Abstract
Background:
Staphylococcus epidermidis is an important opportunistic pathogenic bacterium causing infections on implanted medical devices by biofilm formation. Sodium houttuyfonate (SH), which is the active modified compound of Houttuynia cordata is commonly applied for treating chronic bronchitis, purulent skin infections, and respiratory tract infections in China.Objectives:
We aimed to investigate the in vitro effects of SH in combination with erythromycin on the production of IcaA and expression of icaA to shed the light on the mechanism of SH and erythromycin in inhibiting biofilm formation of S. epidermidis.Methods:
The morphology of the biofilm of S. epidermidis was examined under a scanning electron microscope. Furthermore, inhibitory effects of SH and erythromycin on the production of IcaA and the expression of icaA were detected by Western blotting and quantitative reverse transcription PCR, respectively.Results:
First, the morphological results indicated that combing SH and erythromycin at sub-MIC concentrations can effectively inhibit biofilm formation of S. epidermidis. Furthermore, our presented results showed that combing SH and erythromycin at sub-MIC concentration can significantly downregulate the expression of icaA and repress the protein production of IcaA at irreversible attachment and maturation stages of biofilm formation of S. epidermidis.Conclusions:
Therefore, our findings suggest that the potent antibiofilm activity of SH in combination with erythromycin against S. epidermidis may be partially due to inactivation of icaA.Keywords
Natural Product Sodium Houttuyfonate Biofilm Staphylococcus epidermidis IcaA
1. Background
Staphylococcus epidermidis is an abiding Gram-positive bacterium of the normal human microbiota, usually found on mucosal membranes and skin (1). Staphylococcus epidermidis can establish a lifelong commensal relationship with its host that begins from born by adhering to tissue surface moieties of the mucosal membranes and skin via specific adhesions. Although commensal S. epidermidis strains display high rates of antibiotics resistance, their features as commensal bacteria render this phenomenon largely unconcerned with the normal healthy host (2). However, S. epidermidis has become an important opportunistic pathogen, causing infections on medical implants such as contact lenses, urinary catheters, prosthetic heart valves, central venous catheters, and orthopedic devices in specific persons, which is hard to cure (3-5).
The first step in biofilm formation is the capability of adhering to the surface of the implant, commonly believed to be the most dominant virulence factor of S. epidermidis. Biofilm formation of S. epidermidis facilitates resistance against the host immune system (6-8) and confers resistance to antibiotic (6, 9). Medical and surgical treatment protocols of implant medical device are also complicated by biofilm formation due to remove the biofilm. Biofilms of microorganism are defined as intricate communities of adherent microorganism cells enclosed in a matrix of self-biosynthesized extracellular polymeric substances (10). The adhesion and maturation of the S. epidermidis biofilm occur by several mechanisms.
Polysaccharide intercellular adhesin (PIA), synthesized by icaADBC coding enzymes (11) is responsible for biofilm formation in most S. epidermidis strains and was proved to be the most common macromolecule associated with the adhesion and maturation of S. epidermidis biofilm (12). This was demonstrated by the finding that the ica operon was absent in most non-pathogenic S. epidermidis isolates (13, 14). IcaA, which is an N-acetylglucosaminyltransferase that synthetizes PIA oligomers from UDP-N-acetylglucosamine, exerts a primary role in the PIA synthesis to form biofilm (12). Thus, IcaA is a potential candidate for targeting antibiofilm agents against S. epidermidis.
In China, Houttuynia cordata Thunb (Saururaceae family) is an edible medicinal vegetable, which has been widely applied in traditional Chinese medicine for centuries to cure plenty of inflammatory and infectious diseases (15, 16). Sodium houttuyfonate [SH, CH3(CH2)8COCH2CHOHSO3Na], is a product of addition reaction of sodium bisulfite and houttuynin [decanoyl acetaldehyde, CH3(CH2)8COCH2CHO], which is the most important active constituent of the volatile oil derived from H. cordata (17). Thus, SH is commonly used for treating chronic bronchitis and respiratory tract and purulent skin infections (17). Recently, SH are found to inhibit LPS-induced mastitis in mouse via the NF-κB signaling pathway (18) and alleviate post-infarct remodeling in rats depending on MAP-activated protein kinase pathway (19). However, despite its effective and widespread medical applications especially antimicrobial application, the detailed mechanism of antimicrobial effects of SH remains unknown.
Previously, our group found that SH is promising and effective at repressing pathogenic related biofilm formation and motility of Pseudomonas aeruginosa (20-22), and can inhibit S. epidermidis and Candida albicans (20, 23), and act synergistically with Na2-EDTA and levofloxacin against biofilm formation of P. aeruginosa, S. aureus and C. albicans (23, 24). In addition, we found that SH is a possible natural analog of N-acyl-homoserine lactone, and can effectively inhibit the Las quorum sensing (QS) system and regulated virulence factors of P. aeruginosa (25). However, owing to the high resistance of antimicrobial agents of biofilm, we found that SH alone cannot effectively inhibit the biofilm formation of S. epidermidis. Thus, we have chosen the commonly used macrolide antibiotic, erythromycin to combine with SH to inhibit the biofilm formation of S. epidermidis.
Interestingly, our previous results indicate that SH alone can only mildly inhibit the biofilm formation of S. epidermidis, and SH in combination with erythromycin can significantly inhibit the biofilm formation of S. epidermidis, and up-regulate the transcription of luxS, and down-regulate the expression of agr/RNA III, which are members of regulatory QS system involved in biofilm formation of S. epidermidis (26, 27). However, the mechanism of SH in combination with erythromycin against biofilm development cycle and PIA biosynthesis, especially the adhesion and maturation stages of S. epidermidis remains unclear.
2. Objectives
Therefore, we investigated in vitro effects of SH in combination with erythromycin on the production of IcaA and expression of icaA to shed the light on the mechanism of SH and erythromycin in inhibiting biofilm formation of S. epidermidis in this research. Our results may explore the mechanism of anti-biofilm effect of SH and erythromycin against S. epidermidis.
3. Methods
3.1. Bacterial Strains and Cultures
Staphylococcus epidermidis strain ATCC 35984, which was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP, Beijing, China), was normally cultured in tryptone soy broth (TSB) (Aoboxing Bio-tech, Beijing, China) and grown in a shaker under normal condition (37ºC, 5.4 g) unless otherwise mentioned. Minimum inhibitory concentrations (MIC) of SH and erythromycin against S. epidermidis were determined by CLSI standard method according to our previous research (24, 25).
The concentrations of different drug treatments were set as follows: 64 µg SH mL-1 (1 × MIC), 32 µg SH mL-1 (1/2 × MIC), 16 µg SH mL-1 (1/4 × MIC), 8 µg SH mL-1 (1/8 × MIC), 8 µg erythromycin mL-1 (1 × MIC), 4 µg erythromycin mL-1 (1/2 × MIC), 2 µg erythromycin mL-1 (1/4 × MIC), 1 µg erythromycin mL-1 (1/8 × MIC), 32 µg SH mL-1 (1/2 × MIC) in combination with 4 µg erythromycin mL-1 (1/2 × MIC), 16 µg SH mL-1 (1/4 × MIC) and 2 µg erythromycin mL-1 (1/4 × MIC), 8 µg SH mL-1 (1/8 × MIC) and 1 µg erythromycin mL-1 (1/8 × MIC). Moreover, 1 × MIC of erythromycin was set as the positive control for its promising anti-biofilm formation activity. Erythromycin was dissolved in sterile deionized water as store liquor of 16 mg.mL-1 to dilute working concentrations. Based on the method of Shao (18), preparation for the SH were as follows: one drop of polyoxyethylene sorbitan fatty acid esters and glycerol added to 102.4 mg SH, and then 1 mL deionized sterile water was added to the SH, and the solution was heated to dissolve the SH drugs as store liquor for diluting working concentrations.
3.2. Biofilm Formation
Staphylococcus epidermidis with overnight culturing in liquid TSB was diluted to 0.05 of OD600 with fresh liquid TSB, and then 100 µL of diluted bacterial culture was cultured in six-well polystyrene plates within a coverglass and 2 mL fresh liquid TSB in each well for 3 days for biofilm formation. Then, the mature biofilm on cover glass was removed and washed gently with phosphate buffer saline (PBS), and then used to isolate RNA to perform gene expression analysis.
3.3. Scanning Electron Microscope Analysis of Biofilm Morphology
Basically, preparation of the carrier coverglass with biofilm was also performed as described above, and the planktonic bacteria were rinsed gently with PBS. The prepared biofilm samples of control, (1/4 × MIC) SH, (1/4 × MIC) erythromycin and 1/8 × MIC SH in combination with 1/8
3.4. Gene Expression Analysis
Total RNA was isolated by RNAprep Bacteria Kit (Tiangen, Beijing, China), according to the manufacturer’s protocols. The quality and quantity of extracted RNA were measured by the concentration and OD 260/280 of samples using Nanodrop One (Thermo Fisher, Waltham, USA). A FastQuant RT Kit (Tiangen, Beijing, China) was used to remove genomic DNA, and reverse transcript to cDNA. The resulting cDNA was separated by electrophoresis on 1% agarose gel and imaged. Quantitative RT-PCR (qRT-PCR) was performed using SYBR Premix ExTaq II (Tli Rnase H Plus) (TaKaRa, Kusatsu, Japan) under the conditions as follows: the initial step of 5 min at 95ºC, followed by 40 cycles of 95ºC for 15 s, 60ºC for 15 s, and 72ºC for 30 s.
The qRT-PCR reactions were performed in QuantStudio TM 6 Flex thermal cyclers (Applied Biosystems, Foster City, USA). The real-time PCR was validated by sequencing the PCR products of icaA and gyrB. The calculated cycle threshold (CT) of icaA was normalized to the CT for gyrB amplified from the corresponding sample. GyrB is a housekeeping gene in S. epidermidis, and commonly used as a control in qRT-PCR experiment of bacteria (27). Fold changes of icaA were calculated by relative quantification method, according to the
Oligonucleotide Primers used During qRT-PCR
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| icaA | CAATGAGGGAATCAAACAAGCA | AAAGGCGCATCGTCATCAA |
| gyrB (25) | AGCGGTTCGTAAAAGACCGGGTATG | CCTGCTAATGCCTCGTCAATAC |
3.5. Western Blotting Assay
The western blotting assay of IcaA was performed according to the method of Hnasko and Hnasko (29). First, the customized IcaA polyclonal antibody (HuaAn Biotechnology Co., Ltd, Hangzhou, China) was prepared by standard polyclonal antibody preparation method of synesthetic IcaA immunizing rabbit and validated by western blotting assay. For protein isolation, bacterial planktonic cells treated by different concentration drugs were collected and adjusted to the same OD600 value in PBS. Then, the PBS with bacterial cells was replaced by fresh liquid TSB with different concentrations of erythromycin and SH at 37ºC for 24 h as mentioned before.
Bacterial cells were lysed in Lysis buffer (Sigma, St. Louis, MO) containing protease inhibitors to extract the protein. Same amounts (60 µg) of proteins of each group were loaded in each lane and then subjected to electrophoresis on a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA), and then blocked with non-fat dry milk (5%, w/v) at room temperature for 2 h.
The membrane was incubated at 4ºC overnight with the primary antibody i.e., customized IcaA polyclonal antibody. After washing with PBS with 0.1% Tween, the membranes were individually incubated in the horseradish peroxidase-conjugated secondary goat anti-rabbit antibody (Santa Cruz Biotechnology, CA, USA) for 2 h at room temperature. The detection of IcaA protein was performed by ECL-Plus Western blotting reagents (GE Healthcare, CT, USA) and the image was captured and analyzed using Image Quant Software (Bio-Rad, CA, USA).
3.6. Statistical Analysis
One-way ANOVA followed by Dunnett’s multiple comparisons test of several groups was analyzed by GraphPad Prism version 6.02 (GraphPad Software, La Jolla, USA). All individual experiments were carried out at least in triplicate.
4. Results
4.1. Sodium Houttuyfonate in Combination with Erythromycin Affected the Morphology of Staphylococcus epidermidis Biofilm
Previously, we found that SH can effectively inhibit S. epidermidis (27). To demonstrate that any apparent reduction is genuinely a result of inhibition of biofilm formation and not reduction of bacterial growth of sub-MIC SH and erythromycin, we perform a checkboard test containing alone and in combination treatments of 2 ×, 1 MIC, 1/2 × MIC, 1/4 × MIC, 1/8 × MIC, 1/8 × MIC of SH and 2 ×, 1 MIC, 1/2 × MIC, 1/4 × MIC, 1/8 × MIC, 1/8 × MIC of erythromycin. The results showed that alone and especially in combination of sub-MIC, SH and erythromycin cannot inhibit the growth of S. epidermidis strain (27). Here, we determined the effect of SH in combination with erythromycin against the biofilm formation of S. epidermidis by SEM. According to the SEM images, the morphology of bacterial biofilm was clearly different among drug treatment groups at 3 d of biofilm maturation stage of S. epidermidis.
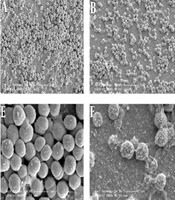
In the control group (Figures 1A and 1E), the bacterial accumulation was dense, and form a thick biofilm structure by multiple layer cells and intracellular matrix. Compared with the control group, the dense biofilm structure of 1/4 × MIC erythromycin group (Figures 1C and 1G) was dramatically damaged, and some of the mucosa properties remained. The biofilm structure of 1/4 × MIC SH group (Figures 1B and 1F) is substantially damaged, and none of the mucosa properties remained. Notably, biofilm structure of the SH in combination with erythromycin group at sub-inhibitory concentration (Figures 1D and 1H) did not exist and bacteria were rare and shrinking with defective cell structure. Therefore, the morphology of the biofilm cells of S. epidermidis was significantly destroyed by SH in combination with erythromycin at sub-inhibitory concentration.
Microscopic morphology changes of Staphylococcus epidermidis biofilm treated by SH in combination with erythromycin. A and E, Negative control group without drug treatment; B and F, 1/4 × MIC SH treatment group; C and G, 1/4 × MIC erythromycin treatment group; D and H, 1/8 × MIC SH in combination with 1/8 × MIC erythromycin group. The A, B, C, D figures were magnified 10,000 times, and the E, F, G, H figures were magnified 50,000 times. The morphology of S. epidermidis biofilm in the different groups was detected at 24 h after drug treatments.

4.2. Sodium Houttuyfonate in Combination with Erythromycin Repressed the Expression of icaA
Here, we determined the effects of SH on the expression of icaA of S. epidermidis that was responsible for biofilm formation at adhesion and mature stages of biofilm development (Figure 2A). The amplification curve of qRT-PCR is presented in Figure 2B, and the melting curves of icaA and igrB are presented in Figures 2C and 2D. At 6 h of the irreversible attachment stage of biofilm formation, the gene expression results (Figure 2A) indicated that the expression of icaA was downregulated by SH in combination with erythromycin.
Compared with the control group, SH at 1 × MIC concentration and erythromycin at 1 × MIC, 1/2 × MIC and 1/4 × MIC concentrations alone could significantly repress the expression of icaA (P < 0.001). Notably, SH in combination with erythromycin groups at all three sub-inhibitory concentrations could significantly repress the expression of icaA (P < 0.001). At 24 h of the maturation stage of biofilm formation, all SH and erythromycin alone and in combination treatments could significantly downregulate the gene expression icaA. According to the results (Figure 2A), downregulation of icaA expression was dose-dependent with SH in combination with erythromycin. Thus, the gene expression icaA of S. epidermidis was significantly repressed by SH alone and in combination with erythromycin.
Different expression levels of the icaA of Staphylococcus epidermidis treated by SH in combination with erythromycin. A, Expression of icaA was monitored in response to SH in combination with erythromycin treatments by qRT-PCR assay. Housekeeping gene, gyrB, was set as the internal reference gene for each sample. The concentrations of different drug treatments were as follows: negative control group (without any drug), 1 × MIC SH ml, 1/2 × MIC SH mL-1, 1/4 × MIC SH, 1/8 × MIC SH, 1 × MIC erythromycin, 1/2 × MIC erythromycin, 1/4 × MIC erythromycin, 1/8 × MIC erythromycin, 1/2 × MIC SH and 1/2 × MIC erythromycin, 1/4 × MIC SH and 1/4 × MIC erythromycin, 1/8 × MIC SH mL-1 and 1/8 × MIC erythromycin. The expression of icaA in the different groups was detected at 6 h and 24 h after drug treatments. *, P < 0.05; **, P < 0.01; ***, P < 0.01; n = 4; B, amplification curve of the real time PCR; C, melting curve of icaA gene; D, melting curve of gyrB gene.

Interestingly, the different effects between 6 h and 24 h drug treatment were intriguing as the action of SH alone, flipping from minimally inhibitory effect (or promotional at low concentration) at 6 h to substantially inhibitory at 24 hours of all concentrations. Thus, SH appeared to provide protection against the inhibitory action of erythromycin in all combination treatments at 6 h. This effect was reversed at 24 h that the icaA expression of erythromycin alone treatment vs. erythromycin (1/2 × MIC) in combination with SH were statistically significant (P < 0.05). Therefore, these findings might suggest that SH counteracted the inhibitory action of erythromycin at the 6 h of the adhesion stage of biofilm formation, but amplified the inhibition at the 24 h of the mature stage of biofilm formation.
4.3. Sodium Houttuyfonate in Combination with Erythromycin Repressed the Production of IcaA
Furthermore, we performed a western blotting assay to determine the production changes of IcaA after combining SH and erythromycin treatments. At 6 h of adhesion stage of biofilm, our presenting results (Figure 3) indicated that the production of IcaA was not significantly reduced by SH and erythromycin alone but significantly reduced SH in combination with erythromycin groups. At 24 h of the mature stage of biofilm formation, the production of IcaA (Figure 3) was significantly reduced by SH and erythromycin alone in a concentration-dependent manner and was substantially repressed by SH in combination with erythromycin in all three sub-inhibitory groups. Therefore, the production of IcaA was significantly inhibited by SH alone and in combination with erythromycin, especially at the mature stage of biofilm formation of S. epidermidis.
Repressive effects of SH in combination with erythromycin on the production of IcaA of Staphylococcus epidermidis. The production of IcaA was monitored in response to SH in combination with erythromycin treatments by Western blotting assay. Distinct drug concentrations of treatments representing different bands were as bellow: negative control group (without any drug), A, 1 × MIC SH mL; B, 1/2 × MIC SH mL-1; C, 1/4 × MIC SH mL-1; D, 1/8 × MIC SH; E, 1×MIC erythromycin; F, 1/2 × MIC erythromycin; G, 1/4 × MIC erythromycin; H, 1/8 × MIC erythromycin; I, 1/2 × MIC SH and 1/2 × MIC erythromycin; J, 1/4 × MIC SH and 1/4 × MIC erythromycin; K, 1/8 × MIC SH and 1/8 × MIC erythromycin; L, the gels of A-H and I-L were grouped together. The production of IcaA in different groups was detected at 6 h and 24 h after drug treatment.

5. Discussion
Staphylococcus epidermidis is an important conditional pathogen involved in device-related infections due to biofilm formation activity (1). The formation of epidermal Staphylococcus biofilm is a dynamic process (30). Firstly, the bacteria surface hydrophobic protein or polysaccharide adhesion to the host organism. The initial attachment of the material forms the bacterial community; then the bacterial cells gather together to build biofilm structure (30). Among them, PIA is a bacterial biofilm material which is necessary for the aggregation stage (30). IcaA is the first gene of the operon and plays a decisive role in the biosynthesis of PIA, and could be a possible target for antibiofilm formation of S. epidermidis. Blocking the expression of the biofilm formation-related gene requires the inhibition of QS mechanism and biosynthesis of polysaccharides and extracellular proteins of biofilm compositions (31).
Currently, macrolide antibiotics, including erythromycin and vancomycin are common anti-staphylococci agents in the clinic by repressing protein biosynthesis of bacterial cell; however, staphylococci easily get resistant to the antibiotics (32), for example, medical devices-related infections by S. epidermidis strains. Therefore, it is important to seek effective anti-biofilm drugs or multi-drug combination application in traditional alternative medicines in order to achieve high drug efficiency against S. epidermidis biofilm and reduce the toxicity of drug treatments. Because the commonly used antibiotic, erythromycin, has lower adverse reaction and price than the third line antibiotics, like vancomycin (32). Thus we used erythromycin as the positive control of antimicrobial agents and selected combination agents in this research.
Recently, SH was found to inhibit the biofilm formation of P. aeruginosa, S. aureus, and C. albicans in vitro (20-25), but the detail of mechanisms remains unclear. Previously, we found that SH in combination with erythromycin groups at sub-inhibitory concentrations could effectively inhibit the biofilm formation of S. epidermidis at adhesion and maturation stages (26, 27). Furthermore, we have explored the effect of SH in combination with erythromycin on QS system, which regulates biofilm system of S. epidermidis. The results (27) indicate that SH in combination with erythromycin can quickly upregulate the expression of luxS at adhesion stage, and significantly reduce the expression of agr and RNAIII at adhesion and maturation stages. These three genes encode the protein and RNA, which are key members of QS system of S. epidermidis. Notably, LuxS can inhibit biofilm formation of S. epidermidis via inactivation of gene expression of icaADBC operon by an icaR-activation pathway (33, 34). Therefore, our previous results suggested that SH may affect QS system to repress the biofilm formation of S. epidermidis.
Here, our results indicated that the morphology of the biofilm cells of S. epidermidis were significantly destroyed by SH alone and in combination with erythromycin at sub-inhibitory concentrations. Furthermore, the qRT-PCR data showed that the gene expression icaA of S. epidermidis was significantly repressed by SH alone and in combination with erythromycin at sub-inhibitory concentrations because SH has been proved to affect QS system in bacteria (25). Therefore, the inhibitory effects of SH against S. epidermidis may be due to the cell density and anti-QS dependent of promotion in the early stage and repression in the maturation stage to cause the intriguing effects of SH against transcript levels of icaA at 6 and 24 hours of exposure. Further, SH interestingly counteracts the inhibitory action of erythromycin at the time point of 6 h but amplifies the inhibition at the 24 h, which may be due to the QS system.
Consistent with the gene expression results, the production of IcaA is also significantly inhibited by SH alone and in combination with erythromycin at sub-inhibitory concentrations, especially at maturation stage of biofilm formation of S. epidermidis. Based on our previous results of repressing of QS system of S. epidermidis by SH in combination with erythromycin (27), the down-regulation of icaA may be due to the repressing of QS system of S. epidermidis by combining SH and erythromycin. Furthermore, reducing the production of IcaA may lead to the decreasing of biofilm matrix PIA, and result in decreasing of biofilm formation. Additional in vivo experiments will be required to assess the future applicability of SH in combination with erythromycin, and effective activity of combining SH and erythromycin against biofilm formation of bacteria may provide a promising approach.
5.1. Conclusions
Our results indicate that the potent antimicrobial activity of natural plant products, SH, in combination with erythromycin may be partially due to its inhibitory effect on biofilm formation in S. epidermidis via inactivation of icaA. Therefore, combining SH and erythromycin may provide a new possible option for the treatment of medical devices-related infections by S. epidermidis biofilm. Furthermore, the above results also imply that IcaA could be a potent drug target to explore new treatment application against biofilm formation of S. epidermidis.
References
-
1.
Sabate Bresco M, Harris LG, Thompson K, Stanic B, Morgenstern M, O'Mahony L, et al. Pathogenic mechanisms and host interactions in Staphylococcus epidermidis device-related infection. Front Microbiol. 2017;8:1401. [PubMed ID: 28824556]. [PubMed Central ID: PMC5539136]. https://doi.org/10.3389/fmicb.2017.01401.
-
2.
Morgenstern M, Erichsen C, Hackl S, Mily J, Militz M, Friederichs J, et al. Antibiotic resistance of commensal Staphylococcus aureus and coagulase-negative Staphylococci in an International Cohort of Surgeons: A prospective point-prevalence study. PLoS One. 2016;11(2). e0148437. [PubMed ID: 26840492]. [PubMed Central ID: PMC4739597]. https://doi.org/10.1371/journal.pone.0148437.
-
3.
Hogan S, Stevens NT, Humphreys H, O'Gara JP, O'Neill E. Current and future approaches to the prevention and treatment of staphylococcal medical device-related infections. Curr Pharm Des. 2015;21(1):100-13. [PubMed ID: 25189861]. https://doi.org/10.2174/1381612820666140905123900.
-
4.
Montanaro L, Speziale P, Campoccia D, Ravaioli S, Cangini I, Pietrocola G, et al. Scenery of Staphylococcus implant infections in orthopedics. Future Microbiol. 2011;6(11):1329-49. [PubMed ID: 22082292]. https://doi.org/10.2217/fmb.11.117.
-
5.
Otto M. Staphylococcus epidermidis--the 'accidental' pathogen. Nat Rev Microbiol. 2009;7(8):555-67. [PubMed ID: 19609257]. [PubMed Central ID: PMC2807625]. https://doi.org/10.1038/nrmicro2182.
-
6.
Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect Immun. 2006;74(8):4849-55. [PubMed ID: 16861673]. [PubMed Central ID: PMC1539625]. https://doi.org/10.1128/IAI.00230-06.
-
7.
Paharik AE, Horswill AR. The Staphylococcal biofilm: Adhesins, regulation, and host response. Microbiol Spectr. 2016;4(2). [PubMed ID: 27227309]. [PubMed Central ID: PMC4887152]. https://doi.org/10.1128/microbiolspec.VMBF-0022-2015.
-
8.
Schommer NN, Christner M, Hentschke M, Ruckdeschel K, Aepfelbacher M, Rohde H. Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infect Immun. 2011;79(6):2267-76. [PubMed ID: 21402760]. [PubMed Central ID: PMC3125858]. https://doi.org/10.1128/IAI.01142-10.
-
9.
Mack D, Rohde H, Harris LG, Davies AP, Horstkotte MA, Knobloch JK. Biofilm formation in medical device-related infection. Int J Artif Organs. 2006;29(4):343-59. [PubMed ID: 16705603]. https://doi.org/10.1177/039139880602900404.
-
10.
Buttner H, Mack D, Rohde H. Structural basis of Staphylococcus epidermidis biofilm formation: Mechanisms and molecular interactions. Front Cell Infect Microbiol. 2015;5:14. [PubMed ID: 25741476]. [PubMed Central ID: PMC4330918]. https://doi.org/10.3389/fcimb.2015.00014.
-
11.
Arciola CR, Campoccia D, Ravaioli S, Montanaro L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front Cell Infect Microbiol. 2015;5:7. [PubMed ID: 25713785]. [PubMed Central ID: PMC4322838]. https://doi.org/10.3389/fcimb.2015.00007.
-
12.
Rohde H, Frankenberger S, Zahringer U, Mack D. Structure, function and contribution of polysaccharide intercellular adhesin (PIA) to Staphylococcus epidermidis biofilm formation and pathogenesis of biomaterial-associated infections. Eur J Cell Biol. 2010;89(1):103-11. [PubMed ID: 19913940]. https://doi.org/10.1016/j.ejcb.2009.10.005.
-
13.
O'Gara JP. ica and beyond: Biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007;270(2):179-88. [PubMed ID: 17419768]. https://doi.org/10.1111/j.1574-6968.2007.00688.x.
-
14.
Zhang YQ, Ren SX, Li HL, Wang YX, Fu G, Yang J, et al. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol Microbiol. 2003;49(6):1577-93. [PubMed ID: 12950922]. https://doi.org/10.1046/j.1365-2958.2003.03671.x.
-
15.
Gao JP, Chen CX, Wang Y, Lu J, Gu WL. Effect of sodium houttuyfonate on myocardial hypertrophy in mice and rats. J Pharm Pharmacol. 2009;61(5):677-83. [PubMed ID: 19406008]. https://doi.org/10.1211/jpp/61.05.0018.
-
16.
Gao JP, Chen CX, Wu Q, Gu WL, Li X. Effect of sodium houttuyfonate on inhibiting ventricular remodeling induced by abdominal aortic banding in rats. Can J Physiol Pharmacol. 2010;88(7):693-701. [PubMed ID: 20651817]. https://doi.org/10.1139/y10-049.
-
17.
Wang D, Yu Q, Eikstadt P, Hammond D, Feng Y, Chen N. Studies on adjuvanticity of sodium houttuyfonate and its mechanism. Int Immunopharmacol. 2002;2(10):1411-8. [PubMed ID: 12400871]. https://doi.org/10.1016/s1567-5769(02)00060-7.
-
18.
Liu P, Yang C, Lin S, Zhao G, Zhang T, Guo S, et al. Sodium houttuyfonate inhibits LPSinduced mastitis in mice via the NFkappaB signalling pathway. Mol Med Rep. 2019;19(3):2279-86. [PubMed ID: 30664199]. https://doi.org/10.3892/mmr.2019.9846.
-
19.
Zheng C, Lin JF, Lin ZH, Lin WQ, Thapa S, Lin YZ, et al. Sodium houttuyfonate alleviates post-infarct remodeling in rats via AMP-Activated protein kinase pathway. Front Pharmacol. 2018;9:1092. [PubMed ID: 30319423]. [PubMed Central ID: PMC6170643]. https://doi.org/10.3389/fphar.2018.01092.
-
20.
Shao J, Cheng H, Wang C, Wang Y. A phytoanticipin derivative, sodium houttuyfonate, induces in vitro synergistic effects with levofloxacin against biofilm formation by Pseudomonas aeruginosa. Molecules. 2012;17(9):11242-54. [PubMed ID: 22996347]. [PubMed Central ID: PMC6268569]. https://doi.org/10.3390/molecules170911242.
-
21.
Wu DQ, Cheng H, Duan Q, Huang W. Sodium houttuyfonate inhibits biofilm formation and alginate biosynthesis-associated gene expression in a clinical strain of Pseudomonas aeruginosa in vitro. Exp Ther Med. 2015;10(2):753-8. [PubMed ID: 26622388]. [PubMed Central ID: PMC4509011]. https://doi.org/10.3892/etm.2015.2562.
-
22.
Wu DQ, Huang WF, Duan QJ, Cheng HJ, Wang CZ. [Sodium houttuyfonate inhibits virulence related motility of Pseudomonas aeruginosa]. Zhongguo Zhong Yao Za Zhi. 2015;40(8):1585-8. [PubMed ID: 26281603].
-
23.
Shao J, Cheng H, Wu D, Wang C, Zhu L, Sun Z, et al. Antimicrobial effect of sodium houttuyfonate on Staphylococcus epidermidis and Candida albicans biofilms. J Tradit Chin Med. 2013;33(6):798-803. [PubMed ID: 24660614]. https://doi.org/10.1016/s0254-6272(14)60015-7.
-
24.
Huang W, Duan Q, Li F, Shao J, Cheng H, Wu D. Sodium houttuyfonate and EDTA-Na(2) in combination effectively inhibits Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans in vitro and in vivo. Bioorg Med Chem Lett. 2015;25(1):142-7. [PubMed ID: 25467165]. https://doi.org/10.1016/j.bmcl.2014.10.072.
-
25.
Wu D, Huang W, Duan Q, Li F, Cheng H. Sodium houttuyfonate affects production of N-acyl homoserine lactone and quorum sensing-regulated genes expression in Pseudomonas aeruginosa. Front Microbiol. 2014;5:635. [PubMed ID: 25505457]. [PubMed Central ID: PMC4244979]. https://doi.org/10.3389/fmicb.2014.00635.
-
26.
Guan Y, Li C, Shi JJ, Zhou HN, Liu L, Wang Y, et al. [Effect of combination of sub-MIC sodium houttuyfonate and erythromycin on biofilm of Staphylococcus epidermidis]. Zhongguo Zhong Yao Za Zhi. 2013;38(5):731-5. Chinese. [PubMed ID: 23724685].
-
27.
Xu GF, Wang JJ, Wu DQ, Guan Y. [Effect of sodium houttuyfonate in combination with erythromycin on luxS, agr/RNA of Staphylococus epidermidis]. Zhongguo Zhong Yao Za Zhi. 2017;42(11):2131-8. Chinese. [PubMed ID: 28822159]. https://doi.org/10.19540/j.cnki.cjcmm.2017.0094.
-
28.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-8. [PubMed ID: 11846609]. https://doi.org/10.1006/meth.2001.1262.
-
29.
Hnasko TS, Hnasko RM. The Western Blot. Methods Mol Biol. 2015;1318:87-96. [PubMed ID: 26160567]. https://doi.org/10.1007/978-1-4939-2742-5_9.
-
30.
Le KY, Park MD, Otto M. Immune evasion mechanisms of Staphylococcus epidermidis biofilm infection. Front Microbiol. 2018;9:359. [PubMed ID: 29541068]. [PubMed Central ID: PMC5835508]. https://doi.org/10.3389/fmicb.2018.00359.
-
31.
McCann MT, Gilmore BF, Gorman SP. Staphylococcus epidermidis device-related infections: Pathogenesis and clinical management. J Pharm Pharmacol. 2008;60(12):1551-71. [PubMed ID: 19000360]. https://doi.org/10.1211/jpp/60.12.0001.
-
32.
Oliveira WF, Silva PMS, Silva RCS, Silva GMM, Machado G, Coelho L, et al. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J Hosp Infect. 2018;98(2):111-7. [PubMed ID: 29175074]. https://doi.org/10.1016/j.jhin.2017.11.008.
-
33.
Xu L, Li H, Vuong C, Vadyvaloo V, Wang J, Yao Y, et al. Role of the luxS quorum-sensing system in biofilm formation and virulence of Staphylococcus epidermidis. Infect Immun. 2006;74(1):488-96. [PubMed ID: 16369005]. [PubMed Central ID: PMC1346618]. https://doi.org/10.1128/IAI.74.1.488-496.2006.
-
34.
Yu D, Zhao L, Xue T, Sun B. Staphylococcus aureus autoinducer-2 quorum sensing decreases biofilm formation in an icaR-dependent manner. BMC Microbiol. 2012;12:288. [PubMed ID: 23216979]. [PubMed Central ID: PMC3539994]. https://doi.org/10.1186/1471-2180-12-288.