Abstract
Background:
There is a high worldwide prevalence of chronic Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) infections, among the significant causes of liver-related morbidity and mortality.Objectives:
We aimed to determine the prevalence of HBV and HCV in a referral center hospital in Southeast Anatolia among patients that applied for major or minimally invasive surgery.Methods:
In a tertiary referral state hospital for general purposes, patients undergoing surgical procedures and serologic examinations for HBV and HCV were included in the study between January 2011 and September 2020.Results:
In the general population, hepatitis B surface antigen (HBsAg) and anti-HBs were examined in 220,724 patients, and anti-HCV was examined in 186,017 patients. The mean age was 42.3 ± 20.2 years with a 51.8% male distribution. The frequency of positive HBsAg and anti-HCV in all patients was 9.4 and 0.9%, respectively. On the other hand, the frequency of positive HBsAg and anti-HCV was 4.2 and 0.7%, respectively, among patients admitted for a surgical procedure. The mean age was 46.1 ± 21.1 years with a slightly male predominance (54 vs. 46%). In this group, the frequency of positive HBsAg was higher in males (5.1%) while the lowest was in the 1 - 10 age range (0.4%) and the highest in the 41 - 50 age range (5.7%). Between 2011 and 2019, the prevalence of HBsAg positivity decreased from 6.4 to 4.0%, while anti-HCV positivity was similar in both genders, and its frequency increased with age.Conclusions:
Between 2011 and 2020, the overall prevalence of HBV and HCV decreased in the Southeast Anatolia Region of Turkey.Keywords
Hepatitis B Virus Hepatitis C Virus Seroprevalence Surgery Procedure
1. Background
Due to their high prevalence worldwide, chronic Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) infections are among the significant causes of liver-related morbidity and mortality. Approximately 257 million people are chronically infected with HBV and at increased risk of cirrhosis and Hepatocellular Carcinoma (HCC) (1). Although hepatitis B surface antigen (HBsAg) positivity significantly varies worldwide, the overall prevalence is reported to be 3.5%. Based on the frequency of HBV, the world is divided into three risk categories, including low (< 2%), moderate (2 - 8%), and high (≥ 8%) prevalence (2). Even though Turkey is classified in the moderate prevalence category with a 4% overall prevalence, a wide range of variability can be observed between the country's regions, varying from 2.3 to 7.3% (2, 3). In a community-based prevalence study applied throughout Turkey and published in 2015, the HBV prevalence was 7.3% in the Southeastern Anatolia Region (3).
The worldwide prevalence of anti-HCV is 1%, affecting approximately 71 million people. While the prevalence of anti-HCV in Turkey is similar to global data, chronic HCV infection is observed in 1.5% of the population of European Union Countries (2, 4). The hepatitis B vaccine was first included in the Ministry of Health's routine immunization program in late 1998 in Turkey. Therefore, individuals born after 1998 are mostly vaccinated. However, a limited number of community-based HBV and HCV prevalence studies have been conducted following the country-wide vaccination. Most of these studies were focused on patients admitted to hospitals, blood donors, and specific patient groups (5-11). Patients of all age groups admitted to hospitals for major or minimally invasive surgical procedures are primarily admitted for reasons other than acute or chronic hepatitis symptoms. Therefore, investigating the HBV and HCV prevalence in this patient group will likely yield results close to those in community-based prevalence studies.
2. Objectives
This study aimed to determine the prevalence of HBV and HCV in a reference center hospital in Southeast Anatolia among patients referred for major or minimally invasive surgery.
3. Methods
3.1. The Hospital Included in the Study
This cross-sectional study was conducted among both outpatients and inpatients admitted to our hospital, a tertiary referral state hospital located in the Southeastern Anatolia Region of Turkey. There are also patients visiting from at least nine provinces within the hospital hinterland, reflecting a representation of the Southeastern Anatolia Region.
3.2. Study Population
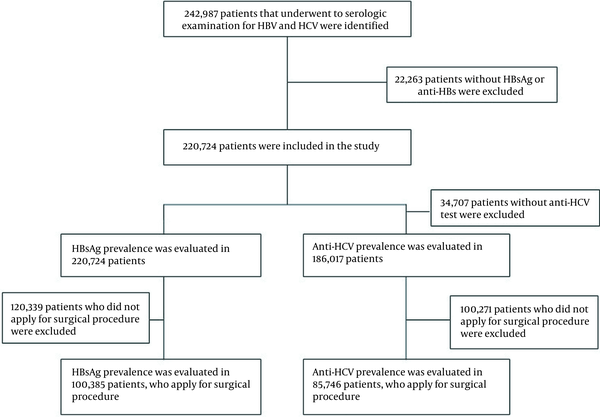
Among patients admitted to our hospital between January 2011 and September 2020, those tested for hepatitis B and hepatitis C serology (at least HBsAg), hepatitis B surface antibody (anti-HBs), and antibody to hepatitis C virus (anti-HCV) were selected as the study population. In the hospital, hepatitis B and C serological testing are routinely performed before specific procedures, like dialysis, invasive, and semi-invasive operations. Also, pregnant women and people with a high risk of hepatitis B and C infection are screened regularly. Patient data were examined retrospectively over the hospital automation system. During the period, hepatitis B and hepatitis C serology were tested in 242,987 patients. Because either HBsAg or anti-HBs tests were not conducted in 22,263 patients, the overall number of patients included in the study was 220,724. We examined the positivity rate of HBsAg among all patients screened for HBsAg besides the positivity rate of anti-HCV among 186,017 patients tested for anti-HCV. Consequently, we evaluated the HBsAg positivity rate in 100,385 patients tested for HBsAg and anti-HBs before the surgical procedure (major or minimally invasive surgery) and the anti-HCV positivity rate in 85,746 patients who were screened for anti-HCV (Figure 1).
Patient selection flowchart
3.3. Serological Tests
Blood samples of the antecubital vein (3 - 5 mL) were collected from hospitalized patients the day after hospitalization and outpatients on the same day. Then, HBsAg, anti-HBs, anti-HBc IgG, and anti-HCV were studied in the sera obtained from blood samples using enzyme immunoassay test kits in the Architect i2000 SR device (Abbott Laboratories, Lake Forest, IL, USA).
3.4. Statistical Analysis
We used SPSS 16.0 (IBM Analytics, New York, USA) for statistical analysis. The gender ratio and positivity rate for HBsAg, anti-HBs, anti-HBc, and anti-HCV markers in different age groups and years are expressed as percentages. The Chi-square test was used to compare the significant differences between groups. A P value of < 0.05 was considered statistically significant.
4. Results
4.1. Characteristics of Patients Admitted to the Hospital
In this observational study, a total of 220,724 patients were referred to the hospital's medical and surgical departments, and those who were tested for HBsAg and anti-HBs tests were identified. Of all patients, 51.8% were males, and the mean age was 42.3 ± 20.2 (Table 1).
The Number and Rate of Patients with Positive HBV and HCV Markers Among Patients Admitted to the Hospital a
| Variables | HBsAg (+); No. (%) | P | Anti-HBs (+); No. (%) | P | Anti-HBc (+); No. (%) | P | Anti-HCV (+); No. (%) | P |
|---|---|---|---|---|---|---|---|---|
| Gender | 0.005 | < 0.001 | < 0.001 | 0.896 | ||||
| Female | 8024 (7.5) | 55,823 (52.5) | 4213 (44.2) | 814 (0.9) | ||||
| Male | 12,618 (11.0) | 61,733 (53.9) | 4233 (53.0) | 898 (0.9) | ||||
| Department | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| Cardiology | 1075 (4.7) | 1224 (53.4) | 431 (56.8) | 170 (0.9) | ||||
| Dermatology | 203 (2.5) | 4929 (61.4) | 26 (35.6) | 27 (0.4) | ||||
| Emergency | 351 (4.3) | 4715 (57.9) | 174 (44.1) | 70 (1.1) | ||||
| Endocrinology | 31 (4.8) | 406 (62.5) | 14 (43.8) | 3 (0.5) | ||||
| Gastroenterology | 2945 (31.5) | 3586 (38.3) | 954 (55.3) | 125 (1.7) | ||||
| Gynecology and obstetrics | 29 (2.7) | 495 (45.7) | 3 (37.5) | 0 (0.0) | ||||
| Infectious diseases | 7429 (36.1) | 8772 (42.7) | 1296 (70.4) | 327 (2.0) | ||||
| Nephrology | 182 (3.9) | 3055 (65.3) | 289 (43.5) | 90 (2.1) | ||||
| Neurology | 219 (4.3) | 2800 (55.3) | 128 (52.5) | 53 (1.1) | ||||
| Oncology | 154 (6.2) | 1296 (52.2) | 205 (44.0) | 16 (0.8) | ||||
| Ophthalmology | 523 (3.9) | 7853 (58.0) | 188 (59.1) | 88 (0.8) | ||||
| Pediatrics | 20 (3.4) | 485 (82.1) | 1 (50) | 1 (0.5) | ||||
| Chest diseases | 148 (6.1) | 1346 (55.2) | 153 (47.5) | 43 (2.1) | ||||
| Rheumatology | 202 (2.9) | 3475 (49.7) | 2043 (41.4) | 29 (0.4) | ||||
| Surgery | 2077 (4.4) | 24,754 (52.1) | 1490 (41.3) | 247 (0.6) | ||||
| İnternal medicine | 4268 (9.2) | 25,701 (55.1) | 762 (48.1) | 300 (0.8) | ||||
| Psychiatry | 38 (5.8) | 358 (54.6) | 43 (34.7) | 3 (0.9) | ||||
| Hematology | 128 (5.1) | 1333 (53.2) | 92 (46.5) | 26 (1.1) | ||||
| Anesthesia | 35 (4.1) | 498 (58.9) | 44 (48.4) | 13 (1.8) | ||||
| Physical therapy | 65 (4.1) | 867 (55.1) | 32 (40.0) | 10 (0.8% | ||||
| Otorhinolaryngology | 226 (3.1) | 4461 (61.9) | 30 (39.0) | 16 (0.3) | ||||
| Traumatology | 294 (4.0) | 4124 (56.5) | 48 (47.5) | 55 (0.8) |
4.2. Distribution of Positive HBsAg and Anti-HCV Among Participants
In this study, HBsAg was positive in 9.4% (n = 20,642) of all patients who applied to any hospital department (7.5% female, 11.0% male). The mean age of the patients with positive HBsAg was 40.2 ± 16.1. A statistically significant difference was observed between genders in terms of HBsAg positivity (P = 0.005). Also, anti-HCV was found positive in 0.9% (n = 1,712) of 186,017 patients tested for anti-HCV (0.9% female, 0.9% male), and the mean age of these patients was 53.5 ± 18.8. No statistically significant difference was observed between genders regarding anti-HCV positivity. The HBsAg positivity was highly observed in infectious diseases, gastroenterology, internal medicine, and oncology outpatient departments, with incidence rates of 36.1, 31.5, 9.2, and 6.2%, respectively. The differences between all departments were statistically significant (P < 0.001) (Table 1).
4.3. Characteristics of Patients Admitted to the Hospital for Surgical Procedure
It was determined that 100,385 out of 220,724 patients were admitted to the departments of cardiology, gynecology, obstetrics, ophthalmology, general surgery, anesthesia, otolaryngology, and traumatology for major or minimally invasive surgical procedures, with a mean age of 46.1 ± 21.1, 46% females, and 54% males. The anti-HCV screening was performed on 85,746 patients in this group.
4.4. Distribution of HBsAg and Anti-HCV in Different Groups of Patients Applying for Surgical Procedure
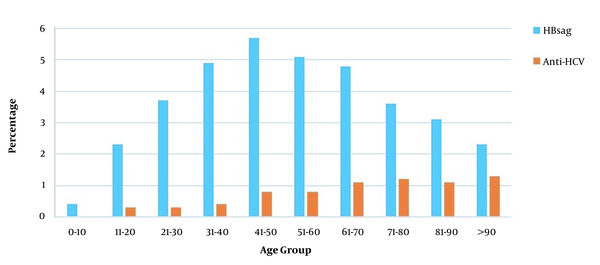
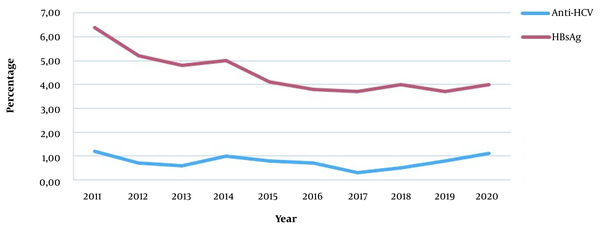
In this study, HBsAg was positive in 4.2% of the patients admitted for a surgical procedure (3.2% female, 5.1% male), and the mean age was 48.7 ± 17.6. A statistically significant difference was observed between the genders regarding HBsAg positivity (P < 0.001). Anti-HCV was positive in 0.7% (n = 589) of 85,746 patients (0.7% female, 0.7% male). The mean age of anti-HCV-positive patients was 57.4 ± 17.6, with a statistically similar gender distribution. Besides, HBsAg and anti-HCV positivity rates of patients applying for surgical procedures are given in Table 2. There was a statistically significant difference between departments in terms of HBsAg and anti-HCV positivity (P < 0.001) (Table 2). The highest HBsAg positivity rate (5.7%) was in the 41 - 50 age group, while it was the lowest (0.4%) in the 0 - 10 age group (Figure 2). A statistically significant difference was observed between age groups regarding HBsAg positivity (P < 0.001). Besides, the HBsAg positivity rate was 6.4% in 2011 and 4.0% in 2020 (Figure 3). A statistically significant difference was observed in terms of HBsAg positivity by years (P < 0.001). When the patients were divided into two groups of born before and after 1999, the HBV prevalence was 1.0% in the latter group and 4.5% in the former group (P < 0.001).
The Number and Rate of Patients with Positive HBV and HCV Markers in Patients Admitted for Surgical Purposes (Major and Minimally Invasive Surgery) a
| Variables | HBsAg; No. (%) | P | Anti-HBs (+); No. (%) | P | Anti-HBc (+); No. (%) | P | Anti-HCV (+); No. (%) | P |
|---|---|---|---|---|---|---|---|---|
| Gender | < 0.001 | < 0.001 | < 0.001 | 0.886 | ||||
| Male | 2769 (5.1) | 30,384 (56.1) | 1225 (51.0) | 325 (0.7) | ||||
| Female | 1490 (3.2) | 24,048 (52.1) | 1009 (41.9) | 264 (0.7) | ||||
| According to date of vaccination | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| Born in 1999 and after | 79 (1.0) | 5564 (68.7) | 29 (17.1) | 6 (0.1) | ||||
| Born before 1999 | 4180 (4.5) | 48,868 (53.0) | 2205 (47.5) | 583 (0.7) | ||||
| Age groups | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| 0 - 10 | 17 (0.4) | 3124 (77.8) | 7 (7.5) | 1 (0.0) | ||||
| 11 - 20 | 205 (2.3) | 6271 (70.4) | 91 (24.5) | 20 (0.3) | ||||
| 21 - 30 | 552 (3.7) | 7855 (52.8) | 218 (32.5) | 38 (0.3) | ||||
| 31 - 40 | 676 (4.9) | 5686 (41.0) | 312 (39.9) | 48 (0.4) | ||||
| 41 - 50 | 825 (5.7) | 6762 (46.7) | 419 (48.1) | 85 (0.8) | ||||
| 51 - 60 | 772 (5.1) | 7880 (52.2) | 422 (54.3) | 103 (0.8) | ||||
| 61 - 70 | 716 (4.8) | 8211 (55.6) | 394 (59.2) | 145 (1.1) | ||||
| 71 - 80 | 375 (3.6) | 6181 (59.7) | 285 (63.2) | 110 (1.2) | ||||
| 81 - 90 | 113 (3.1) | 2249 (61.2) | 81 (65.8) | 35 (1.1) | ||||
| > 91 | 8 (2.3) | 213 (61.0) | 5(71.4) | 4 (1.3) | ||||
| By Years | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| 2011 | 307 (6.4) | 2474 (51.5) | 1360 (49.0) | 52 (1.2) | ||||
| 2012 | 371 (5.2) | 3729 (52.7) | 193 (46.5) | 52 (0.7) | ||||
| 2013 | 526 (4.8) | 6079 (55.9) | 139 (50.0) | 65 (0,6) | ||||
| 2014 | 290 (5.0) | 3286 (57.0) | 184 (50.1) | 58 (1.0) | ||||
| 2015 | 371 (4.1) | 5207 (57.2) | 3 (60.0) | 31 (0.8) | ||||
| 2016 | 528 (3.8) | 7557 (54.7) | 33 (24.4) | 35 (0.7) | ||||
| 2017 | 514 (3.7) | 7484 (54.1) | 33 (24.4) | 40 (0.3) | ||||
| 2018 | 545 (4.0) | 7152 (53.0) | 126 (35.6) | 73 (0.5) | ||||
| 2019 | 616 (3.7) | 8907 (52.9) | 135 (43.7) | 131 (0.8) | ||||
| 2020 | 190 (4.0) | 2557 (53.3) | 47 (37.9) | 52 (1.1) | ||||
| Department | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| Cardiology | 1075 (4.7) | 12,247 (53.4) | 431 (56.8) | 170 (0.9) | ||||
| Gynecology and obstetrics | 29 (2.7) | 495 (45.7) | 3 (37.5) | 0 (0.0) | ||||
| Ophthalmology | 523 (3.9) | 7853 (58.0) | 188 (59.1) | 88 (0.8) | ||||
| Surgery | 2077 (4.4) | 24,754 (52.1) | 1490 (43.1) | 247 (0.6) | ||||
| Anesthesia | 35 (4.1) | 498 (58.9) | 44 (48.4) | 13 (1.8) | ||||
| Otorhinolaryngology | 226 (3.1) | 4461 (61.9) | 30 (39.0) | 16 (0.3) | ||||
| Traumatology | 294 (4.0) | 4124 (56.5) | 30 (39.0) | 55 (0.8) |
HBsAg and anti-HCV positivity rates by age groups
HBsAg and anti-HCV positivity rates by years
4.5. Distribution of Anti-HBs Positivity by Groups of Patients Applying for Surgical Procedure
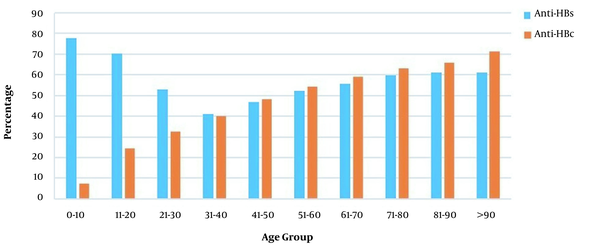
The rate of anti-HBs positivity varied significantly between age groups, with the most common frequency in the 0 - 10 age group (77.8%) (Figure 4). The anti-HBs positivity rates varied between 51.5 and 57.2% over the years 2011 and 2020. The incidence of positive anti-HBs in those born before and after 1999 was 68.7 and 53%, respectively (P < 0.001) (Table 2).
4.6. Distribution of Anti-HBc Positivity in Groups of Patients Applying for Surgical Procedure
Among 4,812 patients screened for anti-HBc, 2,234 patients (46.4%) were tested positive. The highest anti-HBc positivity rate (71.4%) was detected in the age group over 90 years, while the lowest rate (7.5%) was in the 0-10 age group (Figure 4) (P < 0.001). The prevalence of anti-HBc was 17.1% in those born in 1999 or after and 47.5% in those born before 1999 (P < 0.001) (Table 2).
Anti-HBs and anti-HBc positivity rates by age groups
5. Discussion
This cross-sectional study represented about nine million people since it was conducted in a tertiary referral hospital in Turkey's Southeastern Anatolia Region. To the best of our knowledge, this is the first report comprehensively examining the frequency of HBV and HCV in patients referred to a referral center for surgical and nonsurgical purposes from different age groups in a 10-year period in Turkey. In the present study, HBsAg and anti-HCV positivity was 9.4% and 0.9%, respectively, among all patients admitted to the hospital. In other hospital-based prevalence studies from different regions of Turkey, the rate of HBsAg positivity was stated to vary between 4 and 13.4% (5-9). In a population-based study from Turkey that was conducted in all seven regions between 2009 and 2010, the prevalence of HBsAg positivity was 2.3% in the Aegean region, 6.3% in the Blacksea region, 4.3% in the Middle Anatolian region, 3.4% in the East Anatolian region, 3.8% in Marmara region, 3.1% in Mediterranean region, and 7.3% in Southeast Anatolian region (3).
Between 2005 and 2012, the rate of HBsAg positivity was 10%, while the figure for anti-HCV positivity was 1.2% in our hospital (6). Also, the frequency of hepatitis B and C in different departments separately was evaluated (Table 1). In a study examining the HBsAg positivity in 218,267 individuals from a Chinese population, the highest rates were observed in hepatology (42.35%), endocrinology (12.96%), emergency service (12.17%), and gastroenterology (10.1%) outpatient departments (12). However, in the current study, HBsAg positivity was most common in infectious diseases (36.1%), gastroenterology (31.5%), and internal medicine (9.2%) outpatient services. The anti-HCV positivity was highest in the infectious diseases department (19%). Furthermore, 76.8% of the patients who were HbsAg-positive and 43.9% of those who were anti-HCV-positive were among the patients admitted to the departments of infectious diseases, gastroenterology, and internal medicine. The management and treatment of hepatitis B and C in Turkey are performed by the departments of infectious diseases, internal medicine, gastroenterology, and hepatology, which is a natural explanation of higher rates in these outpatient services. The hospital-based HBV and HCV prevalence studies do not reflect the population's true prevalence since the departments where patients with HBV and HCV infection are followed and treated are not evaluated separately.
In two different studies conducted in the studied region, with one-year intervals, HBsAg seropositivity was 7.3% in the community-based prevalence study and 10.4% in the hospital-based study (3, 13). The difference in the seropositivity rate between these studies supports our claims. Based on this result, since the HBV and HCV prevalence in patients scheduled for major or minimally invasive surgical procedures might be close to the community prevalence, the seroprevalence of HBV and HCV in this patient group was investigated. We observed these figures for positive cases in the patient group who applied for the surgical procedure: HBsAg 4.2%, anti-HCV 0.7%, anti-HBs 54.2%, and anti-HBc 46.4%.
In the community-based prevalence study conducted in the region in 2003 by Mehmet et al. (14), they observed the HBsAg prevalence of 7.0% while Tozun et al. (3), in their community-based prevalence study covering all parts of Turkey in 2009 - 2010, found that the rate of HBsAg prevalence was 7.3% in Southeast Anatolia. Compared to these studies, the current study's low rate of HBsAg positivity can be due to increased HBV awareness and effective vaccination programs for newborns and adults. There are also studies reporting that HBsAg positivity is more common in males (7, 15-17). Likewise, HBsAg positivity was significantly higher in males in this study (5.1 vs. 3.2%), which can be attributed to the fact that males are more active in business and social life in Turkey or more commonly engage in hepatitis-risky activities and reaching medical services quickly.
The mean age of patients with positive and negative HBsAg was 48.7 ± 17.6 and 45.9 ± 21.22, respectively, and the difference was statistically significant. Studies report a strong relationship between age and HBsAg positivity (18, 19). Nonetheless, a significant variation exists between regions and countries regarding the relationship of age groups with HBsAg positivity. The lowest HBsAg positivity rates were 0.4, 2.3, and 2.3% in the age groups of 1 - 10, 10 - 20, and over 90 years, respectively, in the present study. The low HBsAg positivity rates in the 1 - 10 and 10 - 20 age groups possibly are due to the vaccination of those born after 1998 within the routine vaccination program framework. The fact that HBsAg positivity was 0.4% in the age range of 1 - 10 indicates that the hepatitis B vaccine was effectively administered to newborns. According to the Ministry of Health data, the HBV vaccination rate in 2018 was as high as 98% (20).
The low rate of HBsAg positivity in patients over the age of 90 suggests that the survival rate of patients with chronic HBV infection is lower due to infection-related complications than that of the healthy population. The HBsAg positivity was higher in middle-aged patients with 5.7% frequency in the 41 - 50 age group. In a study conducted in the region in 2003, the HBsAg positivity was most common in the 25 - 34 age group in rural areas and the 35-44 age group in urban areas (14). Similarly, in China's hospital-based HBV prevalence study, the highest HBsAg positivity was found in the 41 - 50 age group (12).
Likewise, in the hospital-based seroprevalence study conducted in Artvin, located in the northeast of Turkey, the most frequent rate of HBsAg positivity was observed in the 41 - 50 age group (9). The high rate of HBsAg positivity in this group can be explained by the fact that they were infected with viruses in childhood because the most critical transmission route in our country is the vertical and in-family horizontal transmission, and they did not acquire immunity since they were born before the start of the vaccination program. In addition, the fact that people do riskier activities in terms of getting infected with hepatitis B when they reach adulthood may be another reason for high HBsAg positivity in adults.
In this study, HBsAg prevalence was examined by dividing the patients into two groups: those born before 1999 and those born after 1999. The HBsAg prevalence was 4.5% in those born before 1999 and 1.0% in those born after 1999. The hepatitis B vaccine used in the newborns' vaccination program after 1998 in Turkey has significantly decreased the frequency of HBsAg positivity for those born in 1999 and after. It is reported in the literature that the frequency of anti-HBc increases with age (12). The anti-HBc frequency was 46.4%, and it increased with age in the current study, which supports the former report. This may be because people born after 1998 and infected with HBV are fewer due to the HBV vaccine, and people who are not immunized may encounter HBV at a later age.
In the community-based seroprevalence study of Tozun et al. (3) conducted across Turkey, the rate of anti-HCV positivity was observed as 1%. However, it was 1.2% in a hospital-based seroprevalence study conducted in the region the current study applied (6). The anti-HCV seropositivity rate was 0.9% in all patients admitted to our hospital, and 0.7% in patients only admitted for surgical procedures. These low rates may be related to increased awareness and decreased surgical spreading of hepatitis infections due to proper sterilization of surgical material. In an epidemiological study conducted in Turkey for anti-HCV positivity, the positivity rate was more frequent over 54 years of age (21). There are publications in the literature stating that anti-HCV positivity is higher in older ages (22, 23). In the current study, the mean age of patients with positive and negative anti-HCV was 57.4 ± 17.6 and 46.4 ± 21.01, respectively, and the difference was statistically significant. The anti-HCV positivity rate increased after the age of 61, indicating increased cumulative effects of exposure to multiple risk factors (such as surgery, dental interventions, blood transfusion, intravenous drug use) with age. The literature states that anti-HCV positivity is not different between men and women (16). In line with the literature, anti-HCV positivity was 0.7% in both genders in our study, and there was no significant difference between genders.
The current study is limited in a few senses. The first is that the study was conducted retrospectively, leading to missing socioeconomic, educational, and marital status data that may significantly impact the risk of HBV and HCV seroprevalence. As the importance of increased awareness was addressed in several parts of the manuscript, awareness against infectious diseases is significantly better among college graduates, high-income, and married individuals. Second, although patients from different cities in the region apply routinely to the hospital where the study was conducted, it was still a single-center study. Third, although there is a belief that patients admitted to the hospital for major and minimally invasive surgical procedures may be close to the general population in terms of hepatitis B and hepatitis C serology, we have failed to support this claim.
5.1. Conclusions
This research found positive HBsAg incidence in the study region to be much lower than in the previous population-based prevalence studies. The most important reason for this decrease is the effective administration of hepatitis B vaccines and the decrease in the frequency of positive HBsAg in the young population. Male gender and older ages were closely related to HBsAg positivity. While the frequency of positive anti-HCV decreases in the region, it is equally observable in both genders, and its frequency increases with elder ages. If this study is evaluated with previous studies conducted in the Southeastern Anatolian region of Turkey, a decline in the positivity of HBsAg from 7.3 to 4.2% can be observed. Similarly, it is probable to see the same decrease in the frequency of HBsAg positivity in other regions of Turkey, and Turkey may have been listed among the low-prevalence (< 2%) countries. Finally, a community-based study to determine the current prevalence of HBV and HCV in Turkey is essential to reach a more precise conclusion.
References
-
1.
World Health Organization. Global hepatitis report, 2017. Geneva, Switzerland: World Health Organization; 2017.
-
2.
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546-55. [PubMed ID: 26231459]. https://doi.org/10.1016/S0140-6736(15)61412-X.
-
3.
Tozun N, Ozdogan O, Cakaloglu Y, Idilman R, Karasu Z, Akarca U, et al. Seroprevalence of hepatitis B and C virus infections and risk factors in Turkey: A fieldwork TURHEP study. Clin Microbiol Infect. 2015;21(11):1020-6. [PubMed ID: 26163105]. https://doi.org/10.1016/j.cmi.2015.06.028.
-
4.
Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45-57. [PubMed ID: 25086286]. https://doi.org/10.1016/j.jhep.2014.07.027.
-
5.
Ergunay K, Balaban Y, Cosgun E, Alp A, Simsek H, Sener B, et al. Epidemiologic trends in HBV infections at a reference centre in Turkey: An 11-year retrospective analysis. Ann Hepatol. 2012;11(5):672-8. [PubMed ID: 22947528].
-
6.
Turhanoglu M, Onur A, Bilman FB, Ayaydin Z, Aktar GS. Eight-year seroprevalence of HBV, HCV and HIV in Diyarbakir training and research hospital. Int J Med Sci. 2013;10(11):1595-601. [PubMed ID: 24046538]. [PubMed Central ID: PMC3775121]. https://doi.org/10.7150/ijms.6506.
-
7.
Igde FA, Taskin H, Igde M, Yazici Z, Atilla A. Where we are in the fight against Hepatitis B Infection; Trends in Hepatitis B virus seroprevalence in Black Sea Region of Turkey. Niger J Clin Pract. 2018;21(1):87-92. [PubMed ID: 29411730]. https://doi.org/10.4103/njcp.njcp_368_16.
-
8.
Ozturk TC, Guneysel O, Tali A, Yildirim SE, Onur OE, Yaylaci S. Hepatitis B, Hepatitis C and HIV seroprevalence in critically ill emergency medicine department patients in a tertiary inner city hospital in Istanbul, Turkey. Pak J Med Sci. 2014;30(4):703-7. [PubMed ID: 25097500]. [PubMed Central ID: PMC4121681]. https://doi.org/10.12669/pjms.304.4975.
-
9.
İncİ A, GÜven D, Okay M. HBsAg, Anti-HBs, anti-HCV and anti-HIV seroprevalence of the patients applied to Artvin State Hospital. Viral Hepatit Derg. 2013;19(1):41-4. https://doi.org/10.4274/Vhd.03522.
-
10.
Tigen ET, Dogru A, Karadag FY. Hepatitis B, Hepatitis C and human immunodeficiency virus prevalences among first time blood donors in Istanbul, Turkey, 2004-2011. Transfus Apher Sci. 2015;53(2):176-9. [PubMed ID: 25881737]. https://doi.org/10.1016/j.transci.2015.03.013.
-
11.
Ocak S, Kaya H, Çetin M, Inandi T. Seropositivity of Hepatitis A and B according to age and sex in preoperative patients in Antakya. Viral Hepatitis J. 2005;10(3):169-75.
-
12.
Meng J, Xu H, Sui D, Jiang J, Li J, Gao Y, et al. A retrospective serological survey of hepatitis B virus infection in Northeast China. BMC Infect Dis. 2019;19(1):440. [PubMed ID: 31109300]. [PubMed Central ID: PMC6528233]. https://doi.org/10.1186/s12879-019-4091-3.
-
13.
Kaya Ş, Baysal B, Temiz H, Karadağ Ö, Özdemir K, Bilman F. Seroprevalence of hepatitis B and C among Patients admitted to a tertiary hospital. Viral Hepatit Derg. 2014;20(3):120-4. https://doi.org/10.4274/vhd.95967.
-
14.
Mehmet D, Meliksah E, Serif Y, Gunay S, Tuncer O, Zeynep S. Prevalence of hepatitis B infection in the southeastern region of Turkey: Comparison of risk factors for HBV infection in rural and urban areas. Jpn J Infect Dis. 2005;58(1):15-9. [PubMed ID: 15728984].
-
15.
Gogos CA, Fouka KP, Nikiforidis G, Avgeridis K, Sakellaropoulos G, Bassaris H, et al. Prevalence of hepatitis B and C virus infection in the general population and selected groups in South-Western Greece. Eur J Epidemiol. 2003;18(6):551-7. [PubMed ID: 12908721]. https://doi.org/10.1023/a:1024698715741.
-
16.
Demirturk N, Demirdal T, Toprak D, Altindis M, Aktepe OC. Hepatitis B and C virus in West-Central Turkey: Seroprevalence in healthy individuals admitted to a university hospital for routine health checks. Turk J Gastroenterol. 2006;17(4):267-72. [PubMed ID: 17205404].
-
17.
Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212-9. [PubMed ID: 22273662]. https://doi.org/10.1016/j.vaccine.2011.12.116.
-
18.
Khan A, Afzal S, Yaqoob A, Fatima R, Ul Haq M, Junaid K, et al. Epidemiology of viral hepatitis B and C in Punjab, Pakistan: A multicenter cross-sectional study, 2017-18. F1000Research. 2019;8:2065. https://doi.org/10.12688/f1000research.20174.1.
-
19.
Omote V, Kashibu E, Ojumah I, Adda D, Etaghene J, Ukwamedua H. Serological screening of hepatitis B virus and hepatitis C virus among patients attending a tertiary hospital in Jalingo, Taraba state, Nigeria. Saudi J Health Sci. 2018;7(3):167. https://doi.org/10.4103/sjhs.sjhs_39_18.
-
20.
Bİrİncİ Ş, Ulgu M. Health statistics yearbook. Ankara, Turkey: Republic of Turkey Ministry of Health General Directorate of Health Information Systems; 2018.
-
21.
Thomas DL, Mahley RW, Badur S, Palaoglu E, Quinn TC. The epidemiology of hepatitis C in Turkey. Infection. 1994;22(6):411-4. [PubMed ID: 7698839]. https://doi.org/10.1007/BF01715499.
-
22.
Darwish MA, Faris R, Clemens JD, Rao MR, Edelman R. High seroprevalence of hepatitis A, B, C, and E viruses in residents in an Egyptian village in The Nile Delta: a pilot study. Am J Trop Med Hyg. 1996;54(6):554-8. [PubMed ID: 8686770]. https://doi.org/10.4269/ajtmh.1996.54.554.
-
23.
Patino-Sarcinelli F, Hyman J, Camacho LA, Linhares DB, Azevedo JG. Prevalence and risk factors for hepatitis C antibodies in volunteer blood donors in Brazil. Transfusion. 1994;34(2):138-41. [PubMed ID: 8310484]. https://doi.org/10.1046/j.1537-2995.1994.34294143942.x.