Abstract
Background:
Salmonella is one of the main foodborne bacterial pathogens, causing diseases and death. The study used reverse transcription loop-mediated isothermal amplification (RT-LAMP) to detect Salmonella.Objectives:
To design six primers and detect Salmonella using RT-LAMP to facilitate the rapid detection of pathogenic bacteria in food.Methods:
We designed six primers based on the gene coding sequences of inv A, specific to Salmonella. Each reaction solution contained 6.0 mM MgSO4, 1 M betaine, 1.6 mM dNTPs, 160 U/mL Bst DNA polymerase, 0.2 μM of both external primers, 0.8 μM of both internal primers, and 0.2 μM of both loop primers. The reaction temperature was 65°C.Results:
Our amplified products were separated by 2% agarose gel electrophoresis. The detection limit was 10 CFU per reaction.Conclusions:
RT-LAMP exhibited the same accuracy as the Chinese National Standard assay (GB 4789.30-2016) assay in detecting Salmonella in foods. RT-LAMP was highly specific and sensitive; hence, it may serve as an effective tool in detecting Salmonella.Keywords
Salmonella D012475 RT-LAMP Foodborne Pathogens Rapid Detection
1. Background
Salmonella is one of the main foodborne pathogens in the Enterobacteriaceae family. Salmonella spp. cause diarrhea, vomiting, abdominal colic, pneumonia, and bacteremia (1). On the other hand, in the cutting-edge technology of bacterial therapy, scientists utilize bacteria such as Salmonella typhimurium and Shigella flexneri as antiproliferative and potential chemotherapeutic agents to treat gastrointestinal cancers (2, 3). Livestock and poultry products are susceptible to Salmonella contamination. Foods leading to disease outbreaks are mainly eggs and poultry meat; however, the predominant foodborne diseases are enteritis and typhoid fever (4, 5). Due to its excellent survival characteristics, Salmonella is found in various fresh vegetables and meat products. When adhered to the surface of heat-treated foods, the bacteria rapidly are multiplies even at low temperatures (6). Accordingly, the detection of Salmonella is of utmost importance in the food industry (7).
Routine culture is the gold standard for detecting, isolating, and identifying Salmonella spp. However, the cultural process is time-consuming. Samples should be placed in an enrichment solution for 18 - 24 h, transferred to chromogenic plates for 18 - 24 h, and purified for 18 - 24 h. Following these steps, the pathogen can be preliminarily and serologically identified before its identity is confirmed. In this regard, it takes 6 - 7 days to reach results, and specialized laboratories are required (8). Compared with routine culture, polymerase chain reaction (9), DNA microarrays (10), and antibody assays (11) are more appropriate detection methods; however, they are time-consuming. Accordingly, we need a more efficient and straightforward protocol.
In 2000, loop-mediated isothermal amplification (LAMP) was proposed as a novel approach to molecular identification (12). LAMP is specific, sensitive, and cost-effective. However, it fails to distinguish between live and dead cells, and this method often obtains false-positive results from food samples (13). Accordingly, a more accurate method is required to detect and monitor pathogens. Studies have reported that reverse transcription LAMP (RT-LAMP) may overcome the LAMP limitations. RT-LAMP can be used to detect viruses (14). In this study, we collected samples from Hangzhou, Zhejiang Province, China, and used RT-LAMP to detect Salmonella to facilitate the rapid detection of pathogenic bacteria in food.
2. Objectives
To design six primers and detect Salmonella using RT-LAMP to facilitate the rapid detection of the pathogen in food.
3. Methods
3.1. Strains and Culture
We collected 41 strains from different areas in Hangzhou, China. Ten Salmonella spp. were used as positive samples; other bacterial species were selected to evaluate the specificity of RT-LAMP (Table 1). Salmonella strains were stored in the LB broth/agar medium, and additional species such as Listeria were stored in the BHI broth/agar medium. The bacterial solutions were cultured at 37°C and diluted. We measured bacterial concentrations by ultraviolet spectrophotometry. All chemicals and media were acquired from Sangon Co., Ltd. (Shanghai, China).
Bacterial Strains Used in this Study
| Types and Species | Strains |
|---|---|
| Target- Salmonella spp. | |
| Salmonella enterica | ZFM New1 |
| S. enterica | CGMCC 1.1552 |
| S. enterica | CGMCC 1.10603 |
| S. typhimurium | CGMCC 1.1174 |
| S. typhimurium | CGMCC 1.1190 |
| S. pullorum | ZFM 124694 |
| S. anatum | ZFM New2 |
| Non- Salmonella spp. | |
| Micrococcus luteus | CGMCC 1.193 |
| Staphylococcus aureus | ATCC 6538P |
| S. aureus | CGMCC 1.2386 |
| S. aureus | CGMCC 1.879 |
| S. aureus | CGMCC 1.128 |
| Lactobacillus plantarum | CGMCC 1.11 |
| L. plantarum | CGMCC 1.124 |
| L. plantarum | CGMCC 1.551 |
| L. plantarum | CGMCC 1.556 |
| L. plantarum | CGMCC 1.511 |
| L. lactis | ATCC 15577 |
| Bacillus subtilis | CGMCC 1.1627 |
| Enterococcus faecalis | ZFM BM13 |
| E. faecalis | CGMCC 1.125 |
| Shigella flexneri | CGMCC 1.1868 |
| Pseudomonas aeruginosa | CGMCC 1.647 |
| S. dysenteriae | ATCC 9753 |
| Escherichia coli | CGMCC 1.1580 |
| E. coli | O104 |
| E. coli | JM109 |
| Listeria seeligeri | ZFM 51335 |
| L. monocytogenes | ATCC 7648 |
| P. putida | CGMCC 1.645 |
| Rhodotorula Rubra | CGMCC 2.1034 |
| Saccharomyces cerevisiae | CGMCC 2.1643 |
3.2. RNA Extraction and cDNA Synthesis
We extracted total RNA from all 41 bacterial strains using an RNA Rapid Extraction Kit (Sangon Co., Ltd. Shanghai, China) and measured yield using a NanoDrop spectrophotometer (Eppendorf, Germany). To minimize the RNA degradation, total RNA was converted into cDNA using a PrimeScript II First Strand cDNA Synthesis Kit (TaKaRa, China) (15). The reaction volume (20 μL) consisted of 6 µL of RNA, 4 μL of 5× RT buffer, 2 μL of dNTPs (10 mM each), 50 pmol of random hexamers, 10 U of AMV reverse transcriptase XL (TaKaRa), and 10 U of RNase inhibitor (TaKaRa). The reaction solution was maintained for 60 min at 42°C and 15 min at 70°C.
3.3. Primer Design
We designed primers based on an internally transcribed spacer sequence (GenBank Accession No. NC_987035) using Primer Explorer V5 (Eiken Chemical Co. Ltd., Tokyo, Japan). Six pairs of primers were created, which targeted distinct regions of the LAMP sequence and corresponded to two outlying primers (F3 and B3), two internal primers (FIP and BIP), and two loop primers. Table 2 and Figure 1 show the primer sequences and locations, respectively.
Sequence of inv A gene for detecting Salmonella and primer locations. The sequences of each primer-binding site are provided under the line.
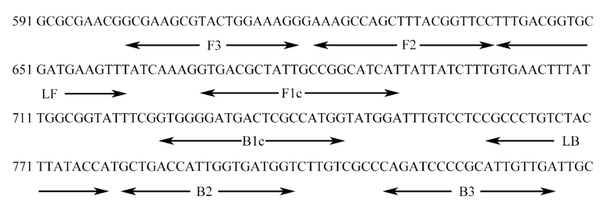
Primers Positions of inv A Gene Coding Sequences of Salmonella
| Primer Name | Type | Sequences (5' - 3') | Length (bp) |
|---|---|---|---|
| F3 | Forward outer | GCGAAGCGTACTGGAAAGG | 19 |
| B3 | Reverse outer | TCAACAATGCGGGGATCTG | 19 |
| FIP | Composed of F1c and F2 | ATGATGCCGGCAATAGCGTCACAAAGCCAGCTTTACGGTTCC | 40 |
| BIP | Composed of B1c and B2 | GTGGGATGACYCGCCATGGACCATCACCAATGGTCAGC | 38 |
| LF | AAACTTCATCGCACCGTCAAA | 21 | |
| LB | CCGCYCTGTCTACTTATACCA | 21 |
3.4. LAMP Assay
The following six primer pairs were added to a 25-μL tube: 1.6 mM dNTPs, 0.8 M betaine (Sigma-Aldrich, USA), 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 4 mM MgSO4, 0.1% Triton X-100, 80 U Bst DNA polymerase (New England Biolabs, USA), and 2 μL of target cDNA. Different assay conditions, including different temperatures (59 to 65°C) and concentrations of MgSO4 (2 to 8 mM), dNTPs (0.8 to 2.0 mM), and Bst DNA polymerase (40 to 320 U), were evaluated. The reaction solution was incubated for 1 h and 5 min at 80°C (16, 17). All experiments were performed in triplicate.
3.5. Detection of RT-LAMP Products
The amplified products were separated using 2% agarose gel electrophoresis and analyzed under ultraviolet light after 40 min. A white precipitate (magnesium pyrophosphate) was produced during the reaction, which was visible with the naked eye.
3.6. Specificity of RT-LAMP
We used the RNA templates of the 41 strains to perform RT-LAMP. A total of 25 non-Salmonella strains were tested to assess potential cross-reactions (Table 1). The other strains were used for determining the specificity of the assay.
3.7. Sensitivity of RT-LAMP
We determined the detection limit of RT-LAMP by preparing a 10-fold dilution series (106 CFU/μL to 100 CFU/μL) of Salmonella (ZFM New1) in sterile phosphate-buffered saline. The strain was measured by plating it onto LB agar directly (Huankai, Guangzhou, China) followed by incubation at 37°C for 24 h. The RNA templates were obtained from 100 μL of each dilution, and 2 μL fractions were amplified using RT-LAMP. Each sensitivity test was performed four times.
3.8. Salmonella Detection in Ready-to-Eat Meats
We used the RT-LAMP assay to detect Salmonella spp. in ready-to-eat (RTE) meats and other foods and compared the results with those obtained from the Chinese National Standard assay (GB 4789.30-2016). The RTE meats were obtained from a local market in China. We divided each sample into 25-g portions, and each portion was isolated for the RNA extraction to act as a template for RT-LAMP. The GB assay was performed by incubating 25 g of each sample at 30°C for 24 h in 225 mL of Listeria enrichment broth I (LB1). In the next phase, we incubated 100 μL of the samples in 10-mL of Listeria enrichment broth II (LB2) at 30°C for 24 h. Positive LB2 cultures aliquots (50 μL) were inoculated on PALCAM agar (Huankai) and Listeria chromogenic agar (Huankai) and incubated at 37°C for 48 h. To confirm the presence of Salmonella spp., we used characteristic colony morphologies, Gram staining, and additional tests, including sugar and motility tests. The strains were finally identified using API Listeria (bioMérieux, France).
3.9. Statistical Analysis
SPSS and Origin were used to analyze the experimental data and process the figures.
4. Results
4.1. Optimization of RT-LAMP Reaction Conditions
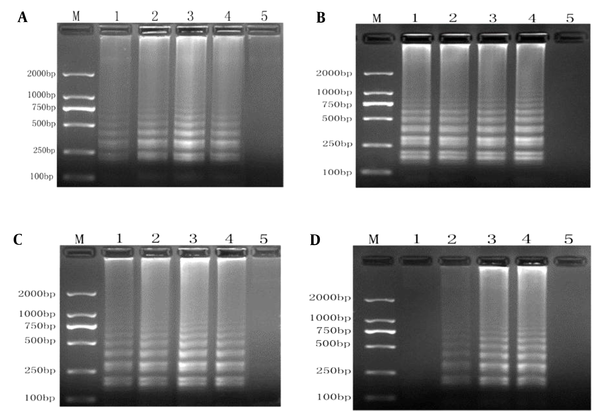
We optimized the RT-LAMP conditions by evaluating different reaction conditions. The optimal Mg and dNTP concentrations were 6.0 mM MgSO4 (Figure 2A) and 1.6 mM dNTPs (Figure 2C), respectively. Figure 2B shows a minor difference in temperature (63 and 65°C). No positive product was amplified at 40 U/mL Bst DNA polymerase, while the optimal results were obtained at 160 U/mL Bst DNA polymerase (Figure 2D). Accordingly, optimum conditions were 6.0 mM MgSO4, 1.6 mM dNTPs, and 160 U/mL Bst DNA polymerase at 65°C.
RT-LAMP system optimization diagram. A, optimization of Mg2+ concentrations (lane M, 2,000-bp ladder marker; lanes 1 - 4, the results of RT-LAMP with Mg2+ concentrations of 2.0 mmol/L, 4.0 mmol/L, 6.0 mmol/L, and 8.0 mmol/L; lane 5, negative control); B, optimization of annealing temperatures (lane M, 2000-bp ladder marker; lanes 1 - 4, the results of RT-LAMP at 59, 61, 63, and 65°C; lane 5, negative control); C, optimization of dNTP concentrations (lane M, 2000-bp ladder marker; lanes 1 - 4, the results of RT-LAMP with dNTP concentrations of 0.8 mmol/L, 1.2 mmol/L, 1.6 mmol/L, and 2.0 mmol/L; lane 5, negative control); D, optimization of Bst enzyme concentrations (lane M, 2,000-bp ladder marker; lanes 1-4, the results of RT-LAMP with Bst enzyme concentrations of 40, 80, 160, and 320 U/mL; lane 5, negative control).
4.2. Detection of RT-LAMP Products
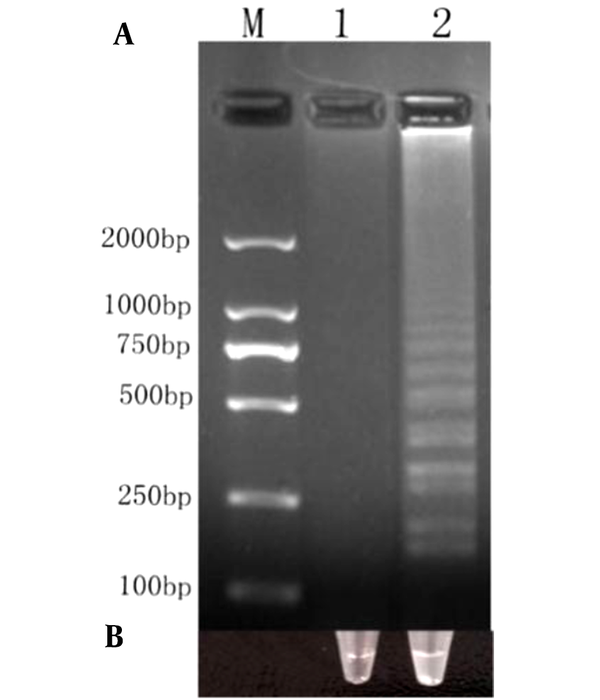
Our amplified products were separated by 2% agarose gel electrophoresis (Figure 3A), and the strains were observed with the naked eye (Figure 3B). We noted turbidity changes under normal lighting conditions. Positive cDNA samples were successfully amplified. The results revealed that Salmonella could be rapidly and easily detected using RT-LAMP.
Detection of RT-LAMP products. A, lane M, 2000-bp ladder marker; lane 1, negative control; lane 2, the results of LAMP optimization reaction; B, physical map of corresponding lanes.
4.3. Specificity of RT-LAMP Assay
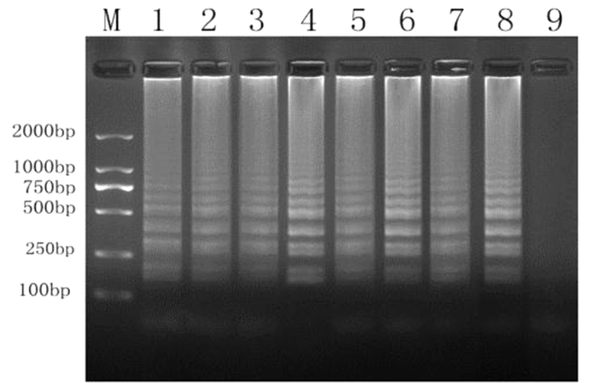
Primer specificity (Figure 1) was investigated by extracting cDNA from eight strains of Salmonella and incorporating 33 strains of other species as templates (Table 1). Each of the eight Salmonella strains (Figure 4) was successfully amplified, whereas none of the other species were amplified. Accordingly, our primers were highly specific to Salmonella.
Specificity test of RT-LAMP assay (lane M, 2,000-bp ladder marker; lane 1, WS1; lane 2, F2365; lane 3, F8027; lane 4, J1723; lane 5, HF550; lane 6, J4045; lane 7, clip11262; lane 8, J0161; and lane 9, negative control).
4.4. Sensitivity of RT-LAMP Assay
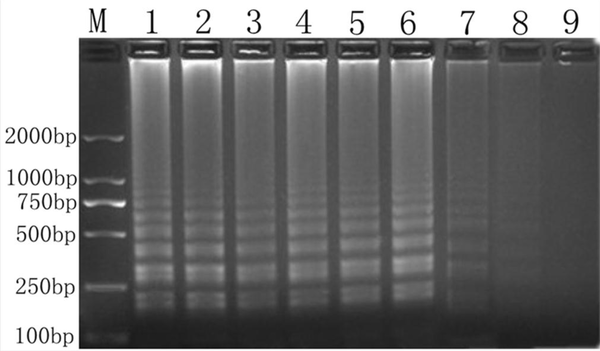
We performed sensitivity analysis by incorporating 10-fold serial dilutions of Salmonella, corresponding to 500 ng, 50 ng, 5 ng, 0.5 ng, 50 pg, 5 pg, 0.5 pg, and 50 fg (equivalent to 1 × 106 CFU/μL to 1 × 100 CFU/μL) of RNA. The RT-LAMP assay resulted in the DNA bands at all dilutions. However, the bands became weaker with decreasing RNA concentration. The sensitivity limit was 50 fg (Figure 5), equivalent to 1 CFU/μL.
Sensitivity test of RT-LAMP assay. Lane M, 2000-bp ladder marker; lanes 1–8, diluted samples of Salmonella with 4.36 × 107, 4.36 × 106, 4.36 × 105, 4.36 × 104, 4.36 × 103, 4.36 × 102, 4.36 × 101, and 4.36; lane 9, negative control
4.5. Detection of viable Salmonella in RTE Food Samples
We assessed the capability of the RT-LAMP assay in detecting pathogenic microorganisms in the RTE foods, including a variety of vegetables, fruits, and meats (Table 3). The RT-LAMP and GB assays detected two Salmonella-positive chicken samples. Similar results were obtained for the other three types of food, indicating that the RT-LAMP and GB assays had similar detection capabilities.
Detection of Salmonella in RTE Foods Using RT-LAMP and GB 4789.4-2016 Assay
| Sample (Tested Number) | No. of Samples Tested Positive for Salmonella | |
|---|---|---|
| RT-LAMP | GB 4789.4-2016 | |
| Beef (25) | 0 | 0 |
| Pork (25) | 1 | 1 |
| Egg (25) | 2 | 2 |
| Fish (25) | 0 | 0 |
| Total (100) | 3 | 3 |
5. Discussion
Microbial contaminants have long been a health concern. Food poisoning has been studied for a long time, and plants are fully used as drugs in traditional treatment methods (18). In recent years, the abuse of antibiotics has led to the rapid spread of antibiotic resistance in pathogens (19) as such, it is necessary to change the traditional treatment strategy. Experts have conducted in-depth studies on the pathogenicity and defense mechanisms. Hassan Mahmoudi found out that the pathogenicity of Escherichia coli with iuc A and iut A genes may be associated to this issue (20). Propolis and garlic water extract can play a role in preventing pathogenic bacteria (21, 22). In the present study, a rapid detection method was designed for pathogenic bacteria in food to expand food safety methods further.
Developing detection methods for Salmonella, one of the most common contaminants in fruits and meats, is vital to minimize outbreaks (23). From a food safety and human health perspective, detecting foodborne bacterial pathogens requires immediate attention. This study proposed the use of RT-LAMP for detecting Salmonella in foods. The assay had high specificity and sensitivity in detecting Salmonella. Moreover, RT-LAMP was accurate and fast enough to be used in routine inspections and quarantine situations. Accordingly, it may serve as a potential diagnostic tool in the food industry to detect Salmonella in food products rapidly.
The proposed detection method of Salmonella uses inv A as the target gene, specific to Salmonella. Previous studies on inv A-based molecular detection assays have demonstrated their high specificity and sensitivity (24). When bacteria die, RNA cleaves quickly. Accordingly, the RT-LAMP assay for detecting Salmonella has lower false-positive results. Mg2+ concentration has the largest impact on primer annealing and DNA polymerase activity. A preliminary study revealed no target amplification at high Mg2+ concentrations since Mg2+ is bound to chelating agents such as ethylenediaminetetraacetic acid and anionic groups. Hence, the DNA polymerase activity and amplification results were affected. After evaluating a range of concentrations (2 - 8 mM), we found that 6 mM MgSO4 was optimal, indicating that the Bst DNA polymerase was effective at 61 - 65°C. We selected 65°C as the optimal temperature based on the AT and GC contents of the primers. The number of inv A mRNA copies was significantly higher than the number of inv A genes in the cell. Copy number is the major determinant of sensitivity.
5.1. Conclusions
The findings revealed that the detection limit for Salmonella in RT-LAMP was 1 CFU per reaction in pure cultures, which was 10-fold lower than that in PMA-LAMP (25). As an alternative to PCR, RT-LAMP is more sensitive, may be superior to PMA-LAMP in detecting Salmonella, and is further used to detect microorganisms in the RTE meats. Compared to GB 4789.4-2016, it was accurate; however, it took considerably less time.
References
-
1.
Bayrac C, Eyidogan F, Avni Oktem H. DNA aptamer-based colorimetric detection platform for Salmonella Enteritidis. Biosens Bioelectron. 2017;98:22-8. [PubMed ID: 28646719]. https://doi.org/10.1016/j.bios.2017.06.029.
-
2.
Khodavirdipour A, Rouhani nejad H, Zandi M, Khodavirdipour A, Khayyam N. To study in vitro anti-proliferative and pro-apoptotic properties of Salmonella typhi in human pancreatic cancer cell line. Avicenna J Clin Microbiol Infect. 2019;6(3):77-82. https://doi.org/10.34172/ajcmi.2019.13.
-
3.
Khodavirdipour A, Jamshidi F, Nejad HR, Zandi M, Zarean R. To study the anti-cancer effects of Shigella flexneri in AspC-1 pancreatic cancer cell line in approach to bax and bcl-2 genes. J Gastrointest Cancer. 2021;52(2):593-9. [PubMed ID: 32524303]. https://doi.org/10.1007/s12029-020-00433-9.
-
4.
Jarquin R, Hanning I, Ahn S, Ricke SC. Development of rapid detection and genetic characterization of salmonella in poultry breeder feeds. Sensors (Basel). 2009;9(7):5308-23. [PubMed ID: 22346699]. [PubMed Central ID: PMC3274138]. https://doi.org/10.3390/s90705308.
-
5.
Ocana-de Jesus RL, Gutierrez-Ibanez AT, Sanchez-Pale JR, Mariezcurrena-Berasain MD, Laguna-Cerda A, Hernandez-Chinas U, et al. Motility and survival of Salmonella enterica subspecies enterica serovar enteritidis in tomato plants (Solanum lycopersicum L). Int Microbiol. 2019;22(3):363-8. [PubMed ID: 30811003]. https://doi.org/10.1007/s10123-019-00059-3.
-
6.
Burnett SL, Chopskie JH, Podtburg TC, Gutzmann TA, Gilbreth SE, Bodnaruk PW. Use of octanoic acid as a postlethality treatment to reduce Listeria monocytogenes on ready-to-eat meat and poultry products. J Food Prot. 2007;70(2):392-8. [PubMed ID: 17340874]. https://doi.org/10.4315/0362-028x-70.2.392.
-
7.
Khan I, Khan J, Miskeen S, Tango CN, Park YS, Oh DH. Prevalence and control of Listeria monocytogenes in the food industry – a review. Czech J Food Sci. 2016;34(No. 6):469-87. https://doi.org/10.17221/21/2016-cjfs.
-
8.
Liu A, Shen L, Zeng Z, Sun M, Liu Y, Liu S, et al. A Minireview of the Methods for Listeria monocytogenes Detection. Food Anal Methods. 2017;11(1):215-23. https://doi.org/10.1007/s12161-017-0991-2.
-
9.
Shoukat S, Ali M, Wani H, Ali U, Para PA, Ganguly S. Food-borne listeriosis, an upcoming public health problem: A review. J Immunol Immunopathol. 2017;19(1):1-9. https://doi.org/10.5958/0973-9149.2017.00001.6.
-
10.
Dzupova O, Rozsypal H, Smiskova D, Benes J. Listeria monocytogenes meningitis in adults: the Czech Republic experience. Biomed Res Int. 2013;2013:846186. [PubMed ID: 24106719]. [PubMed Central ID: PMC3784069]. https://doi.org/10.1155/2013/846186.
-
11.
Savas S, Ersoy A, Gulmez Y, Kilic S, Levent B, Altintas Z. Nanoparticle enhanced antibody and DNA biosensors for sensitive detection of Salmonella. Materials (Basel). 2018;11(9). [PubMed ID: 30150524]. [PubMed Central ID: PMC6163637]. https://doi.org/10.3390/ma11091541.
-
12.
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12). E63. [PubMed ID: 10871386]. [PubMed Central ID: PMC102748]. https://doi.org/10.1093/nar/28.12.e63.
-
13.
Mei X, Zhai X, Lei C, Ye X, Kang Z, Wu X, et al. Development and application of a visual loop-mediated isothermal amplification combined with lateral flow dipstick (LAMP-LFD) method for rapid detection of Salmonella strains in food samples. Food Control. 2019;104:9-19. https://doi.org/10.1016/j.foodcont.2019.04.014.
-
14.
Parida MM, Santhosh SR, Dash PK, Tripathi NK, Saxena P, Ambuj S, et al. Development and evaluation of reverse transcription-loop-mediated isothermal amplification assay for rapid and real-time detection of Japanese encephalitis virus. J Clin Microbiol. 2006;44(11):4172-8. [PubMed ID: 17005741]. [PubMed Central ID: PMC1698363]. https://doi.org/10.1128/JCM.01487-06.
-
15.
Zhang Y, Chen M, Liu C, Chen J, Luo X, Xue Y, et al. Sensitive and rapid on-site detection of SARS-CoV-2 using a gold nanoparticle-based high-throughput platform coupled with CRISPR/Cas12-assisted RT-LAMP. Sens Actuators B Chem. 2021;345:130411. [PubMed ID: 34248284]. [PubMed Central ID: PMC8257267]. https://doi.org/10.1016/j.snb.2021.130411.
-
16.
Itano T, Kawakami H, Kono T, Sakai M. Detection of fish nocardiosis by loop-mediated isothermal amplification. J Appl Microbiol. 2006;100(6):1381-7. [PubMed ID: 16696687]. https://doi.org/10.1111/j.1365-2672.2006.02872.x.
-
17.
Yeh HY, Shoemaker CA, Klesius PH. Evaluation of a loop-mediated isothermal amplification method for rapid detection of channel catfish Ictalurus punctatus important bacterial pathogen Edwardsiella ictaluri. J Microbiol Methods. 2005;63(1):36-44. [PubMed ID: 16157211]. https://doi.org/10.1016/j.mimet.2005.02.015.
-
18.
Jalali H, Mozaffari Nejad AS, Ebadi AG, Laey G. Ethnobotany and folk pharmaceutical properties of major trees or shrubs in northeast of Iran. Asian J Chem. 2009;21(7):5632-8.
-
19.
Kavyani B, Arabestani MR, Mozaffari Nejad AS, Moradkhani S, Kamarehei F, Taheri M, et al. The effect of Cinnamon bark on the expression of quorum sensing system and virulence genes in pseudomonas aeruginosa strain PAO1. Res J Biotechnol. 2019;14(2):42-8.
-
20.
Mahmoudi H, Hossainpour H, Moradi M, Alikhani MY. Distribution of iron uptake systems encoding genes among the clinical isolates of Escherichia coli compared to foodstuffs isolates. Infect Disord Drug Targets. 2020;20(4):517-22. [PubMed ID: 30659552]. https://doi.org/10.2174/1871526519666190119112542.
-
21.
Shariatifar N, Janghorban A, Rahimnia R, Mozaffari Nejad AS. Antimicrobial, antifungal and antioxidant activities of bee glue ethanol and aqueous extracts. J Biol Res. 2018;90(2). https://doi.org/10.4081/jbr.2017.6452.
-
22.
Mozaffari Nejad AS, Shabani S, Bayat M, Hosseini SE. Antibacterial effect of garlic aqueous extract on Staphylococcus aureus in hamburger. Jundishapur J Microbiol. 2014;7(11). e13134. [PubMed ID: 25774277]. [PubMed Central ID: PMC4332239]. https://doi.org/10.5812/jjm.13134.
-
23.
Tamariz J, Guevara V, Guerra H. Rapid detection of salmonellosis due to Salmonella enterica serovar Typhimurium in Peruvian commercially bred cavies, using indigenous wild bacteriophages. Germs. 2018;8(4):178-85. [PubMed ID: 30775336]. [PubMed Central ID: PMC6363402]. https://doi.org/10.18683/germs.2018.1144.
-
24.
Bai J, Trinetta V, Shi X, Noll LW, Magossi G, Zheng W, et al. A multiplex real-time PCR assay, based on invA and pagC genes, for the detection and quantification of Salmonella enterica from cattle lymph nodes. J Microbiol Methods. 2018;148:110-6. [PubMed ID: 29621581]. https://doi.org/10.1016/j.mimet.2018.03.019.
-
25.
Wan C, Yang Y, Xu H, Aguilar ZP, Liu C, Lai W, et al. Development of a propidium monoazide treatment combined with loop-mediated isothermal amplification (PMA-LAMP) assay for rapid detection of viableListeria monocytogenes. Int J Food Sci Technol. 2012;47(11):2460-7. https://doi.org/10.1111/j.1365-2621.2012.03123.x.