Abstract
Background:
Plant extracts or compounds derived from plants are a valuable source for finding new anti-leishmaniasis drugs.Objective:
In this study, Capparis spinosa, Ricinus communis, and Solanum luteum were used as lethal agents for the promastigotes of Leishmania major parasites in the culture medium.Methods:
Diluted extracts of 12.5, 100, and 500 mg/mL were prepared from medicinal plant dried extracts. Glucantime at a concentration of 500 mg/mL was used as a positive control.Results:
For all three extracts, concentrations of 100 and 500 mg/mL could kill promastigotes at higher rates and speeds compared to other concentrations. The high concentrations of extracts (500 mg/mL) presented similar effects. According to the findings, hydroalcoholic extract of C. spinosa presented considerably lower antiparasitic effects, and S. luteum and R. communis extracts could kill most of the parasitic promastigotes at higher doses. The ANOVA test did not show any significant viability percentage difference of Leishmania extracts between different extract types.Conclusions:
In this study, the lethal effects of R. communis and S. luteum hydroalcoholic extracts on L. major promastigotes were found to be stronger than the C. spinosa L. extract.Keywords
Capparis spinosa Ricinus communis Solanum luteum Leishmania major Promastigotes In Vitro
1. Background
Sand flies transmit species of Leishmania parasites that cause the cutaneous leishmaniasis disease, which is a global issue originating from Leishmania agents. The World Health Organization (WHO) mentioned cutaneous leishmaniasis as the most important re-emerging tropical disease that needs tailored control measures. Additionally, reports of new leishmaniasis cases by WHO are estimates at 700,000 to 1 million cases annually (1). In 2019, WHO reported that over 87% of new cutaneous leishmaniasis cases occurred in 10 countries: Afghanistan, Algeria, Brazil, Colombia, Iran (the Islamic Republic of), Iraq, Libya, Pakistan, the Syrian Arab Republic, and Tunisia. Its global incidence ranges from 600 000 to 1 million new cases (1). According to the research conducted in 2018, Cutaneous Leishmaniasis (CL) and Visceral Leishmaniasis (VL) were endemic in 92 and 83 countries or territories, respectively (1).
Nowadays, more than 1 billion people live in areas endemic for leishmaniasis, which indicates their higher risk of infection. Scientists estimated that 30, 000 new cases of VL and more than 1 million new cases of CL occur annually in these areas (1).
Using medicinal plants to fight against the disease has a long history, particularly in developing countries, mainly due to their safety and low cost. Given the extensive benefits of drugs derived from medicinal plants, it is of profound significance to discover new drug sources against Leishmania infection (2-4). The long course of treatment, need for frequent and painful injections, high cost, and increased resistance to common drugs, as well as isolation of antimonial resistant strains from patients with CL have all increased the complicacy of treating the disease (5). The possibility of the recurrence of this disease has led to extensive research and evaluation of simple, topical, and appropriate therapeutic methods in the field of herbal products (5).
The arrival of plants, as the most evolutionary diverse creatures in the world, on land has a history of 400 million years. Plants use a wide range of protective mechanisms to defend themselves against insect attacks, such as chemical defense by insect repellents and pesticides. Additionally, they are safer for the environment. Interestingly, plants like Neem (Azadirachta indica, Meliaceae) have been observed to be remarkably effective (6, 7) in commercial products based on them. Several other plants like Capparis spinosa, Bougainvillea glabra (Nyctaginaceae), Solanum jasminoides (Solanaceae), and Ricinus communis (Euphorbiaceae) (8, 9) have been reported as future alternatives to control sand flies.
Feeding on R. communis (Castor), C. spinosa, and S. luteum increases sand flies' mortality and causes stomach deformities for the parasite (10). In 1987, Schlein and Yuval studied leishmaniasis, and the number of P. papatasi (Diptera: Psychodidae) attracted to plants in the Jordan Valley (11). Using plant-baited traps, they examined 12 plant species attractive for P. papatasi. They observed that of 12 tested species, eight could significantly attract more sand flies than the unbaited control, with C. spinosa var. aravensis, S. nigrum, Prosopis farcta, and Atriplex halimus having the highest catches. Their experiments revealed that 86.4%, 75.6%, 71%, 52.1%, and 43% of P. papatasi fed on Prosopis farcta, C. spinosa, R. communis, S. nigrum, and Kochia indica, respectively. Sand flies were also attracted to such plants in the field (11). In sand flies, the developmental cycle of Leishmania parasites only occurs in the gut, sand flies ate various plants and the parasites were exposed to the ingested plant tissues in the midgut of the sand flies. Studies on artificial parasite-infected sand flies showed that Leishmania major parasites agglutinate and die after sand flies fed on some plants found in their natural habitat. Hence, it can be argued that sand fly plant-feeding may have toxic effects on parasites in the form of agglutinated and dead parasites that are found in 20% of infected P. papatasi caught in the field (12, 13). Schlein et al. evaluated sand flies feed on noxious plants as a potential method of controlling leishmaniasis (8). They found that feeding on the branches of R. communis, S. jasminoides, or B. glabra overnight barely shortened the longevity of sand flies (8). Their results showed that B. glabra’s flower attracted sand flies in the field. Interestingly, in some L. major endemic regions with abounding sand flies, the number of P. papatasi was eight times less near B. glabra hedges (62 versus 502 flies trapped) than in the control area (8). Therefore, it was postulated in this study that the above-mentioned plants can be utilized as a natural method to lower the transmission rate of leishmaniasis. Accordingly, ornamental plants endemic for leishmaniasis, capable of growing in arid areas, were studied for their anti-Leishmania effects with initial screening for plants that sand flies readily feed upon. Therefore, we decided to further investigate the effects of certain plants on Leishmania promastigotes in laboratory conditions.
Capparis spinosa is a medicinal plant with a background of usage due to its many biologically active chemical groups, including alkaloids, glycosides, tannins, phenolic, flavonoids, triterpenoids steroids, carbohydrates, saponins, and a wide range of minerals and trace elements. This plant Extract exerts many pharmacological effects, including anti-inflammatory, antimicrobial, cytotoxic, antidiabetic, antioxidant, and many other effects (14). The immature flower buds of C. spinosa contain glucosidrotein, pentosam (4%), rutic acid, pectic acid, volatile substances, and saponin. Plant and root tissues and crushed dry leaves have been used for closing and drying wounds that need attention (14).
Treatment of CL is limited to a few compounds as its golden standard, among them, the pentavalent antimonial compounds, Pentostam and Glucantime, used systematically or locally, are considered the first drugs of choice (14). Therefore, the effects of the hydroalcoholic extracts of C. spinosa, R. communis, and S. luteum on Leishmania promastigotes were investigated in this study.
2. Objectives
The present study aimed to investigate the effects of C. spinosa, R. communis, and S. luteum as lethal agents for the promastigotes of the L. major parasite in vitro.
3. Methods
3.1. Collecting and Preparing Plant Samples
The current study is conducted following an experimental design. Castor was collected from Koohpayeh and Alghadir suburbs of Kerman city in 2020 (Figure 1). Solanum luteum was collected from Bagherabad village in Kerman (Figure 2). Capparis spinosa was collected from Koohpayeh (Figure 3). Plant extracts were prepared from the roots, leaves, flowers, and fruits of C. spinosa, R. communis, and S. luteum. All these organs were first separated from thorns and debris and then crushed with a hand mortar and passed through a number 10 sieve.
Fresh Capparis spinosa from the Koohpayeh of Kerman city, in spring and summer of 2019 - 2020

Fresh Castor, Ricinus communis, from Koohpayeh and Alghadir suburbs of Kerman city, in spring and summer of 2019 - 2020

Fresh Solanum nigrum from the BagherAbad village in the suburbs of Kerman city, in spring and summer of 2019 - 2020

3.2. Plant Sampling Processing
The roots, stems, leaves, flowers, and fruits of the plants were dried and powdered separately at room temperature. Each of the dried plant organs was then stored separately in glass containers.
3.3. Preparation of Hydroalcoholic Extracts from the Desired Plants
The obtained powder was mixed in a 1:1 ratio with 70% ethanol and extracted by agitation on a shaker incubator for a week. The obtained extracts were then dried in an oven at 40°C until the alcohol was removed.
3.4. Preparation of Different Hydroalcoholic Extract Dilutions from Plants
Hydroalcoholic extracts were prepared from dried plant extracts with dilutions of 12.5, 100, and 500 mg/mL. The solvent used was 70% ethanol. The solvent was mixed with the extract and sterilized by filtration. The concentrations of 12.5, 100, and 500 mg of all extracts were made in 1 mL of culture medium.
3.5. Parasite Culture
To maintain the parasites in vitro, the standard parasitic L. major strains (MRHO/IR/75/ER) were cultured in the NNN medium. Then, the parasites were transferred from the NNN medium to the RPMI medium. When the number of parasites reached the static stage, the contents of the glass were transferred to special tubes, followed by centrifugation. Then, parasites were counted by a Neobar slide. After the mass culture of the parasite, all samples were collected in the stationary stage. In most RPMI 1640 media, L. major was first cultured in RPMI 1640 medium with 10 - 20% FBS to reach sufficient quantities of parasites. The anti-Leishmania effects of the extracts were investigated in the static phase of the growth curve of promastigotes.
3.6. Investigation of Leishmania major Promastigote Cytotoxic Effects of Plant Hydroalcoholic Extracts
The Promastigotes entered the stationary phase after 72 hours in RPMI 1640 medium. Afterward, the culture medium parasites were counted using a cell-counter device and considered as the initial parasite inoculum. To evaluate the anti-Leishmania effects of C. spinosa, R. communis, and S. luteum extracts in RPMI 1640 culture medium, dried plant extracts with dilutions of 500, 100, 12.5 mg/ mL. Finally, 10 μL of the prepared dilution series was added to all Eppendorfs. The solvent used in the preparation of extracts was 70% ethanol. The solvent was mixed with the extract in small quantities. The anti-Leishmania effect of each dilution was evaluated in three 1.5 mL Eppendorf tubes. The parasitic suspension (80 μL) was added to each Eppendorf tube, and for the negative control, 80 μL of the parasitic suspension and 20 μL of solvent (diluted ethanol) were mixed. Glucantime with dilutions of 12.5, 100, and 500 mg/mL was used as the positive control. After the initial counting of the parasites, the plate was placed in an incubator at 27°C, and the number of parasites per 10 μL was counted for three days by a Neobar slide. Finally, the average number of promastigotes in all three series of plates was estimated, and the percentage of live parasites at each time for each concentration was calculated.
3.7. Cytotoxicity Test
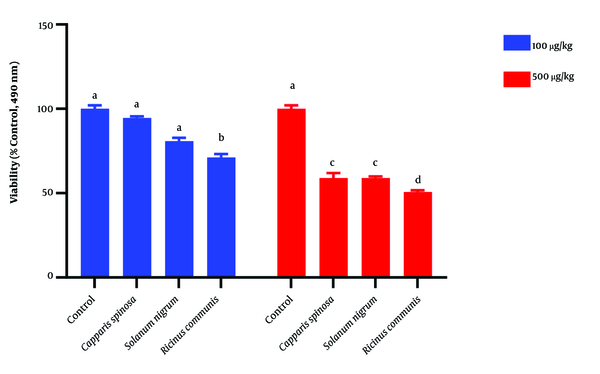
Mosmann has been described using in vitro cytotoxicity tests, following a modified rapid colorimetric assay (15). Promastigotes were cultured and maintained in the RPMI 1640 medium supplemented with 10 % FBS. Then, promastigotes were cultured at 27°C in an incubator for 72 h in a 50 mL vial started with 100 µl of parasite suspension (1 × 106 Promstigote/mL). They were then put into two rows of wells (A-H) in a 96-well microtiter Nunc-Immuno™ (MaxiSorp™ Surface) plate, the medium was aspirated, and 150 µL of the highest concentration of the C. spinosa, R. communis, and S. luteum extracts was added into the same row in a serial dilution. Promastigotes with no extract and only medium were used as controls. Next, each well with promastigote received 10 µL of the M.T.T reagent (3-4, 5-dimethgylthiaol-2-yl-2, 5-diphenyltetrazolium bromide) and plates were incubated for 4 h. The medium and M.T.T were then aspirated. Dimethyl Sulfoxide (DMSO) (100 µL) was added to the plates and agitated for five min. A microtiter plate reader was used for measuring the absorbance of each well at 490 nm (16).
3.8. Statistical Analysis
For all traits, analysis of variance was performed using SPSS version 22. Data were analyzed using repeated measures test and ANOVA test. Average comparison of the viability percentage of Promastigotes at different concentrations of plant extract and different time points was investigated using Post Hoc tests and the Tukey test. Statistical significance was considered when P ≤ 0.05.
4. Results
4.1. Growth Responses of Promastigotes to Plant Extracts of Different Concentrations and Time Points
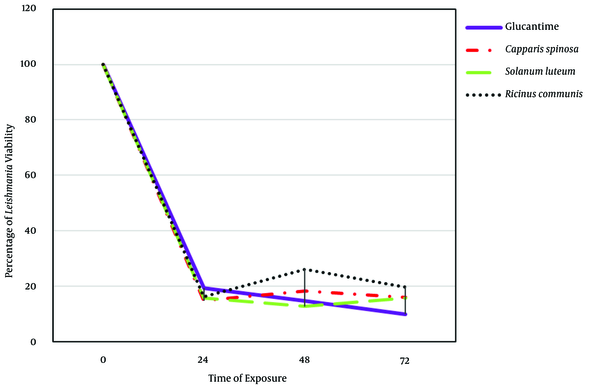
This study demonstrated in vitro inhibitory effects of plant extracts and the Glucantime against L. major promastigotes. One-way ANOVA showed that the time and concentration of plant extracts significantly affect the viability percentage of Leishmania (P < 0.05) (Table 1). Also, the ANOVA test did not show any significant difference between extracts and Glucantime on viability percentage of Leishmania extract (Table 1). The repeated Measure model showed interactional effects between different time points and extracts. This model also showed a significant difference between different concentrations and extract types (P < 0.05) (Table 2). This test also showed a significant difference between extracts in different time points and concentrations (P < 0.05) (Table 2). Tukey means comparison test was performed to determine the viability percentage of Promastigotes in different concentrations of plant extract. The Tukey test showed that 500 mg/mL concentrations were more effective than other concentrations in killing promastigotes (P < 0.05). Repeated Measure test showed significant differences between 500 mg/mL with zero and 12.5 mg/mL (P < 0.05). This test also showed no significant difference between 500 and 100 mg/mL (P > 0.05) (Figure 4). The Post Hoc test showed no significant difference between exposure times to plant extracts (24, 48, 72 hours) (P > 0.05). The plant extracts yielded the best results at 100 and 500 mg/mL concentrations during in vitro experiments. As shown in Figures 4 and 5, the plant extracts inhibited the growth of L. major promastigotes in a range of about 12.5 to about 500 mg/mL. In the positive control group (Glucantime), Glucantime (500 mg/mL) could kill approximately all Promstigote 24 h post-exposure. Promastigotes number decrease was not observed in the negative control group. The positive effects of 100 and 500 mg/mL concentrations of the R. communis and S. luteum on promastigotes of L. major are depicted in Figure 4. The results of the cytotoxicity assay also showed that 500 mg/mL is more effective than 100 mg/mL concentrations in killing promastigotes than the three other extracts (Figure 4).
Evaluation of Plant Extracts Effects on Viability Percentage of Leishmania Promastigotes in In Vitro and in Different Time Points and Concentrations, Kerman, 2020
| Variables | N | Mean ± SD | F | df | P-Value |
|---|---|---|---|---|---|
| Time | 404.34 | 3 | < 0.001 | ||
| 0 | 48 | 100.00 ± 00.00 | |||
| 24 | 48 | 16.54 ± 18.49 | |||
| 48 | 48 | 17.98 ± 17.29 | |||
| 72 | 48 | 15.33 ± 13.62 | |||
| Extract of plant | 0.145 | 3 | 0.933 | ||
| Capparis spinosa | 48 | 37.25 ± 38.09 | |||
| Solanum nigrum | 48 | 39.65 ± 5.72 | |||
| Ricinus communis | 48 | 40.57 ± 39.90 | |||
| Glucantime | 48 | 35.96 ± 40.06 | |||
| Concentration | 4.71 | 3 | 0.003 | ||
| 0 | 48 | 25.00 ± 43.76 | |||
| 12.5 | 48 | 32.72 ± 39.80 | |||
| 100 | 48 | 39.14 ± 36.51 | |||
| 500 | 48 | 52.98 ± 29.87 |
Evaluation of Liner Interaction Between Time, Concentration, and Plant Extract on Viability Percentage of Leishmania Promastigotes In Vitro, Kerman, 2020 (Tests of Within-Subjects Effects)
| Source (Greenhouse-Geisser) | Type III Sum of Squares | df | Mean Square | F | P-Value |
|---|---|---|---|---|---|
| Time | 250476.28 | 2.39 | 104723.01 | 2971.46 | < 0.001 |
| Time * extract | 1336.73 | 7.18 | 186.29 | 5.29 | < 0.001 |
| Time * concentration | 8654.64 | 7.18 | 1206.16 | 34.22 | < 0.001 |
| Time * extract * concentration | 2280.422 | 21.53 | 105.94 | 3.01 | < 0.001 |
| Error (Time) | 2697.41 | 76.54 | 35.24 |
Promastigote viability subjected to various extract tests (cytotoxicity test), Kerman, 2020
Leishmania major promastigote viability (MRHO/IR/75/ER) in different exposure times to ethanolic extracts of leaves of Ricinus communis, Capparis spinosa, and Solanum luteum. Each point represents the means of three independent tests, 2020.
5. Discussion
The findings of the present study agree with previous studies (17) and corroborate the use of Promastigote and amastigote-phagocyte assays for evaluating the anti-leishmanial potential efficiency of plant extracts. Bahmani et al. investigated Iranian traditional medicine plants for leishmaniasis. Several native plants have been used for the treatment of CL, and some recently performed clinical trials have proven the efficacy of some of them. Research conducted on these plants showed that garlic, shallots, wormwood, yarrow, walnuts, thyme, henna plant, mimosa, aloe, wood betony, medlar, periwinkle, yeah, savory, black beans, etc. are effective on CL (18). Jacobson and Schlein found that extracts of R. communis (Euphorbiaceae), C. spinosa (Capparaceae), Prosopis farcta (Mimosaceae), and Tamarix nilotica (Tamaricaceae) in in vitro tests resulted in parasite Promastigote agglutination and death (13). Specific carbohydrates and different lectins in the extracts could inhibit the activity of the parasite. S. luteum (Solanaceae) extract could lyse the Leishmania Promastigote in vitro (13).
The glucose test did not reduce cytotoxicity. When infected, sand flies fed an extract of Castor (R. communis) maximized the mortality of parasites happened (13). A carbohydrate in plant extracts inhibited the agglutination caused by the extract in vitro. This study showed that the lectins and toxins found in the vegetation in L. major foci may decrease the transmission of the parasite (13). Rajesh et al. studied chemical and medico biological usage of the Capparidaceae family (19). Capparis spinosa belongs to this family, and they have several active constituents like flavonoids such as Kaempferal and quercetin (19).
Rahnavard and Razavi evaluated the medical effects of C. spinosa (20). This plant has a lot of traditional and medical uses and contains several biologically active chemical groups, including alkaloids, glycosides, tannins, phenolics, flavonoids, triterpenoids steroids, carbohydrates, and saponins, as well as a wide range of minerals and trace elements. The main pharmacological properties of C. spinosa extracts included antimicrobial, antifungal activity, inflammatory disorders, antiparasitic and cytotoxic, antidiabetic, and antioxidant effects. Plant parts used for medicinal purposes include fruits, flower buds, aerial parts, roots, whole plant, and leaves (20).
Azaizeh et al. showed that S. nigrum extract, when used as a foliage originating decoction, can be used as a Wound and sunburn treatment (21). Clementino et al. studied Solanum lycocarpum glycoalkaloids activity against promastigotes of L. infantum (anti-Leishmania activity) in in vitro conditions (22). They isolated alkaloids solasodine, solamargine, and solasonine against promastigotes and intracellular amastigotes of L. infantum (22). Previous Researchers reported the antileishmanial activity of extracts obtained from other Solanum species (23-27). Kheiri Manjili et al. prepared Hydroalcoholic extract of C. spinosa L. from Leaves or twigs. They investigated the effect of 5 to 500 mg/mL plant extracts against L. major (MRHO/IR/75/ ER) promastigotes after 28 to 30 h and calculated IC50 with the formula IC50 = 375 ± 2.96 mg/mL (28).
A study conducted in Iran investigated the effects of Methanolic extracts obtained from the root of C. spinosa in different concentrations (0.1, 0.3, 0.5, 0.7, and 0.9 mg/mL) on L. major (MRHO/IR/75/ ER) in exposure times of 24, 48, and 72 h. This study showed that the anti-protozoal activity of Caparis extracts (900 mg/mL) could kill 97.8% of promastigotes after 72 h (29). In contrast to the previously reported findings, this study (as shown in Figure 5) revealed that after exposure to hydroalcoholic extracts at different time points, the lethal effect of R. communis (Castor) and S. luteum hydroalcoholic extracts on L. major Promstigotes was stronger than that of the C. spinosa extracts. As demonstrated in Figure 4, an increase in the concentration of plant extracts increased the mortality rate of promastigotes. Concentrations of 100 and 500 mg/mL were more effective against promastigotes.
Yakhchali and Ranjbari Ki-Jandabeh investigated the effects of Nerium oleander (Oleander) leaves, R. communis oil (Castor oil plan), Capsicum annuum (Kapsa) seeds, and Amygdalus communis (almond) compounds on CL caused by Leishmania species under laboratory conditions (30). They showed that the number of Leishmania promastigotes was reduced compared to the control group (30). Soosaraei et al. published a review article on medicinal plants with promising antileishmanial activity in Iran (31). He investigated anti-leishmanial activities of Iranian plants, including Artemisia spp, Allium sativum, Achilleamille folium, Peganum harmala, and Thymus vulgaris, R. communis, C. spinosa (31). In addition, this study listed various Iranian plants researched by the title of the list of medicinal herbs and natural products with anti-leishmanial activity (31).
Sozangar et al. studied the effect of Abu Khalsa and yarrow on L. major in vitro (32) and determined the effects of different concentrations of its extracts on Leishmania parasites in comparison with the control environment. They observed that all concentrations of this extract could decrease the number of Leishmania parasites (32). The results of direct counting and M.T.T tests in their study demonstrated that the number of promastigotes in the groups treated with concentrations of 10, 20, 30, and 40% boiled black tea plant was decreased 24, 48, and 72 hours after culture, but the control group had normal growth. The results of the study showed a significant difference between the two trait groups (P < 0.05). Furthermore, the results revealed that boiled black tea was strongly effective in killing L. major Promstigotes in vitro (32).
5.1. Conclusions
In general, this study demonstrated that the hydroalcoholic extracts of R. communis (Castor) and S. luteum are more effective in killing the promastigotes of L. major parasites than C. spinosa. Such extracts can exert lethal effects on the flagellate form of parasites. Hence, future studies can investigate active compounds of various parts of this plant. Additionally, future studies need to be more extensive in evaluating the effect of these alkaloids on the infectious types of the parasite either in vitro or in vivo.
Acknowledgements
References
-
1.
WHO. Leishmaniasis. Geneva: World Health Organization; 2021, [cited 20 May, 2021]. Available from: http://www.who.int/leishmaniasis/en/.
-
2.
Accioly MP, Bevilaqua CM, Rondon FC, de Morais SM, Machado LK, Almeida CA, et al. Leishmanicidal activity in vitro of Musa paradisiaca L. and Spondias mombin L. fractions. Vet Parasitol. 2012;187(1-2):79-84. [PubMed ID: 22521971]. https://doi.org/10.1016/j.vetpar.2011.12.029.
-
3.
Date AA, Joshi MD, Patravale VB. Parasitic diseases: Liposomes and polymeric nanoparticles versus lipid nanoparticles. Adv Drug Deliv Rev. 2007;59(6):505-21. [PubMed ID: 17574295]. https://doi.org/10.1016/j.addr.2007.04.009.
-
4.
Corral MJ, Benito-Pena E, Jimenez-Anton MD, Cuevas L, Moreno-Bondi MC, Alunda JM. Allicin induces calcium and mitochondrial dysregulation causing necrotic death in Leishmania. PLoS Negl Trop Dis. 2016;10(3). e0004525. [PubMed ID: 27023069]. [PubMed Central ID: PMC4811430]. https://doi.org/10.1371/journal.pntd.0004525.
-
5.
Sadeghian G, Ziaei H, Sadeghi M. Electrocardiographic changes in patients with cutaneous leishmaniasis treated with systemic glucantime. Ann Acad Med Singap. 2008;37(11):916-8. [PubMed ID: 19082196].
-
6.
Isman MB. Neem and other botanical insecticides: Barriers to commercialization. Phytoparasitica. 1997;25(4):339-44. https://doi.org/10.1007/bf02981099.
-
7.
Jaglan MS, Khokhar KS, Malik MS, Singh R. Evaluation of neem (Azadirachta indica A. Juss) extracts against American Bollworm, Helicoverpa armigera (Hubner). J Agric Food Chem. 1997;45(8):3262-8. https://doi.org/10.1021/jf9700539.
-
8.
Schlein Y, Jacobson RL, Muller GC. Sand fly feeding on noxious plants: a potential method for the control of leishmaniasis. Am J Trop Med Hyg. 2001;65(4):300-3. [PubMed ID: 11693873]. https://doi.org/10.4269/ajtmh.2001.65.300.
-
9.
Schlein Y, Jacobson RL. Mortality of Leishmania major in Phlebotomus papatasi caused by plant feeding of the sandflies. Am J Trop Med Hyg. 1994;50:20-7. https://doi.org/10.4269/ajtmh.2001.65.300.
-
10.
Dinesh DS, Kumari S, Kumar V, Das P. The potentiality of botanicals and their products as an alternative to chemical insecticides to sandflies (Diptera: Psychodidae): a review. J Vector Borne Dis. 2014;51(1):1-7. [PubMed ID: 24717195].
-
11.
Schlein Y, Yuval B. Leishmaniasis in the Jordan Valley. IV. Attraction of Phlebotomus papatasi (Diptera: Psychodidae) to plants in the field. J Med Entomol. 1987;24(1):87-90. [PubMed ID: 3820245]. https://doi.org/10.1093/jmedent/24.1.87.
-
12.
Schlein Y, Jacobson RL. Mortality of Leishmania major in Phlebotomus papatasi caused by plant feeding of the sand flies. Am J Trop Med Hyg. 1994;50(1):20-7. [PubMed ID: 8304569].
-
13.
Jacobson RL, Schlein Y. Lectins and toxins in the plant diet of Phlebotomus papatasi (Diptera: Psychodidae) can kill Leishmania major promastigotes in the sandfly and in culture. Ann Trop Med Parasitol. 1999;93(4):351-6. [PubMed ID: 10656036]. https://doi.org/10.1080/00034989958348.
-
14.
Anwar F, Muhammad G, Hussain MA, Zengin G, Alkharfy KM, Ashraf M, et al. Capparis spinosa L.: A plant with high potential for development of functional foods and nutraceuticals/pharmaceuticals. Int J Pharmacol. 2016;12(3):201-19. https://doi.org/10.3923/ijp.2016.201.219.
-
15.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55-63. [PubMed ID: 6606682]. https://doi.org/10.1016/0022-1759(83)90303-4.
-
16.
Wang X, Ge J, Wang K, Qian J, Zou Y. Evaluation of MTT assay for measurement of emodin-induced cytotoxicity. Assay Drug Dev Technol. 2006;4(2):203-7. [PubMed ID: 16712424]. https://doi.org/10.1089/adt.2006.4.203.
-
17.
Van den Bogaart E, Schoone GJ, England P, Faber D, Orrling KM, Dujardin JC, et al. Simple colorimetric trypanothione reductase-based assay for high-throughput screening of drugs against Leishmania intracellular amastigotes. Antimicrob Agents Chemother. 2014;58(1):527-35. [PubMed ID: 24189262]. [PubMed Central ID: PMC3910791]. https://doi.org/10.1128/AAC.00751-13.
-
18.
Bahmani M, Saki K, Ezatpour B, Shahsavari S, Eftekhari Z, Jelodari M, et al. Leishmaniosis phytotherapy: Review of plants used in Iranian traditional medicine on leishmaniasis. Asian Pac J Trop Biomed. 2015;5(9):695-701. https://doi.org/10.1016/j.apjtb.2015.05.018.
-
19.
Rajesh P, Selvamani P, Latha S, Saraswathy A, Kannan V. A review on chemical and medicobiological applications of capparidaceae family. Pharmacogn Rev. 2009;3(6):378.
-
20.
Rahnavard R, Razavi N. A review on the medical effects of Capparis spinosa L. Adv Herb Med. 2017;3(1):44-53.
-
21.
Azaizeh H, Saad B, Khalil K, Said O. The state of the art of traditional arab herbal medicine in the eastern region of the mediterranean: a review. Evid Based Complement Alternat Med. 2006;3(2):229-35. [PubMed ID: 16786053]. [PubMed Central ID: PMC1475945]. https://doi.org/10.1093/ecam/nel034.
-
22.
Clementino LDC, Velásquez AMA, Passalacqua TG, de Almeida L, Graminha MA, Martins GZ, et al. In vitro activities of glycoalkaloids from the Solanum lycocarpum against Leishmania infantum. Rev Bras farmacogn. 2018;28(6):673-7. https://doi.org/10.1016/j.bjp.2018.07.008.
-
23.
Abdel-Sattar E, Maes L, Salama MM. In vitro activities of plant extracts from Saudi Arabia against malaria, leishmaniasis, sleeping sickness and Chagas disease. Phytother Res. 2010;24(9):1322-8. [PubMed ID: 20127723]. https://doi.org/10.1002/ptr.3108.
-
24.
Hubert DJ, Celine N, Michel N, Gogulamudi VR, Florence NT, Johnson BN, et al. In vitro leishmanicidal activity of some Cameroonian medicinal plants. Exp Parasitol. 2013;134(3):304-8. [PubMed ID: 23562881]. https://doi.org/10.1016/j.exppara.2013.03.023.
-
25.
Estevez Y, Castillo D, Pisango MT, Arevalo J, Rojas R, Alban J, et al. Evaluation of the leishmanicidal activity of plants used by Peruvian Chayahuita ethnic group. J Ethnopharmacol. 2007;114(2):254-9. [PubMed ID: 17889471]. https://doi.org/10.1016/j.jep.2007.08.007.
-
26.
Filho VC, Meyre-Silva C, Niero R, Bolda Mariano LN, Gomes do Nascimento F, Vicente Farias I, et al. Evaluation of antileishmanial activity of selected brazilian plants and identification of the active principles. Evid Based Complement Alternat Med. 2013;2013:265025. [PubMed ID: 23840252]. [PubMed Central ID: PMC3690643]. https://doi.org/10.1155/2013/265025.
-
27.
Abreu Miranda M, Tiossi RF, da Silva MR, Rodrigues KC, Kuehn CC, Rodrigues Oliveira LG, et al. In vitro leishmanicidal and cytotoxic activities of the glycoalkaloids from Solanum lycocarpum (Solanaceae) fruits. Chem Biodivers. 2013;10(4):642-8. [PubMed ID: 23576350]. https://doi.org/10.1002/cbdv.201200063.
-
28.
Kheiri Manjili H, Jafari H, Ramazani A, Davoudi N. Anti-leishmanial and toxicity activities of some selected Iranian medicinal plants. Parasitol Res. 2012;111(5):2115-21. [PubMed ID: 22875395]. https://doi.org/10.1007/s00436-012-3059-7.
-
29.
Shemshadi B, Ranjbar-Bahadory S, Ahmadi H. Effect of Caparis spinosa root extract on promastigotes and amastigotes of Leishmania major. Arch Adv Biosci. 2015;6(1).
-
30.
Yakhchali M, Ranjbari Ki-Jandabeh M. [Effects of Nerium oleander leaf, Ricinus communis oil, Capsicum spp. seeds, and almond compound on cutaneous leishmaniasis caused by Leishmania species under laboratory condition and its effect on cutaneous lesion progression in mice]. Sci J Kurdistan Univ Medical Sci. 2013;18(3). Persian.
-
31.
Soosaraei M, Fakhar M, Hosseini Teshnizi S, Ziaei Hezarjaribi H, Banimostafavi ES. Medicinal plants with promising antileishmanial activity in Iran: a systematic review and meta-analysis. Ann Med Surg (Lond). 2017;21:63-80. [PubMed ID: 28794869]. [PubMed Central ID: PMC5536386]. https://doi.org/10.1016/j.amsu.2017.07.057.
-
32.
Sozangar N, Jeddi F, Reaghi S, Khorrami S, Arzemani K. Abulkhalsa and Yarrow plant effect on Leishmania major in vitro. J North Khorasan Univ Med Sci. 2012;4(3):329-33. https://doi.org/10.29252/jnkums.4.3.329.