Abstract
Background:
Helicobacter pylori is an important pathogen in the upper digestive tract. It is of great significance to properly understand the risk factors for the transformation of Barrett esophagus into esophageal carcinoma. However, the relationship between H. pylori and gastroesophageal reflux disease (GERD) and Barrett esophagus remains controversial, and the correlation with immune function has been rarely reported.Objectives:
This study investigated the effect of H. pylori infection on Barrett esophagus and its correlation with immune function.Methods:
We recruited 40 patients with Barrett esophagus (Barrett esophagus group) and 40 patients with GERD (GERD group). In addition, 40 healthy controls were selected for the control group. Esophageal function and its correlation with immune function were measured in each group.Results:
The positivity rate of H. pylori (P < 0.05) and sphincter pressure were lower in both Barrett esophagus and GERD groups than in the control group, while the levels of PGI, PGII, PGI/II, and G-17 were higher (P < 0.05). The levels of CD3+, CD4+, and CD4+/CD8+ were lower in the Barrett esophagus group than in the GERD group, but they were negatively correlated (P < 0.05) with H. pylori infection. The level of CD8+ was higher in the Barrett esophagus group, and it was positively correlated (P < 0.05) with H. pylori infection.Conclusions:
Helicobacter pylori infection may protect against Barrett esophagus by reducing gastric acid secretion and increasing lower esophageal sphincter pressure. Besides, it has a certain correlation with immune function.Keywords
Helicobacter pylori Barrett Esophagus Pepsinogens Lower Esophageal Sphincter
1. Background
Studies have shown that the risk of esophageal cancer in patients with Barrett esophagus is 30 - 120 times the risk in ordinary people (1, 2). Therefore, it is of great significance to properly understand the risk factors of the transformation of Barrett esophagus into esophageal carcinoma to reduce the incidence of malignant tumors (3). Helicobacter pylori is an important pathogen in the upper digestive tract. It can increase the incidence of peptic ulcer, atrophic gastritis, and gastric cancer after infection (4). However, the relationship between H. pylori and gastroesophageal reflux disease (GERD) and Barrett esophagus remains controversial.
The research of domestic scholars (5) has revealed that H. pylori infection is a pathogenic factor of Barrett esophagus. However, some researchers have considered that H. pylori infection is not correlated with Barrett esophagus. Furthermore, the incidence of Barrett esophagus and esophageal carcinoma can easily increase after the removal of H. pylori. Therefore, some researchers (6) suggest that H. pylori infection has a protective effect against Barrett esophagus. However, this conclusion has not been confirmed, and H. pylori correlation with immune function has been rarely reported. In the present study, 80 patients who underwent gastroscopy in the Gastroscopy Center of cooperative hospitals from March 2016 to September 2017, and 40 healthy controls who were admitted to a hospital in the same period were selected as study subjects.
2. Objectives
This study aimed to investigate the effect of H. pylori infection on Barrett esophagus and its correlation with immune function.
3. Methods
Patients with Barrett esophagus were recruited in this study. Healthy controls were selected for the control group. Esophageal function and its correlation with immune function were measured in each group.
3.1. Inclusion and Exclusion Criteria
The inclusion criteria included patients who met the diagnostic criteria for Barrett esophagus established by the Chongqing National Barrett Esophagus Conference in 2005 (7), patients who met the diagnostic criteria of gastroesophageal reflux disease described in Internal Medicine (8), all of whom confirmed by gastroscopy, and patients who could follow the doctor’s instructions to complete the relevant examinations and diagnoses. The exclusion criteria included patients suffering from severe heart, liver, and kidney dysfunction and obvious mental disorders, patients who suffered from chronic diseases, such as diabetes, hypertension, and chronic obstructive pulmonary disease, and esophageal adenocarcinoma patients with an expected survival time of fewer than three months.
3.2. Sampling
In the Barrett esophagus group, biopsy samples, along with two pieces of antral gastric mucosae were obtained from four quadrants of the esophageal lesion during gastroscopy. In the GERD group and control group, biopsy samples along with two pieces of antral gastric mucosae were obtained below the dentate line of the lower segment of the esophagus. The obtained samples were fixed in 10% neutral buffer formalin fixation solution. After paraffin embedding, the slices were prepared and stored. The sampling date was the first day when the subjects were admitted to Jingzhou City of Hubei Province in China.
3.3. Rapid Urease Test (RUT)
Fresh biopsy tissues from gastroscopy were placed in the center of the yellow rapid urease detection paper (provided by Zhuhai Kaidi Technology Development Co., Ltd.). Then, the adhesive was combined on the film to make it closely combined with the film, and the color change was observed within 1 ~ 3 minutes. The urease released by H. pylori could decompose the urease in the test paper into ammonia, which made the phenol red indicator turn red. If there was no H. pylori, the test paper would not change the color. The tissue edge of the test paper changing from yellow to fuchsia within one minute indicated strong positivity, within three minutes indicated weak positivity, and non-discoloration indicated negativity.
3.4. Warthin-Starry Silver Staining Method
The prepared paraffin sections were dewaxed with xylene and then treated with 100%, 95%, 90%, 80%, and 70% ethanol for two minutes, in sequence. Next, they were washed with distilled water for three minutes. The samples were stained with 0.06 mol/L silver nitrate solution for 60 min under the condition of constant temperature and dark at 43°C. Then, the samples were placed in the developer solution for development at 55°C. When the tissue was brown in 2 ~ 5 min, the developer solution was immediately removed, and the tissue was washed with distilled water at 55°C. Finally, the slices were dehydrated with ethanol and sealed with neutral resin. Under certain conditions, H. pylori cells adsorb silver ions from the silver nitrate solution, and adsorbed silver ions in H. pylori are reduced to black metal silver to develop color. Therefore, the background color of sections was mostly light yellow while stained H. pylori cells were brown and black, and were judged to be positive. Otherwise, they were negative.
3.5. Measurement of pH and Lower Esophageal Sphincter Pressure
The 24-h pH values of the lower esophagus in the three groups were measured by a 24-h dynamic pH detector (MMS, Netherlands). The patients fasted for more than eight hours, and gastric motor drugs and H2 receptor blockers were discontinued for two days. The lower esophageal sphincter pressure of patients in the three groups was measured by a gastrointestinal manometer (MMS, Netherlands) (9).
3.6. Concentrations of Pepsinogen I/II and Gastrin
We collected 3 mL of venous blood on an empty stomach the next morning after patient admission in the three groups. Then, the collected blood was centrifuged for 15 min at 4,500 rpm. After separating the serum, the sample was stored in a refrigerator at -20°C. The levels of serum pepsinogen I (PGI), pepsinogen II (PGII), PGI/PGII, and gastrin-17 (G-17) in three groups were measured by Enzyme-linked Immunosorbent assay (ELISA). The above-mentioned serum samples were collected, and the levels of CD3+, CD4+, CD8+, and CD4+/CD8+ of patients in the three groups were measured by flow cytometry. The procedures were completed in strict accordance with the operating instructions of the instrument (10).
3.7. Statistical Analysis
We used SPSS 20.0 software program (IBM, Chicago, USA) to conduct the statistical analysis. The correlation between H. pylori infection and the immune function of patients with Barrett esophagus was analyzed by Pearson’s correlation analysis. The W-test was used for the normality test, F-test for the homogeneity test of variances, one-way Analysis of Variance (ANOVA) for comparisons among multiple groups, and the lysergic acid diethylamide (LSD) test for backtesting. Furthermore, the nonparametric tests were used to compare the mean values of multiple samples that were not normally distributed or were normally distributed with an uneven variance. A P < 0.05 was considered statistically significant.
4. Results
4.1. General Characteristics
A total of 40 healthy controls were admitted to a hospital in the same period of patient admission and were selected as the control group. These subjects included 21 males and 19 females, and their ages ranged from 17 to 84 years, with a median of 56.08 ± 5.77 years. The difference in gender and age between the three groups was not statistically significant (P > 0.05). There were 23 males and 17 females in the Barrett esophagus group, and their ages ranged from 14 to 79 years, with a median age of 53.57 ± 5.71 years. The course of the disease was 1 - 7 years, with a median of 4.53 ± 0.98 years. Based on the type of Barrett esophagus, 17 patients were classified as gastric fundic-type, 18 patients as cardia-type, and five patients as special intestinal metaplasia. There were 22 males and 18 females in the GERD group, and their ages ranged from 16 to 82 years, with a median age of 55.17 ± 5.75 years. The course of the disease was 1 - 8 years, with a median of 4.49 ± 0.94 years.
4.2. Comparison of the Positivity Rate of Helicobacter pylori
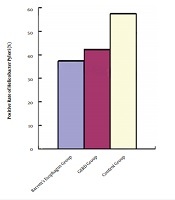
There were 15 [37.5% (15/40)] Barrett esophagus patients who were positive for H. pylori by rapid urease and Warthin-Starry silver staining method. The positivity detection rate was 42.5% (17/40) in the GERD group and 57.5% (23/40) in the normal control group. The difference in the positivity rate of Hp detected by the rapid urease test and Warthin-Starry silver staining method was not statistically significant (P > 0.05) between the Barrett esophagus group and GERD group. The positivity rate of Hp detected by the rapid urease test and Warthin-Starry silver staining method was lower in both Barrett esophagus and GERD groups than in the control group (P < 0.05, Table 1, Figure 1).
| Group | Number | RUT Positivity Rate | Warthin-Starry Silver Staining | χ2 | P |
|---|---|---|---|---|---|
| Barrett esophagus group | > 0.05 | ||||
| H. pylori infection | 32 | 1 (15.63)b | 2 (6.25)c | 0.836 | |
| Non-H. pylori infection | 8 | 1 (12.50) | 1 (12.50) | ||
| GERD group | > 0.05 | ||||
| H. pylori infection | 30 | 2 (6.67) | 2 (6.67) | ||
| Non-H. pylori infection | 10 | 1 (10.00)b | 0 (0.00)c | 0.295 | |
| Control group | > 0.05 | ||||
| H. pylori infection | 4 | 3 (75.00) | 2 (50.00) | ||
| Non-H. pylori infection | 36 | 5 (13.89) | 9 (19.57) | 0.551 |
Comparison of the positivity rate of Helicobacter pylori among the three groups [No. (%)]. GERD group, gastroesophageal reflux disease group.
![Comparison of the positivity rate of Helicobacter pylori among the three groups [No. (%)]. GERD group, gastroesophageal reflux disease group. Comparison of the positivity rate of Helicobacter pylori among the three groups [No. (%)]. GERD group, gastroesophageal reflux disease group.](https://services.brieflands.com/cdn/serve/31516/68dee3c08b8ab594cea15028dcd92d64777d9922/jjm-13-6-98422-i001-preview.png)
4.3. Comparisons of pH, Lower Esophageal Sphincter Pressure, Pepsinogen I/II, and Gastrin Concentration
The difference in pH value was not statistically significant (P > 0.05) when the Barrett esophagus group and GERD group were compared to the control group. Sphincter pressure was lower in the Barrett esophagus group than in the GERD group and control group (P < 0.05), and it was lower in the GERD group than in the control group (P < 0.05). The levels of PGI, PGII, PGI/II, and G-17 were higher in the Barrett esophagus group than in the GERD group and control group (P < 0.05), and they were higher in the GERD group than in the control group (P < 0.05, Table 2).
Comparison of pH Values, Lower Esophageal Sphincter Pressure, Pepsinogen I/II, and Gastrin Concentrations Among Three Groups (
| Group | pH Value < 4 Total Time, % | Sphincter Pressure, mmHg | PGI, g/L | PGII, g/L | PG I/II | G-17, pmol/L |
|---|---|---|---|---|---|---|
| Barrett esophagus group | ||||||
| H. pylori infection | 2.44 ± 0.95 | 11.87 ± 3.46 | 125.21 ± 9.73 | 16.73 ± 4.61 | 13.58 ± 1.18 | 12.21 ± 0.23 |
| Non-H. pylori infection | 2.43 ± 0.94 | 10.55 ± 3.43 | 129.68 ± 9.84 | 18.24 ± 5.71 | 14.11 ± 1.20 | 14.83 ± 0.25 |
| GERD group | ||||||
| H. pylori infection | 2.41 ± 0.91 | 14.39 ± 3.61 | 120.61 ± 8.31 | 14.31 ± 3.41 | 10.88 ± 1.10 | 7.31 ± 2.07 |
| Non-H. pylori infection | 2.42 ± 0.90 | 12.41 ± 2.94 | 123.39 ± 8.56 | 15.29 ± 3.43 | 12.95 ± 1.21 | 9.38 ± 2.15 |
| Control group | ||||||
| H. pylori infection | 2.42 ± 0.93 | 16.12 ± 3.38 | 118.35 ± 7.57 | 13.23 ± 5.63 | 8.94 ± 1.21 | 3.95 ± 0.55 |
| Non-H. pylori infection | 2.42 ± 0.93 | 17.24 ± 3.21 | 112.41 ± 7.32 | 10.84 ± 4.21 | 6.13 ± 0.52 | 2.19 ± 0.41 |
| F | 1.291 | 5.692 | 6.898 | 5.019 | 7.392 | 5.195 |
| P | 0.195 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
4.4. Comparisons of Immune Cell Levels
The levels of CD3+, CD4+, and CD4+/CD8+ were lower in the Barrett esophagus group and GERD group than in the control group (P < 0.05). The level of CD8+ was higher in the Barrett esophagus group and GERD group than in the control group (P < 0.05). The levels of CD3+, CD4+, and CD4+/CD8+ were lower in the Barrett esophagus group than in the GERD group (P < 0.05). The level of CD8+ was higher in the Barrett esophagus group than in the GERD group (P < 0.05, Table 3).
| Group | CD3+, % | CD4+, % | CD8+, % | CD4+/CD8+ |
|---|---|---|---|---|
| Barrett esophagus group | ||||
| Helicobacter pylori infection | 52.12 ± 3.68 | 44.21 ± 4.09 | 34.21 ± 3.21 | 1.29 ± 0.31 |
| Non-H. pylori infection | 48.34 ± 3.41b, c | 41.14 ± 3.29b, c | 36.49 ± 3.25b, c | 1.13 ± 0.23b, c |
| GERD group | ||||
| Helicobacter pylori infection | 51.21 ± 3.50 | 47.39 ± 4.58 | 30.58 ± 2.96 | 1.55 ± 0.30 |
| Non-H. pylori infection | 56.43 ± 3.54b | 45.95 ± 4.53b | 29.35 ± 2.93b | 1.57 ± 0.31b |
| Control group | ||||
| Helicobacter pylori infection | 64.35 ± 4.32 | 49.81 ± 4.55 | 22.12 ± 2.84 | 2.25 ± 0.35 |
| Non-H. pylori infection | 67.21 ± 4.43 | 52.09 ± 4.56 | 21.09 ± 2.81 | 2.47 ± 0.41 |
4.5. Correlation Analysis Between Helicobacter pylori Infection and Immune Function in Patients With Barrett Esophagus
Pearson’s correlation analysis was performed by SPSS. The results indicated that H. pylori infection was negatively correlated (P < 0.05) with CD3+, CD4+, and CD4+/CD8+. However, there was a positive correlation between H. pylori infection and CD8+ (P < 0.05, Table 4).
Correlation Analysis Between Helicobacter pylori Infection and Barrett’s Esophageal Immune Function (r, P)
| Correlation | CD3+, % | CD4+, % | CD8+, % | CD4+/CD8+ |
|---|---|---|---|---|
| r | -0.291 | -0.574 | 0.291 | -0.447 |
| P | 0.043 | 0.032 | 0.039 | 0.041 |
5. Discussion
In the present study, the difference in the positivity rate of H. pylori detected by the rapid urease test and Warthin-Starry silver staining method was not statistically significant (P > 0.05) between the Barrett esophagus group and GERD group. The positivity rate of pH detected by the rapid urease test and Warthin-Starry silver staining method was lower in the Barrett esophagus group and GERD group than in the control group. Thus, H. pylori infection could decrease the incidence of Barrett esophagus and GERD. Furthermore, ideal results can be achieved in detecting the positivity rate of pH detected by the rapid urease test and Warthin-Starry silver staining method.
At present, the mechanism of Barrett esophagus remains unknown in clinical settings (11-13). Its pathogenesis is mainly due to the inappropriate transient relaxation of the lower esophageal sphincter, which leads to excessive contact between acidic gastric contents and esophageal mucosa, resulting in varying degrees of damage to the gastric mucosa and exacerbation of corresponding symptoms (5, 14-16). In the present study, sphincter pressure was lower in the Barrett esophagus group than in the GERD group and control group (P < 0.05). Furthermore, sphincter pressure was lower in the GERD group than in the control group (P < 0.05). The levels of PGI, PGII, PGI/II, and G-17 were higher in the Barrett esophagus group than in the GERD group and control group (P < 0.05). Furthermore, the levels of PGI, PGII, PGI/II, and G-17 were higher in the GERD group than in the control group (P < 0.05). Hence, it can be observed that H. pylori infection has a protective effect against Barrett esophagus. When the body is infected with H. pylori, this would lead to a decrease in gastric acid secretion and an increase in lower esophageal sphincter pressure in the body, thereby playing a protective role.
The study (17) has shown that T cells are related to esophagitis and Barrett’s esophagus. In Barrett’s esophagus, the level of CD4 + T cells and IL-6 secretion produced by IL-4 is increased, while the activation rate of CD4+ T cells in the infiltrating esophageal adenocarcinoma is decreased, indicating that the phenotype and function of T cells play an important role in the progression of esophageal diseases. In the present study, the levels of CD3+, CD4+, and CD4+/CD8+ were lower in the Barrett esophagus group and GERD group than in the control group (P < 0.05). The level of CD8+ was higher in the Barrett esophagus group and GERD group than in the control group (P < 0.05). The levels of CD3+, CD4+, and CD4+/CD8+ were lower in the Barrett esophagus group than in the GERD group (P < 0.05). The level of CD8+ was higher in the Barrett esophagus group than in the GERD group (P < 0.05).
Pearson’s correlation analysis was performed by SPSS. The results indicated that H. pylori infection was negatively correlated (P < 0.05) with CD3+, CD4+, and CD4+/CD8+ but positively correlated (P < 0.05) with CD8+. Thus, it can be concluded that H. pylori infection could protect against Barrett esophagus through preventing the significant immune inflammatory damage in esophageal mucosa (18, 19). Therefore, the T lymphocyte level and H. pylori infection should be intensively measured in patients with Barrett esophagus to understand the changes in patient condition, improve the corresponding measures performed according to the test results, and take timely effective measures for the treatment of Barrett esophagus to avoid missing the best treatment opportunities. There were some shortcomings in the present study. First, the present study was a case-control study rather than a randomized controlled trial. Second, the present study is a single-center clinical study with a small sample size. Finally, research on mechanisms in the present study was insufficient, and thus further studies are needed from the perspective of molecular biology.
5.1. Conclusions
In summary, H. pylori infection may protect against Barrett esophagus by reducing gastric acid secretion and increasing lower esophageal sphincter pressure. Moreover, it has a certain correlation with immune function. Therefore, H. pylori may be beneficial for patients with Barrett esophagus.
References
-
1.
Cerrone SA, Trindade AJ. Advanced imaging in surveillance of Barrett's esophagus: Is the juice worth the squeeze? World J Gastroenterol. 2019;25(25):3108-15. [PubMed ID: 31333304]. [PubMed Central ID: PMC6626724]. https://doi.org/10.3748/wjg.v25.i25.3108.
-
2.
Menon S, Nightingale P, Trudgill N. Chronic obstructive pulmonary disease and the risk of esophagitis, Barrett's esophagus, and esophageal adenocarcinoma: A primary care case-control study. J Clin Gastroenterol. 2019;53(10):e451-5. [PubMed ID: 31008868]. https://doi.org/10.1097/MCG.0000000000001215.
-
3.
Sutton RA, Sharma P. Imaging for Barrett's esophagus: state of the art. Curr Opin Gastroenterol. 2019;35(5):395-400. [PubMed ID: 31306160]. https://doi.org/10.1097/MOG.0000000000000557.
-
4.
Lv J, Guo L, Wang JH, Yan YZ, Zhang J, Wang YY, et al. Biomarker identification and trans-regulatory network analyses in esophageal adenocarcinoma and Barrett's esophagus. World J Gastroenterol. 2019;25(2):233-44. [PubMed ID: 30670912]. [PubMed Central ID: PMC6337015]. https://doi.org/10.3748/wjg.v25.i2.233.
-
5.
Steele D, Baig KKK, Peter S. Evolving screening and surveillance techniques for Barrett's esophagus. World J Gastroenterol. 2019;25(17):2045-57. [PubMed ID: 31114132]. [PubMed Central ID: PMC6506582]. https://doi.org/10.3748/wjg.v25.i17.2045.
-
6.
Zhang C, Ye JQ, Li YA, Ye ZN. Effect of H. pylori infection on the level of movement in patients with Parkinson's disease. Chin J Nosocomiol. 2017;27(5):1149-52. https://doi.org/10.11816/cn.ni.2016-163281.
-
7.
Holmberg D, Ness-Jensen E, Mattsson F, Lagergren J. Adherence to clinical guidelines for Barrett's esophagus. Scand J Gastroenterol. 2019;54(8):945-52. [PubMed ID: 31314608]. https://doi.org/10.1080/00365521.2019.1641740.
-
8.
Solomon SS, Kothari S, Smallfield GB, Inamdar S, Stein P, Rodriguez VA, et al. Liquid nitrogen spray cryotherapy is associated with less postprocedural pain than radiofrequency ablation in Barrett's esophagus: A multicenter prospective study. J Clin Gastroenterol. 2019;53(2):e84-90. [PubMed ID: 29351156]. https://doi.org/10.1097/MCG.0000000000000999.
-
9.
Petrick JL, Li N, Anderson LA, Bernstein L, Corley DA, El Serag HB, et al. Diabetes in relation to Barrett's esophagus and adenocarcinomas of the esophagus: A pooled study from the International Barrett's and Esophageal Adenocarcinoma Consortium. Cancer. 2019;125(23):4210-23. [PubMed ID: 31490550]. [PubMed Central ID: PMC7001889]. https://doi.org/10.1002/cncr.32444.
-
10.
Reed CC, Shaheen NJ. Durability of endoscopic treatment for dysplastic Barrett's esophagus. Curr Treat Options Gastroenterol. 2019;17(2):171-86. [PubMed ID: 31077058]. https://doi.org/10.1007/s11938-019-00226-5.
-
11.
Zheng Q, Zhou Z, Liu S, Lin W, Chen S, Yunbin YE. Association of microsatellite polymorphism of MICA gene with susceptibility to esophageal cancer. Chin J Immunol. 2017;33(5):738-41. 745.
-
12.
Saller J, Al Diffalha S, Neill K, Bhaskar RA, Oliveri C, Boulware D, et al. CDX-2 expression in esophageal biopsies without goblet cell intestinal metaplasia may be predictive of Barrett's esophagus. Dig Dis Sci. 2020;65(7):1992-8. [PubMed ID: 31691172]. https://doi.org/10.1007/s10620-019-05914-x.
-
13.
Li S, Chung DC, Mullen JT. Screening high-risk populations for esophageal and gastric cancer. J Surg Oncol. 2019;120(5):831-46. [PubMed ID: 31373005]. https://doi.org/10.1002/jso.25656.
-
14.
Liu M, Wu Y. Diagnostic value of FICE with magnifying endoscopy for Barrett's esophagus and early esophageal cancer. Pract Cancer J. 2017;32(2):258-60. https://doi.org/10.3969/j.issn.1001-5930.2017.02.025.
-
15.
Samuels TL, Altman KW, Gould JC, Kindel T, Bosler M, MacKinnon A, et al. Esophageal pepsin and proton pump synthesis in barrett's esophagus and esophageal adenocarcinoma. Laryngoscope. 2019;129(12):2687-95. [PubMed ID: 31046139]. https://doi.org/10.1002/lary.28051.
-
16.
Cao HQ, Gu Y, Ma SY. Prevention effect of different doses of simvastatin on esophagus cancer of Barrett's esophagus patients. Chin Fore Med Res. 2015;13(23):16-8. https://doi.org/10.14033/j.cnki.cfmr.2015.23.007.
-
17.
Kavanagh ME, Conroy MJ, Clarke NE, Gilmartin NT, O'Sullivan KE, Feighery R, et al. Impact of the inflammatory microenvironment on T-cell phenotype in the progression from reflux oesophagitis to Barrett oesophagus and oesophageal adenocarcinoma. Cancer Lett. 2016;370(1):117-24. [PubMed ID: 26519754]. https://doi.org/10.1016/j.canlet.2015.10.019.
-
18.
Di Marco V, Calvaruso V, Ferraro D, Bavetta MG, Cabibbo G, Conte E, et al. Effects of eradicating hepatitis c virus infection in patients with cirrhosis differ with stage of portal hypertension. Gastroenterology. 2016;151(1):130-139 e2. [PubMed ID: 27039970]. https://doi.org/10.1053/j.gastro.2016.03.036.
-
19.
Nie XY, Zeng X, Wu QC, Lan HX. Application of modified transparent cap in Barrett's esophagus and nursing. Mod Clin Nur. 2016;15(10):49-51. https://doi.org/10.3969/j.issn.1671-8283.2016.10.013.