at sink condition.Conclusions:
According to release exponent n > 1, diffusion mechanism is super case П. The prepared micronized benzoyl peroxide in foamable formulation was stable according to different pharmaceutical evaluation. The emu oil incorporated in this formulation could reduce drawback of BPO (Benzoyl peroxide). This formulation could be a good candidate in acne treatment.Keywords
1. Background
Acne vulgaris is the most common skin disorder, characterized by the inflammation of the pilosebaceous glands (1). In addition to physical disfigurement, acne vulgaris may be responsible for significant emotional and physiological distress, and can negatively impact social functioning and overall quality of life (2-4). Although a variety of systemic and topical therapies are available, about 10% to 15% percent of patients or even more fail to respond to treatment, largely because of poor adherence to the prescribed therapy. Reasons for noncompliance are various but include dissatisfaction with the available drug therapy (2, 5).
Pharmacotherapy of acne includes a number of drugs administered orally or topically. Topical administration of anti-acne agents comprises an important part of the therapy. Treatment of mild to moderate cases of acne vulgaris usually begins with topical therapy (6). Benzoyl peroxide is an antibacterial agent, which releases free radical oxygen species capable of oxidizing bacterial proteins and have mild keratolytic effect too (7). It is one of the most widely prescribed drugs in acne therapy. The available acne products containing benzoyl peroxide formulations include creams, gels, lotions, and washes from 2.5% to 10%. Benzoyl peroxide may cause skin dryness and irritation. To reduce irritation and dryness of benzoyl peroxide, an emollient may be used in its formulations (8).
The purpose of the following study was to prepare benzoyl peroxide foamable emulsion without propellant by using suitable amounts of foaming agents and emu oil as an emollient effect for reducing skin irritation and dryness. In addition, emu oil has anti-acne properties without blocking sebum outlet duct. Foam in the field of topical drug delivery is an elegant, aesthetic and cosmetically appealing vehicle, which provides an alternative and promising formulation strategy in the highly competitive dermatological market. It can be easily transferred from the container into the skin site of application (9).
In this study, an air spray foam pump was used to create foam without using any gas propellant. This unique patented technology allows for the precise mixing of the liquid and air, resulting in a dose of high-quality foam with each single stroke. The emu oil can easily penetrate into skin because of its large amount of oleic acid and similarity to human sebum. It is an excellent transdermal carrier, which gets into the skin and enhances the potency of topical medications such as benzoyl peroxide and provides long-lasting effectiveness. It has strong anti-inflammatory effect and reduces redness and inflammation of already occurring acne. Emu oil also helps to repair scar tissues and previous scar damages. It has been shown to accelerate the development of new skin cells by delivering the necessary bionutrients deep into the skin where new cells form and reduce the buildup of scar tissue (10).
2. Objectives
Benzoyl peroxide foamable emulsion was prepared with solvent evaporation method to conduct particle size less than 8 micron to be able to penetrate in to follicular unit, reducing skin irritation and increasing consumer compliance.
3. Materials and Methods
Benzoyl peroxide, cocamidopropyl betaine (CAPB) and coconut fatty acid diethanolamine (Lauramid), glycerol monostearate (GMS), sorbitan monolaurate (Span 20), PEG-20 sorbitan monooleate (Tween 80), sorbitan monooleate (Crill 6), and butylated hydroxytoluene (BHT) were obtained from Merck (Germany). Emu oil was obtained from Abyaneh Cosmetic company, Isfahan Iran. All other ingredients used in this study were of analytical grade.
3.1. Authentication of Benzoyl Peroxide
Benzoyl peroxide was authenticated according to USP and BP pharmacopeia by UV and IR spectrum and determination of the melting point guideline.
3.2. Preparation of Standard Curve
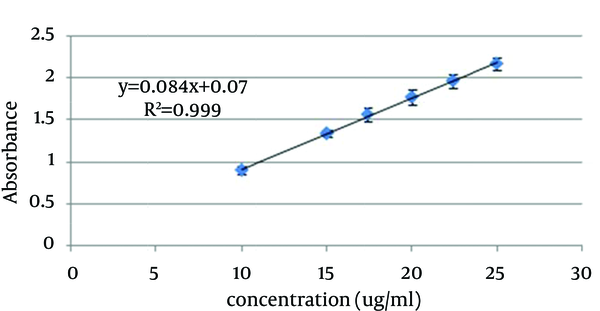
In order to generate the standard curve, 5 mg of benzoyl peroxide was accurately weighted and solubilized in 50 mL ethanol 70%. This solution was diluted to obtain standard solutions in the concentration ranges of 10 - 25 µg/mL. The absorbance of standard solutions were measured by UV/VIS spectrophotometer (Shimadzu, model UV mini-1240CE) at 237 nm and the standard curve was generated (Figure 1).
Benzoyl Peroxide Standard Curve in Ethanol 70%
3.3. Determination of Benzoyl Peroxide Solubility
In this study, we attempted to determine benzoyl peroxide solubility in different solvents such as ethanol, isopropyl myristate, PEG400, glycerin, and propylene glycol. The solubility of benzoyl peroxide in these solvents is slight and even by using the large amount of these solvents in the formulation, we could not prepare suitable foamable Emu oil emulsion. Thus, micronized benzoyl peroxide was prepared and dispersed in the emulsion (11).
3.4. Preparation of Micronized Benzoyl Peroxide by Solvent Evaporation Method
To prepare micronized benzoyl peroxide by the solvent evaporation method, 5 g benzoyl peroxide was dissolved in 50 mL acetone as the solution 1 and 10 g polyvinyl alcohol (PVA) solution (5%) was added to 120 mL water as solution 2. Then, solution 1 was added to solution 2 and was stirred at 1250 rpm for 5 hours. The system was thermally controlled at 25°C in a water bath. Acetone was evaporated and removed from the reaction. The formed micronized benzoyl peroxide was filtered through the filter paper and dried at room temperature (12, 13).
3.5. Preparation of Foamable Emu Oil Emulsion
To develop a stable emulsion, several formulations were prepared using different amounts of emulsifiers. The compositions of the prepared formulations are shown in Table 1. The oil phase of emulsion was prepared by mixing emu oil, GMS, and butylated hydroxytoluene (BHT). The aqueous phase was prepared by mixing betaine, lauramid, and purified water. Both the oil and the aqueous phase were separately heated to 40 - 50°C. Then, the aqueous phase was added to the oily phase with continuous stirring at 1000 rpm. When the emulsion temperature reached the room temperature, the micronized benzoyl peroxide was dispersed in the emulsion and stirring was continued. Based on the primary evaluation of organoleptic and centrifuge test of the prepared emulsions, formulation 13 was selected for final evaluation.
| Ingredient | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzoyl peroxide | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Emu oil | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Tween 80 | 1.5 | 1.8 | 2.1 | 2.4 | 2.7 | 3 | - | - | - | - | - | - | - |
| Crill 6 | 3.5 | 4.2 | 4.9 | 5.6 | 6.3 | 7 | - | - | - | - | - | - | - |
| Span 20 | - | - | - | - | - | - | 1 | 2 | 3 | 4 | 5 | - | - |
| Betaine | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Lauramid | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| GMS | - | - | - | - | - | - | - | - | - | - | - | 0.5 | 1 |
| BHT, % | 0.5 - 1 | 0.5 - 1 | 0.5 - 1 | 0.5 - 1 | 0.5 - 1 | 0.5 - 1 | 0.5 - 1 | 0.5 - 1 | 0.5 - 1 | 0.5 - 1 | 0.5 - 1 | 0.5 - 1 | 0.5 - 1 |
| Purified water up to, g | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
3.6. Evaluation of Selected Formulation
The following physicochemical parameters were used for the evaluation of formulation.
3.6.1. Determination of Particle Size
The particle size of micronized benzoyl peroxide was determined by zeta analyzer (Malvern Instruments Ltd).
3.6.2. Organoleptic Evaluation
The prepared foamable emulsion was inspected visually for color and homogeneity (Table 2).
3.6.3. Centrifuge Test
The prepared formulation was centrifuged at 3000 rpm for 30 minutes (HETTIC D-7200, Germany) 24 hours after preparation and at one-week intervals for 28 days (14) (Table 2).
3.6.4. Determination of pH
pH value of the prepared formulation was measured by a digital pH meter (Metrohm-Switzerland). The determinations were carried out in triplicate, and the average of three readings was recorded (14) (Table 2).
3.6.5. Freeze-Thaw Cycle
Freeze-thaw treatment of the emulsion was performed immediately after preparation. Samples (20 mL) were stored at -20°C for 48 hours. The frozen samples were subsequently thawed at room temperature for 48 hours. This test carried out in triplicate, for each sample (15) (Table 2).
| Parameters | Results |
|---|---|
| Physical appearance | Light white color and a specific odor, completely homogeneous |
| Centrifuge | Excellent |
| Freeze-Thaw | Excellent |
| pH | 5.82 ± 0.18 |
| Drug content | 98.07% ± 0.41 |
3.6.6. Determination of Foam Stability
The cylinder shake method was used to determine foam stability. Fifty milliliter of the formulation was put into a 250 mL graduated cylinder and covered the cylinder with hand and shaken for 10 times. The total volume of the foam content was recorded after 1 minute shaking. Only, the foam volume was calculated. Immediately after the shaking, the foam volume was recorded at 1-minute intervals for 4 minutes (16) (Table 3).
Foam Stability
| Time, min | Foam Volume, mL |
|---|---|
| 1 | 88 ± 1.25 |
| 2 | 88 ± 1.25 |
| 3 | 87 ± 1.25 |
| 4 | 86 ± 1.27 |
| 5 | 84.33 ± 1.41 |
3.6.7. Drug Content
Five sprays of the foamable emulsion, which was equal to 0.5 g was transferred into a screw-capped tube and dispersed in 50 mL ethanol 70%. The tube was kept for 2 hours and shaken well in an orbital water bath (Gallen KAMP-Germany). After suitable dilution, the drug content was measured by UV-spectrophotometer (Shimadzu, model UV mini-1240 CE) at 237 nm against corresponding emulsion formulation as blank (half a gram spray of foamable emulsion without benzoyl peroxide that prepared similar to samples) (Table 2).
3.6.8. In Vitro Drug Release Study
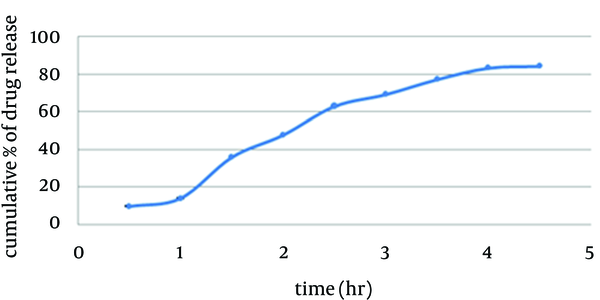
Franz diffusion cells (with 25 mL volume) were used for the drug release studies. We used 25 mL of ethanol 70% as the receptor compartment. One spray of foamable emulsion which was equal to 0.1 g was applied on the surface of cellulose acetate membrane. The membrane was clamped between the donor and the receptor compartment. The donor compartment was kept in contact with a receptor compartment and the temperature was maintained at 37°C. The solution in the receptor compartment was stirred by a magnetic stirrer. At predetermined time intervals, 1 mL of solution from receptor compartment was pipetted out and immediately replaced with 1 mL fresh ethanol 70%. After suitable dilution, the drug concentration in the receptor fluid was determined spectrophotometrically against the appropriate blank (1 spray of foamable emulsion without benzoyl peroxide which was equal to 0.1 g). The experiment was carried out in triplicate. This test was studied under sink condition in the receiver compartment (17) (Figure 2).
In vitro Release of Benzoyl Peroxide Through Cellulose Acetate Membrane
3.6.9. Stability Test
The stability studies were carried out at 8°C (refrigerator), 25°C (room), and 40°C (oven). Drug content and physical appearances (organoleptic characteristic) were evaluated at 1-week intervals for one month (14) (Table 4).
| Condition | Initial | 7 Days | 14 Days | 28 Days |
|---|---|---|---|---|
| Physical appearance | ||||
| 8°C | * | * | * | * |
| 25°C | * | * | * | * |
| 40°C | * | * | * | * |
| Drug content | ||||
| 8°C | 98.07 ± 0.41 | 97.7 ± 1 | 97.6 ± 1.02 | 97 ±1.07 |
| 25°C | 98.07 ± 0.41 | 97.9 ± 1.02 | 97.5 ± 1.16 | 97.56 ± 1.16 |
| 40°C | 98.07 ± 0.41 | 97 ± 1 | 96.88 ± 1.023 | 96.5 ± 1.07 |
3.6.10. Kinetic Analysis of Drug Release
To study drug release kinetics, data obtained from in vitro release study were fitted in Zero model, First model and Higuchi kinetic model equations. In order to evaluate mechanism of drug release, data of drug release were fitted in Korsmeyer-Peppas equation (18).
4. Results
4.1. Authentication of Benzoyl Peroxide
4.1.1. UV Spectrum Benzoyl Peroxide
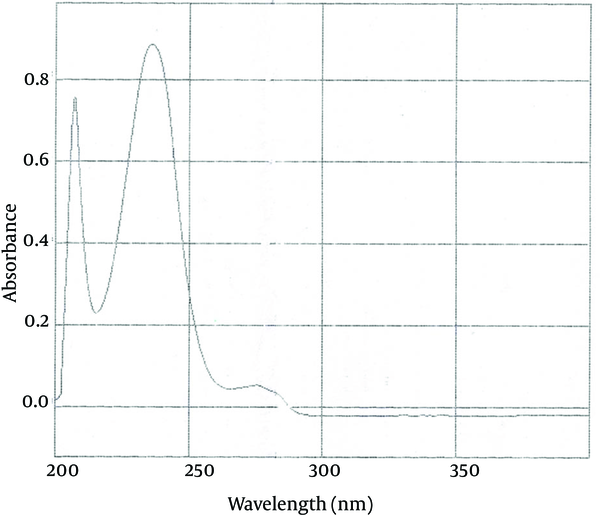
The maximum absorption of benzoyl peroxide with concentration of 8 μg /mL in ethanol 96% in a range of 220 to 250 nm is 235 nm. The UV spectrum obtained from the benzoyl peroxide sample in ethanol 96 % solution was similar to standard benzoyl peroxide (Figure 3).
UV Spectrum of Standard Benzoyl Peroxide
4.1.2. IR Spectrum Benzoyl Peroxide
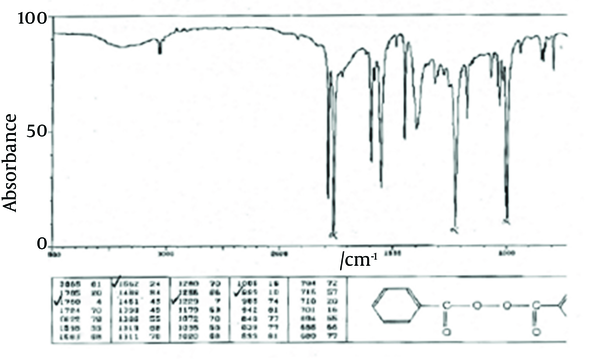
The principle peaks of IR spectrum of benzoyl peroxide are at wave numbers of 1760/cm, 1229/cm, 999/cm, and 1662/cm. All the above characteristic peaks of standard benzoyl peroxide appear in the obtained spectra of benzoyl peroxide sample. Thus, we could conclude that the used benzoyl peroxide was pure (Figures 4 and 5).
IR Spectrum of Standard Benzoyl Peroxide
IR Spectrum Benzoyl Peroxide Sample
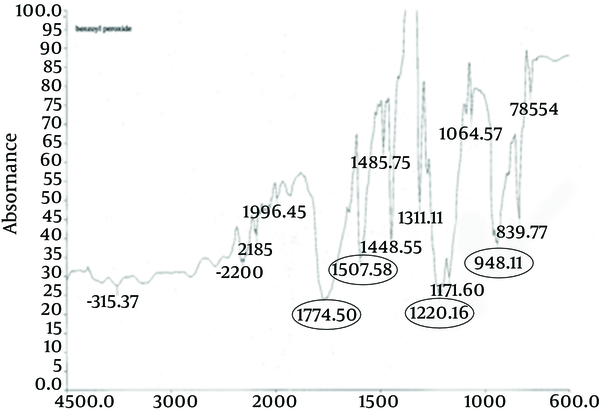
4.1.3. Determination of Melting Point
The range of melting point of standard benzoyl peroxide is 103°C to 106°C. The melting point obtained from benzoyl peroxide sample was 105°C (similar to standard BPO).
4.2. Particle Size of Prepared Micronized Benzoyl Peroxide
In order to reach to particle size less than 10 μm, which is able to penetrate into the follicular structure, all parameters such as solvent volume, stirring rate, and the drug: polymer ratio on the mean particle size were monitored according to preparation of benzoyl peroxide micro sponge by emulsion solvent diffusion method (13). In this study, for preparing micronized benzoyl peroxide with suitable particle size two parameters, i.e. solvent volume and stirring rate were monitored. The results of their change on mean particle size were reported in Table 5.
The Effect of Stirring Rate and Solvent Volume on the Mean Particle Size of Microparticles
| Stirring Rate, rpm | 1000 | 1000 | 1000 | 1250 | 1250 | 1250 |
|---|---|---|---|---|---|---|
| Solvent volume, mL | 30 | 40 | 50 | 30 | 40 | 50 |
| Drug: polymer ratio | 10:1 | 10:1 | 10:1 | 10:1 | 10:1 | 10:1 |
| mean particle size, µm | 8.67 | 7.65 | 5.10 | 7.41 | 5.78 | 4.56 |
Accordingly, the mean particle size of micronized benzoyl peroxide that prepared at stirring rate 1250 rpm, solvent volume 50 mL, and drug: polymer ratio 10:1 was 4.56 ± 0.61 μm. Drug particle size in the final form metered in different time intervals was not significantly different from the micronized particles indicating stability of micro particles.
4.3. Foamable Emulsion Preparation
According to Table 1, formulations F1-F6 containing between 80 and Crill 6 based on RHLB of the system (RHLB of the system is 8) with the ratio of 3:7 with different percents of emulsifiers (5% to 10%), were not stable and immediately phase separation was seen. Therefore, between 80 and Crill 6 were replaced by span 20, but formulations F7-F11 containing different amount of span 20 were not stable too. Therefore, to obtain stable emulsion by increasing viscosity, GMS was added, which resulted in formulations F12-F13. And according to organoleptic appearance, the best formulation was F13. In this study, betaine and lauramid are foaming agents, which also act as amphoteric and nonionic surfactants, respectively. BHT as a preservative was added to the foamable emulsion.
4.4. Quality Control Tests of Selected Formulation
The selected formulation (F13) was light white color and had a specific odor. Observation of the prepared formulation under the microscope revealed homogeneity of globules, internal phases. pH value of 1% (w/w) solution of formulation was in the range of skin and appropriate for application of this emulsion on the skin.
4.5. Evaluation of Foam Stability
Foam generation and retention ability could have more acceptability in consumer’s view; therefore, we design formulation to stabilize for at least 5 minutes to satisfy these criteria (Table 3).
4.5.1. Drug Release Kinetic Analysis
In vitro drug release study of the formulation exhibited a controlled drug release for a period of 4.5 hours at sink condition (Table 6). According to release exponent n > 1, diffusion mechanism is super case П (18).Table 6.
| Kinetic Parameters | R² ± SD | k (h-¹) ± SD |
|---|---|---|
| Zero-order | 0.95 ± 0.01 | 1.01 ± 0.04 |
| First-order | 0.98 ± 0.01 | 0.20 ± 0.01 |
| Higuchi | 0.95 ± 0.01 | 2.03 ± 0.16 |
| Korsmeyer-Peppas | 0.97 ± 0.02 | 1.01 ± 0.07 |
4.5.2. Stability Studies
According to Table 1, Formulation F13 was stable with no sign of change in the physical appearance, organoleptic characteristic, or drug content.
5. Discussion
From above results we can conclude that foamable Benzoyl peroxide formulation with particle size less than 8 µ, included an emu oil as a comedolytic agent and an emollient toward decreasing the skin irritation and dryness causes by drug and increase more compliance of patients could be a good candidate formulation for acne treatment.
Acknowledgements
References
-
1.
Date AA, Naik B, Nagarsenker MS. Novel drug delivery systems: potential in improving topical delivery of antiacne agents. Skin Pharmacol Physiol. 2006;19(1):2-16. [PubMed ID: 16247244]. https://doi.org/10.1159/000089138.
-
2.
Berson DS, Chalker DK, Harper JC, Leyden JJ, Shalita AR, Webster GF. Current concepts in the treatment of acne: report from a clinical roundtable. Cutis. 2003;72(1 Suppl):5-13. [PubMed ID: 12887172].
-
3.
Fried RG, Wechsler A. Psychological problems in the acne patient. Dermatol Ther. 2006;19(4):237-40. [PubMed ID: 17004999]. https://doi.org/10.1111/j.1529-8019.2006.00079.x.
-
4.
Layton AM. Optimal management of acne to prevent scarring and psychological sequelae. Am J Clin Dermatol. 2001;2(3):135-41. [PubMed ID: 11705090].
-
5.
Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient preferences for topical antibiotic treatment for acne. A randomised controlled trial. Br J Dermatol. 2006;154(3):524-32. [PubMed ID: 16445786]. https://doi.org/10.1111/j.1365-2133.2005.07047.x.
-
6.
Berson DS, Shalita AR. The treatment of acne: the role of combination therapies. J Am Acad Dermatol. 1995;32(5 Pt 3):S31-41. [PubMed ID: 7738225].
-
7.
Del Rosso JQ. Benzoyl peroxide cleansers for the treatment of acne vulgaris: status report on available data. Cutis. 2008;82(5):336-42. [PubMed ID: 19090337].
-
8.
Nguyen QH, Kim YA, Schwartz RA. Management of acne vulgaris. Am Fam Physician. 1994;50(1):89-96. 99-100. [PubMed ID: 8017261].
-
9.
Bikowski J. A review of the safety and efficacy of benzoyl peroxide (5.3%) emollient foam in the management of truncal acne vulgaris. J Clin Aesthet Dermatol. 2010;3(11):26-9. [PubMed ID: 21103313].
-
10.
Whitehouse MW, Turner AG, Davis CK, Roberts MS. Emu oil(s): a source of non-toxic transdermal anti-inflammatory agents in aboriginal medicine. Inflammopharmacology. 1998;6(1):1-8. [PubMed ID: 17638122]. https://doi.org/10.1007/s10787-998-0001-9.
-
11.
Chellquist EM, Gorman WG. Benzoyl peroxide solubility and stability in hydric solvents. Pharm Res. 1992;9(10):1341-6. [PubMed ID: 1448436].
-
12.
Arabi H, Hashemi SA, Fooladi M. Microencapsulation of allopurinol by solvent evaporation and controlled release investigation of drugs. J Microencapsul. 1996;13(5):527-35. [PubMed ID: 8864990]. https://doi.org/10.3109/02652049609026038.
-
13.
Jelvehgari M, Siahi-Shadbad MR, Azarmi S, Martin GP, Nokhodchi A. The microsponge delivery system of benzoyl peroxide: preparation, characterization and release studies. Int J Pharm. 2006;308(1-2):124-32. [PubMed ID: 16359833]. https://doi.org/10.1016/j.ijpharm.2005.11.001.
-
14.
Madhav NV, Tangri P. Formulation of escitalopram emulsion using a novel bio-emusifier from unriped fruit pulp of Artocarpus heterophyllus. J Appl Pharm sci. 2011;1(6):194-6.
-
15.
Ghosh S, Rousseau D. Freeze-thaw stability of water-in-oil emulsions. J Colloid Interface Sci. 2009;339(1):91-102. [PubMed ID: 19683718]. https://doi.org/10.1016/j.jcis.2009.07.047.
-
16.
Ken K. Evaluating shampoo foam. Cosmetic and Toiletrics magazine. 2004;119(10).
-
17.
Thakur NK, Bharti P, Mahant S, Rao R. Formulation and characterization of benzoyl peroxide gellified emulsions. Sci Pharm. 2012;80(4):1045-60. [PubMed ID: 23264949]. https://doi.org/10.3797/scipharm.1206-09.
-
18.
Song LH, Du MB, Liu SZ, Ge KY, Wang WP, Cao QC, et al. [Study on in vitro release and percutaneous absorption for Zhitong cataplasm]. Zhongguo Zhong Yao Za Zhi. 2013;38(14):2306-8. [PubMed ID: 24199560].