Abstract
Background:
Candidemia is the most common systemic infection in hospitalized patients causing high mortality. Hence, the diagnosis of this infection in the early stage with appropriate antifungal therapy is paramount.Objectives:
The study aimed at molecular identification of Candida species isolated from candidemia patients and evaluation of the in vitro antifungal susceptibility patterns of these strains to fluconazole, amphotericin B, and caspofungin.Methods:
In the present study, 800 hospitalized patients who were suspected to have candidemia were sampled. Candida species were isolated and identified based on morphological characteristics and PCR-sequencing of the ITS1-5.8S-ITS2 region. Antifungal susceptibility tests for fluconazole, amphotericin B, and caspofungin were performed according to the Clinical and Laboratory Standards Institute protocol M27-A3. Also, clinical data were recorded from the patients' records.Results:
Twenty-seven patients among the sample of hospitalized patients were found to have candidemia. A total of 33.3% of candidemia patients were treated with amphotericin B, in which case the mortality rate was 14.8%. The majority of patients (59%) were from the neonatal intensive care unit, and premature birth was the most common underlying condition. Candida albicans (n = 18; 66.6%) was the most common species isolated from blood cultures, followed by C. parapsilosis (n = 7; 25.9%), C. pelliculosa (n = 1; 3.7%), and C. tropicalis (n = 1; 3.7%). Only one C. albicans isolate resistant to fluconazole (minimum inhibitory concentration = 32 µg/mL).Conclusions:
Generally, C. albicans has been the most frequent causative agent of candidemia. Resistance to antifungal drugs among candidemia agents was rare. Also, the identification of Candida isolates at the species level with in vitro antifungal susceptibility tests helps manage candidemia patients better and decrease the mortality rate among them.Keywords
Candidemia Candida albicans Antifungal Susceptibility Antifungals
1. Background
One of the most prevalent causes of hospital-acquired infections, more specifically in severe cases, is systemic fungal infections. Candida species are the most common causative agents of these infections. Candidemia is the most common systemic infection in hospitalized patients leading to high mortality (1). Prolonged antibiotic therapy, immune dysfunction, use of corticosteroids, renal failure, and dialysis are introduced as the predisposing risk factors for candidemia (2). Candida albicans is the most common causative agent of candidemia. However, the prevalence of candidemia caused by non-albicans species, including C. tropicalis, C. glabrata, C. krusei, and C. parapsilosis, is increasing (3-6).
The change in the epidemiology of Candida infection has contributed to the development of antifungal resistance so that many Candida species are resistant to fluconazole (6, 7). Resistance to antifungal agents can increase the risk of treatment failure, mortality, and prolonged hospitalization (8, 9). Therefore, accurate diagnosis of the fungal infection, identification of the species, monitoring incidence, and in vitro antifungal susceptibility profiles are essential to managing systemic Candida infection (10). DNA-based methods such as PCR-sequencing and restriction fragment length polymorphism (RFLP) are reliable methods for the identification of Candida species (11, 12).
2. Objectives
The present study was conducted to identify Candida species isolated from candidemia patients using molecular methods. Also, the antifungal susceptibility profiles of these species were evaluated.
3. Methods
3.1. Clinical Samples and Candida Isolation
This study focused on patients with candidemia admitted to the medical centers in Yasuj, Shahid Beheshti, Imam Sajjad, and Provincial Reference Laboratory from November 2016 to November 2019. During the study period, 800 blood samples were collected from patients and inoculated into a biphasic blood culture medium (Tebo Sadegh, Iran) and incubated at 37°C aerobically for more than 7 days. During this period, 1 mL of each blood culture was subcultured on CHOROM agar Candida (CHOROM agar, France) and incubated at 37°C. After that, Candida species were initially identified based on classical methods.
3.2. DNA Extraction
DNA was extracted and purified using the boiling method described by Tay et al. (13). Then, a loop full of a fresh colony of isolates of each yeast was added to 1.5-mL tubes containing 300 µL of distilled water. The tubes were then placed in boiling water for 20 min. After that, tubes were vortexed for 5 min and centrifuged at 8000 rpm for 1 min. Finally, supernatant (DNA) was separated and stored at a temperature of -20°C and used for PCR amplification.
3.3. PCR-Sequencing
The ITS1-5.8S-ITS2 region of the rDNA complex was amplified using V9g/LS266 primers (V9g, 5’-TTACGTCCCTGCCCTTTGTA-3’, and LS266, 5’-TCCTCCGCTTATTGATATGC-3’) for all strains (14). Then, the PCR products were sequenced, manually verified, and aligned by the MEGA6 software. All sequence data were compared to reference sequences in the GenBank (NCBI) and CBS databases via the nucleotide BLAST™ algorithm to achieve a definitive identification (similarity values ≥ 99%). Finally, all nucleotide sequences were submitted to the GenBank database, and accession numbers were obtained.
3.4. Antifungal Susceptibility Testing
The antifungal assay was performed according to the Clinical and Laboratory Standards Institute (CLSI) protocol M27-A3 (15). Overnight cultures of Candida species on Sabouraud Dextrose agar (SDA) (Merck, Germany) were used for this purpose. Suspensions were adjusted to 0.5 McFarland and then diluted to 1:1000 (1:20 and 1:50). The serial dilutions of the antifungal agents were prepared from 0.0625 - 32 µg/mL for fluconazole, 0.03 - 16 µg/mL for amphotericin B, and 0.015 - 8 µg/mL for caspofungin (15). A volume of 100 µL of yeast suspension and 100 µL of serial dilution of each tested antifungal agent were added into each well of the microplate. Microplates were incubated at 35°C for 24 - 48 h. Then, the minimum inhibitory concentration (MIC) range, MIC50, MIC90, and MICGM were calculated. Defined CLSI guidelines breakpoints were used to assess susceptibility, dose-dependence, and resistance (16). Besides, C. parapsilosis ATCC 22019 was used for quality control.
3.5. Statistical Analysis
The data were analyzed by the chi-squared and Kruskal-Wallis one-way ANOVA tests using SPSS version 21.0. A default value of 0.05 was considered the P-value for significance.
4. Results
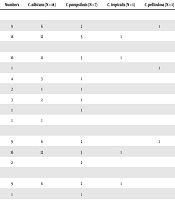
A total of 800 patients who were suspected to have sepsis and candidemia were enrolled for this study. Of these patients, 27 patients were diagnosed with candidemia based on mycological criteria. Of these patients, 18 (66.7%) were male and 9 (33.3%) female (Table 1). Also, the age range of these patients varied from 3 days in the neonates to 80 years in adult patients. A total of 51.8% of candidemia patients had a history of received antimicrobial drugs such as vancomycin, amikacin, gentamycin, and ciprofloxacin. Over 59% of the yeast isolates were recovered from the neonatal intensive care unit (NICU), and the rest (33.3%) were obtained from the intensive care unit (ICU). Among the underlying diseases, premature birth (16,59.3%) and diabetes (4, 14.8%) were the most frequent conditions in admitted patients.
Clinical Properties of Patients with Candidemia in Our Study
| Clinical Properties | Numbers | C. albicans (N = 18) | C. parapsilosis (N = 7) | C. tropicalis (N = 1) | C. pelliculosa (N = 1) |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 9 | 6 | 2 | 1 | |
| Male | 18 | 12 | 5 | 1 | |
| Underline disease | |||||
| Premature | 16 | 11 | 3 | 1 | |
| UTI | 1 | 1 | |||
| Diabetes | 4 | 3 | 1 | ||
| Sepsis | 2 | 1 | 1 | ||
| Cancer | 3 | 2 | 1 | ||
| COPD | 1 | 1 | |||
| ESRD | 1 | 1 | |||
| Ward | |||||
| ICU | 9 | 6 | 2 | 1 | |
| NICU | 16 | 12 | 3 | 1 | |
| Non-evaluated | 2 | 2 | |||
| Antifungal treatment | |||||
| Amphotericin B | 9 | 6 | 2 | 1 | |
| Fluconazole | 1 | 1 | |||
| Amphotericin B + fluconazole | 1 | 1 | |||
| Non-evaluated | 5 | 5 | |||
| Not treated | 11 | 7 | 3 | 1 | |
| Mortality rate | 4 | 4 |
A total of 33.3% of candidemia patients were treated with amphotericin B, followed by fluconazole and a combination of these drugs. The mortality rate was obtained at 14.8%and was associated with C. albicans. According to sequence analysis, C. albicans (n = 18; 66.6%) was the most common isolated species followed by C. parapsilosis (n = 7; 25.9%), C. pelliculosa (n = 1; 3.7%), and C. tropicalis (n = 1; 3.7%). The accession numbers were LC617336-LC617355. The MIC range, MIC50, MIC90, and MICGM were calculated for all isolates, as shown in Table 2. As shown, except for one C. albicans isolate (MIC = 32 µg/mL), all Candida species were sensitive to fluconazole. Also, 100% of Candida isolates were found to be wild-type for amphotericin B. Generally, all Candida isolates were sensitive to caspofungin, and 22.2% of these isolates were dose-dependent (MIC = 0.5 µg/mL).
Minimum Inhibitory Concentration Range, MIC50, MIC90, and MICGM Values Obtained for Candida Isolates
| Species | Numbers | Antifungals | Minimum Inhibitory Concentration (MIC) | R | ECV, % | |||
|---|---|---|---|---|---|---|---|---|
| MIC Range | MIC50 | MIC90 | MICGM | |||||
| Candida albicans | 18 | Fluconazole | 0.0625 - 32 | 0.25 | 1 | 0.145 | 1 | 100 |
| Amphotericin B | 0.03125 - 2 | 0.25 | 0.5 | 0.21 | - | |||
| Caspofungin | 0.03125 - 1 | 0.25 | 0.5 | 0.138 | - | |||
| C. parapsilosis | 7 | Fluconazole | 0.03125 - 0.5 | - | 100 | |||
| Amphotericin B | 0.03125 - 0.5 | - | ||||||
| Caspofungin | 0. 125 - 1 | - | ||||||
| C. tropicalis | 1 | Fluconazole | 1 | - | 100 | |||
| Amphotericin B | 0.125 | - | ||||||
| Caspofungin | 0.03125 | - | ||||||
| C. pelliculosa | 1 | Fluconazole | 0.25 | - | ND | |||
| Amphotericin B | 0.0625 | - | ||||||
| Caspofungin | 0.0625 | - | ||||||
| Total | 27 | Fluconazole | 0.0625 - 32 | 0.093 | 1 | 0.15 | 1 | ND |
| Amphotericin B | 0.03125 - 2 | 0.125 | 0.5 | 0.14 | ||||
| Caspofungin | 0.03125 - 1 | 0.25 | 0.7 | 0.175 | ||||
5. Discussion
Candidemia is one of the most common systemic fungal infections that is common among hospitalized patients, especially premature infants (17). The prevalence of candidemia varies between published reports (18-20). In our study, the incidence of candidemia was 3.4% among hospitalized patients with positive blood cultures. In agreement with our study, this rate was reported to be 3.5% in Motta et al. from Brazil (21). However, the incidence of candidemia in published reports from the US and Europe was lower than in our study (19, 20).
Amphotericin B is one of the main antifungal drugs with broad-spectrum uses in systemic mycosis (22). However, 40.7% of our patients were not treated with antifungal drugs. This result disagrees with other studies that reported fluconazole as the most received antifungal drug in candidemia patients (23, 24). A low mortality rate (14.8%) was obtained in our study, which is lower than most other studies conducted in Iran (28 - 47%) (23, 24). It seems that this difference may be due to different conditions of candidemia patients, such as underlying disease, antifungal therapy, the type of Candida species, and the length of hospitalization. In the present study, C. albicans was found to be the only cause of mortality in candidemia patients. However, C. tropicalis was attributed to high mortality rates found in other studies (25, 26).
In the present study, C. albicans was the most frequent agent of candidemia (78.5%). These results are in agreement with other studies conducted in Iran, such as Razzaghi et al. (27) and Sadrossadati et al. (28), who reported that C. albicans was the most common. Besides, our results are similar to other reports from different countries (29, 30). However, in recent years, the infections caused by non-albicans species have emerged as the common etiology of candidemia (8, 31). In studies such as those performed by Chander et al. (32) in India and Ghahri et al. (8) in Iran, C. tropicalis (40.8%) and C. parapsilosis (34.4%) were found to be the most frequent cause of candidemia, respectively. The reason for the emergence of infections caused by non-albicans species can be associated with some underlying conditions, which are different for different species (27). In our study, the second most frequent Candida species were C. parapsilosis. Similar to our results, Sadrossadati et al. (28) and Kooshki et al. (33) revealed that C. parapsilosis was the second most prevalent isolated species. However, Arastehfar et al. (24) showed that C. glabrata was the second most prevalent causative agent of candidemia in Shiraz. This contrast may be due to the difference in the age groups of patients.
In our study, the MIC range for fluconazole was evaluated between 0.0625 and 32 µg/mL for the 27 Candida strains. Besides, resistance to fluconazole was detected in 5.6% of C. albicans strains; however, all non-albicans strains were sensitive to this drug. Similarly, Arastehfar et al. (24), reported that 4.42% of Candida strains isolated from blood cultures were resistant to fluconazole. Although initially, it seems that triazoles are highly efficient, but overusing them is associated with azole resistance in Candida species (34). Kumar et al. (35) reported 19% of Candida strains found in India were resistant to fluconazole, while Khairat et al. (36) found it to be 38.9%. Generally, it seems that the history of antifungal therapy and the underlying condition of patients could be related to antifungal resistance.
Even though amphotericin B is one of the most toxic antifungal drugs in clinical use, it is still considered a standard and inexpensive treatment (27, 37). In this study, 100% of C. albicans strains were wild-type to amphotericin B. This result is in agreement with Arastehfar et al. (24), who also showed that all candidemia isolates were wild-type against amphotericin B. Also, in international studies such as Motta et al. (21), amphotericin B resistance among Candida bloodstream isolates was found to be rare. Therefore, it seems that amphotericin B can be a therapeutic agent of choice for patients with candidemia.
Echinocandins are one of the main choices of treatment in a patient with candidemia (1, 38). Caspofungin is one of the echinocandin class drugs and uses more in the different forms of candidiasis (39). This drug is more active against Candida species, especially in azole resistance agents (27). In this study, antifungal susceptibility tests indicated that caspofungin was the most efficient drug against Candida species (MIC ≤ 1 µg/mL). In other studies carried out in Iran, the level of resistance was varied among Candida species. The susceptibility profile of caspofungin in our study was similar to that reported in Iran and other countries (21, 27). In some studies, resistance to this drug has been observed in non-albicans species, such as C. parapsilosis and C. krusei (40, 41).
5.1. Conclusions
In conclusion, our study demonstrated that C. albicans has been the most frequent causative agent of candidemia in the southwest of Iran. Resistance to antifungal drugs among candidemia agents was rare. Also, the identification of Candida isolates at the species level with in vitro antifungal susceptibility tests can help manage and decrease the mortality rate among candidemia patients.
References
-
1.
Salehi M, Ghomi Z, Mirshahi R, Dehghan Manshadi SA, Rezahosseini O. Epidemiology and outcomes of candidemia in a referral center in Tehran. Caspian J Intern Med. 2019;10(1):73-9. [PubMed ID: 30858944]. [PubMed Central ID: PMC6386322]. https://doi.org/10.22088/cjim.10.1.73.
-
2.
Lotfi N, Shokohi T, Nouranibaladezaei SZ, Nasrolahi Omran A, Kondori N. High recovery rate of Non-albicans Candida species isolated from burn patients with candidemia in Iran. Jundishapur J Microbiol. 2015;8(10). e22929. [PubMed ID: 26587207]. [PubMed Central ID: PMC4644265]. https://doi.org/10.5812/jjm.22929.
-
3.
Wu Z, Liu Y, Feng X, Liu Y, Wang S, Zhu X, et al. Candidemia: incidence rates, type of species, and risk factors at a tertiary care academic hospital in China. Int J Infect Dis. 2014;22:4-8. [PubMed ID: 24583564]. https://doi.org/10.1016/j.ijid.2013.11.011.
-
4.
Doi AM, Pignatari AC, Edmond MB, Marra AR, Camargo LF, Siqueira RA, et al. Epidemiology and microbiologic characterization of nosocomial candidemia from a Brazilian National Surveillance Program. PLoS One. 2016;11(1). e0146909. [PubMed ID: 26808778]. [PubMed Central ID: PMC4726651]. https://doi.org/10.1371/journal.pone.0146909.
-
5.
Pinhati HM, Casulari LA, Souza AC, Siqueira RA, Damasceno CM, Colombo AL. Outbreak of candidemia caused by fluconazole resistant Candida parapsilosis strains in an intensive care unit. BMC Infect Dis. 2016;16(1):433. [PubMed ID: 27544427]. [PubMed Central ID: PMC4992558]. https://doi.org/10.1186/s12879-016-1767-9.
-
6.
Sanguinetti M, Posteraro B, Lass-Florl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58 Suppl 2:2-13. [PubMed ID: 26033251]. https://doi.org/10.1111/myc.12330.
-
7.
Lin S, Chen R, Zhu S, Wang H, Wang L, Zou J, et al. Candidemia in adults at a tertiary hospital in china: Clinical characteristics, species distribution, resistance, and outcomes. Mycopathologia. 2018;183(4):679-89. [PubMed ID: 29572768]. https://doi.org/10.1007/s11046-018-0258-5.
-
8.
Ghahri M, Mirhendi H, Zomorodian K, Kondori N. Identification and antifungal susceptibility patterns of Candida strains isolated from blood specimens in Iran. Arch Clin Infect Dis. 2013;8(3). https://doi.org/10.5812/archcid.14529.
-
9.
Gharaghani M, Hivary S, Taghipour S, Zarei-Mahmoudabadi A. Luliconazole, a highly effective imidazole, against Fusarium species complexes. Med Microbiol Immunol. 2020;209(5):603-12. [PubMed ID: 32253502]. https://doi.org/10.1007/s00430-020-00672-4.
-
10.
Bassetti M, Merelli M, Ansaldi F, de Florentiis D, Sartor A, Scarparo C, et al. Clinical and therapeutic aspects of candidemia: a five year single centre study. PLoS One. 2015;10(5). e0127534. [PubMed ID: 26010361]. [PubMed Central ID: PMC4444310]. https://doi.org/10.1371/journal.pone.0127534.
-
11.
Wise MG, Healy M, Reece K, Smith R, Walton D, Dutch W, et al. Species identification and strain differentiation of clinical Candida isolates using the DiversiLab system of automated repetitive sequence-based PCR. J Med Microbiol. 2007;56(Pt 6):778-87. [PubMed ID: 17510263]. https://doi.org/10.1099/jmm.0.47106-0.
-
12.
Fatima A, Bashir G, Wani T, Jan A, Kohli A, Khan MS. Molecular identification of Candida species isolated from cases of neonatal candidemia using polymerase chain reaction-restriction fragment length polymorphism in a tertiary care hospital. Indian J Pathol Microbiol. 2017;60(1):61-5. [PubMed ID: 28195093]. https://doi.org/10.4103/0377-4929.200023.
-
13.
Tay ST, Chai HC, Na SL, Ng KP. Molecular subtyping of clinical isolates of Candida albicans and identification of Candida dubliniensis Malaysia. Mycopathologia. 2005;159(3):325-9. [PubMed ID: 15883714]. https://doi.org/10.1007/s11046-004-6269-4.
-
14.
Gharaghani M, Rezaei-Matehkolaei A, Hardani AK, Zarei Mahmoudabadi A. Genotypic diversity and antifungal susceptibility pattern of Candida albicans species isolated from hospitalized paediatric patients with urinary tract infection in Iran. J Appl Microbiol. 2021;131(2):1017-27. [PubMed ID: 33460500]. https://doi.org/10.1111/jam.15006.
-
15.
CLSI. Reference Method for Broth Dilution antifungal susceptibility testing of yeasts, Approved Standard M27-A3. Wayne, PA: Clinical Laboratory Standards Institute; 2008.
-
16.
CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts; CLSI standard M27. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
-
17.
Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis. 2003;37(5):634-43. [PubMed ID: 12942393]. https://doi.org/10.1086/376906.
-
18.
Colombo AL, Guimaraes T, Silva LR, de Almeida Monfardini LP, Cunha AK, Rady P, et al. Prospective observational study of candidemia in Sao Paulo, Brazil: incidence rate, epidemiology, and predictors of mortality. Infect Control Hosp Epidemiol. 2007;28(5):570-6. [PubMed ID: 17464917]. https://doi.org/10.1086/513615.
-
19.
Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, Faure O, et al. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis. 2004;23(4):317-22. [PubMed ID: 15029512]. https://doi.org/10.1007/s10096-004-1103-y.
-
20.
Macphail GL, Taylor GD, Buchanan-Chell M, Ross C, Wilson S, Kureishi A. Epidemiology, treatment and outcome of candidemia: a five-year review at three Canadian hospitals. Mycoses. 2002;45(5-6):141-5. [PubMed ID: 12100528]. https://doi.org/10.1046/j.1439-0507.2002.00741.x.
-
21.
Motta AL, Almeida GM, Almeida Junior JN, Burattini MN, Rossi F. Candidemia epidemiology and susceptibility profile in the largest Brazilian teaching hospital complex. Braz J Infect Dis. 2010;14(5):441-8. [PubMed ID: 21221471].
-
22.
Jafarian H, Gharaghani M, Seyedian SS, Mahmoudabadi AZ. Genotyping, antifungal susceptibility, enzymatic activity, and phenotypic variation in Candida albicans from esophageal candidiasis. J Clin Lab Anal. 2021;35(7). e23826. [PubMed ID: 33988259]. [PubMed Central ID: PMC8274993]. https://doi.org/10.1002/jcla.23826.
-
23.
Kord M, Salehi M, Khodavaisy S, Hashemi SJ, Daie Ghazvini R, Rezaei S, et al. Epidemiology of yeast species causing bloodstream infection in Tehran, Iran (2015-2017); superiority of 21-plex PCR over the Vitek 2 system for yeast identification. J Med Microbiol. 2020;69(5):712-20. [PubMed ID: 32368996]. [PubMed Central ID: PMC7451039]. https://doi.org/10.1099/jmm.0.001189.
-
24.
Arastehfar A, Yazdanpanah S, Bakhtiari M, Fang W, Pan W, Mahmoudi S, et al. Epidemiology of candidemia in Shiraz, southern Iran: A prospective multicenter study (2016-2018). Med Mycol. 2021;59(5):422-30. [PubMed ID: 32692816]. https://doi.org/10.1093/mmy/myaa059.
-
25.
Megri Y, Arastehfar A, Boekhout T, Daneshnia F, Hortnagl C, Sartori B, et al. Candida tropicalis is the most prevalent yeast species causing candidemia in Algeria: the urgent need for antifungal stewardship and infection control measures. Antimicrob Resist Infect Control. 2020;9(1):50. [PubMed ID: 32264966]. [PubMed Central ID: PMC7140370]. https://doi.org/10.1186/s13756-020-00710-z.
-
26.
Arastehfar A, Daneshnia F, Hafez A, Khodavaisy S, Najafzadeh MJ, Charsizadeh A, et al. Antifungal susceptibility, genotyping, resistance mechanism, and clinical profile of Candida tropicalis blood isolates. Med Mycol. 2020;58(6):766-73. [PubMed ID: 31828316]. [PubMed Central ID: PMC7398758]. https://doi.org/10.1093/mmy/myz124.
-
27.
Razzaghi R, Momen-Heravi M, Erami M, Nazeri M. Candidemia in patients with prolonged fever in Kashan, Iran. Curr Med Mycol. 2016;2(3):20-6. [PubMed ID: 28681025]. [PubMed Central ID: PMC5490286]. https://doi.org/10.18869/acadpub.cmm.2.3.20.
-
28.
Sadrossadati SZ, Ghahri M, Imani Fooladi AA, Sayyahfar S, Beyraghi S, Baseri Z. Phenotypic and genotypic characterization of Candida species isolated from candideamia in Iran. Curr Med Mycol. 2018;4(2):14-20. [PubMed ID: 30324152]. [PubMed Central ID: PMC6181062]. https://doi.org/10.18502/cmm.4.2.64.
-
29.
Lindberg E, Hammarstrom H, Ataollahy N, Kondori N. Species distribution and antifungal drug susceptibilities of yeasts isolated from the blood samples of patients with candidemia. Sci Rep. 2019;9(1):3838. [PubMed ID: 30846717]. [PubMed Central ID: PMC6405987]. https://doi.org/10.1038/s41598-019-40280-8.
-
30.
Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20:5-10. https://doi.org/10.1111/1469-0691.12539.
-
31.
Ma CF, Li FQ, Shi LN, Hu YA, Wang Y, Huang M, et al. Surveillance study of species distribution, antifungal susceptibility and mortality of nosocomial candidemia in a tertiary care hospital in China. BMC Infect Dis. 2013;13:337. [PubMed ID: 23875950]. [PubMed Central ID: PMC3723814]. https://doi.org/10.1186/1471-2334-13-337.
-
32.
Chander J, Singla N, Sidhu SK, Gombar S. Epidemiology of Candida blood stream infections: experience of a tertiary care centre in North India. J Infect Dev Ctries. 2013;7(9):670-5. [PubMed ID: 24042103]. https://doi.org/10.3855/jidc.2623.
-
33.
Kooshki P, Rezaei-Matehkolaei A, Mahmoudabadi AZ. The patterns of colonization and antifungal susceptibility of Candida, isolated from preterm neonates in Khorramabad, South West of Iran. J Mycol Med. 2018;28(2):340-4. [PubMed ID: 29530715]. https://doi.org/10.1016/j.mycmed.2018.02.010.
-
34.
Shokohi T, Badali H, Amirrajab N, Ataollahi MR, Kouhpayeh SA, Afsarian MH. In vitro activity of five antifungal agents against Candida albicans isolates, Sari, Iran. Curr Med Mycol. 2016;2(2):34-9. [PubMed ID: 28681018]. [PubMed Central ID: PMC5490303]. https://doi.org/10.18869/acadpub.cmm.2.2.8.
-
35.
Kumar D, Kumar D, Priyadarshini P, Basu S, Tilak R. Distribution and susceptibility to various antifungal agents among blood stream Candida isolates from Neonatal intensive care unit. Asian Pac J Health Sci. 2018;5(3):97-102. https://doi.org/10.21276/apjhs.2018.5.3.13.
-
36.
Khairat SM, Sayed AM, Nabih M, Soliman NS, Hassan YM. Prevalence of Candida blood stream infections among children in tertiary care hospital: detection of species and antifungal susceptibility. Infect Drug Resist. 2019;12:2409-16. [PubMed ID: 31496753]. [PubMed Central ID: PMC6689130]. https://doi.org/10.2147/IDR.S196972.
-
37.
Eksi F, Gayyurhan ED, Balci I. In vitro susceptibility of Candida species to four antifungal agents assessed by the reference broth microdilution method. Sci World J. 2013;2013:236903. [PubMed ID: 24250260]. [PubMed Central ID: PMC3819922]. https://doi.org/10.1155/2013/236903.
-
38.
De Francesco MA, Piccinelli G, Gelmi M, Gargiulo F, Ravizzola G, Pinsi G, et al. Invasive candidiasis in Brescia, Italy: analysis of species distribution and antifungal susceptibilities during seven years. Mycopathologia. 2017;182(9-10):897-905. [PubMed ID: 28597394]. https://doi.org/10.1007/s11046-017-0155-3.
-
39.
Badiee P, Badali H, Boekhout T, Diba K, Moghadam AG, Hossaini Nasab A, et al. Antifungal susceptibility testing of Candida species isolated from the immunocompromised patients admitted to ten university hospitals in Iran: comparison of colonizing and infecting isolates. BMC Infect Dis. 2017;17(1):727. [PubMed ID: 29157206]. [PubMed Central ID: PMC5697407]. https://doi.org/10.1186/s12879-017-2825-7.
-
40.
Amran F, Aziz MN, Ibrahim HM, Atiqah NH, Parameswari S, Hafiza MR, et al. In vitro antifungal susceptibilities of Candida isolates from patients with invasive candidiasis in Kuala Lumpur Hospital, Malaysia. J Med Microbiol. 2011;60(Pt 9):1312-6. [PubMed ID: 21459913]. https://doi.org/10.1099/jmm.0.027631-0.
-
41.
Tavernier E, Desnos-Ollivier M, Honeyman F, Srour M, Fayard A, Cornillon J, et al. Development of echinocandin resistance in Candida krusei isolates following exposure to micafungin and caspofungin in a BM transplant unit. Bone Marrow Transplant. 2015;50(1):158-60. [PubMed ID: 25402414]. https://doi.org/10.1038/bmt.2014.230.