Abstract
Context:
First cases of Coronavirus disease 2019 (COVID-19) were reported in December 2019. With more than 100 million confirmed cases 14 months later, the disease has become the worst public-health dilemma of the century. The rapid global spread of COVID-19 has resulted in an international health emergency, threatening to overwhelm health care systems in many parts of the world, especially poor resource countries.Evidence Acquisition:
Influenza and COVID-19 have similar clinical symptoms, and both cause a respiratory illness that may vary from mild to severe. Both diseases have the same mode of transmission and require similar public health guidelines to prevent their spread, but their treatment strategies are different. In this study, an algorithmic method is proposed for managing patients according to their symptoms for each of these infections.Results:
In fall and winter, infections with seasonal influenza and other respiratory viruses become common. As influenza also causes significant morbidity and mortality, especially at the two extremes of age and in those with compromised immunity, it is of major importance to know the similarities and dissimilarities between COVID-19 and seasonal influenza and plan appropriate public health measures to deal with each of these illnesses.Conclusions:
Will there be a devastating combined epidemic of COVID-19 and influenza (COV-Flu) during the 2020 - 2021 season? Does co-infection increase the risk of severe illness or amplify virus shedding? Actually, we do not yet know the answers to these questions; so, in this article, first, we attempt to define the similarities and differences between COV-Flu. Then, we will have a brief discussion on how to manage patients presenting with symptoms suggestive of both diseases. However, as COVID-19 has been recognized as a pandemic since December 2019, the management of this emerging disease is rapidly evolving as new information is collected from different parts of the world.Keywords
1. Context
Severe acute respiratory syndrome-Coronavirus-2 (SARS-CoV-2) is not a type of influenza virus, but it may be hard to tell the difference between influenza and COVID-19 based on symptoms alone, and testing may be needed to help confirm a diagnosis (1, 2). Fortunately, the Southern hemisphere spent the cold season with the influenza (flu) outbreak severely reduced. In the Northern hemisphere, we now see a very low prevalence of influenza compared to the same time in the last year. This may have been due to the compliance with health protocols, including physical distancing, masking, and handwashing to reduce the rate of infection (3).
During the cold season, both influenza virus and SARS-CoV-2 will co-circulate in the general population. The differential diagnosis of patients presenting with the symptoms of respiratory infection will include influenza, COVID-19, or co-infection. Influenza and COVID-19 overlap clinical manifestations, and both cause respiratory illness, which may vary from mildly symptomatic to life-threatening, resulting in severe respiratory compromise and death. Both diseases have similar modes of transmission that need similar public health guidelines for transmission prevention but require different treatment strategies. In this article, we attempt to define the similarities and differences between them and describe an algorithmic approach for the management of patients presenting with symptoms compatible with either of these infections. The characteristics of both diseases, along with the differences between them, are outlined, as well (4).
2. Evidence Acquisition
2.1. Molecular Virology and Pathophysiology
Seven human coronaviruses (HCoVs) have been identified so far that cause disease in humans, namely HCOV-NL63, HCOV-HKU1, HCOV-OC43, HCOV-229E, SARS-COV, Middle East respiratory syndrome coronavirus (MERS-CoV), and novel Coronavirus (2019-nCOV) (5). Among them, SARS-CoV-2 is a single-stranded RNA virus; it is enveloped with spike (S) protein and hemagglutinin-esterase (HE) protein on the surface. The virus coat contains membrane (M) protein and envelope (E) protein, and the viral genome is attached to the nucleocapsid (N) protein (5, 6). Usually, HCoVs cause mild upper respiratory tract disease and contribute to 15 - 30% of cold cases in adults. After inhalation, the virus binds to the receptor of Angiotensin-converting enzyme 2 (ACE 2) on epithelial cells of the respiratory tract and uses lipids from the host cell to form a new virion (7). In addition to involving the lungs in the form of alveolar damage, SARS-CoV-2 causes endothelial injury in blood vessels with widespread thrombosis (8). In severe infections, the dysregulation of the immune response results in the uncontrolled secretion of cytokines, so-called “cytokine storm”, leading to multi-organ failure.
Influenza viruses, on the other hand, are classified as orthomyxoviruses, types A, B, and C. Influenza A viruses are categorized into subtypes according to two surface antigens of hemagglutinins (HA) and neuraminidase (NA). The subtypes of the virus are labeled according to these surface antigens, for instance, H1N1, H3N2, etc. Influenza A viruses have 18 HA and 11 NA subtypes. They are classified based on antigenic variances in the viral nucleoprotein (NP) and matrix protein (M). An interesting phenomenon in influenza A viruses is a rapid mutation rate, which is about 300 times the rate of other viruses, via two well-defined mechanisms, including antigenic shift and antigenic drift.
The latter results in ongoing point mutations, causing minute changes in antigens on the surface of influenza virus NA and HA that alter the antigenic coat of the virus, enabling it to elude annually acquired immunity in human beings. As an example, AH2N2 Singapore 225/99 has re-emerged as AH2N2 New Delhi 033/01. Antigenic shift occurs less often and only in influenza virus A, as opposed to antigenic drift that may happen in influenza viruses A, B, and C. In antigenic shift, the recombination of influenza genes takes place between two strains, presumably during co-infection in a single host; the change is major and drastic, forms a new subtype, makes no similarity to the previous virus, and may jump from one species to the other one. Since everybody is susceptible to the new virus, it leads to pandemics, and a new vaccine is essential for prevention (9).
2.2. Epidemiology
According to the World Health Organization (WHO), more than 100 million cases of COVID-19 have been confirmed globally, with more than two million deaths (10). Influenza has a different epidemiologic pattern, with outbreaks of varying intensity occurring every year, depending on the shifting antigenic characteristics of the virus and the proportion of susceptible individuals in the population (11).
- Incubation period: With COVID-19, the incubation period varies between two and 14 days, with an average of five to six days, while the incubation period of the flu is one to four days (average of two days) (12).
- Transmission period: Both SARS-CoV-2 and influenza viruses are transmitted through droplets, aerosols, and fomites, in some cases (13).
Significant transmission in the first 3 - 5 days of infection is common with influenza. With COVID-19, although virus shedding occurs 1 - 2 days before the appearance of symptoms, it is not considered as the main driver of infection in the population. Pre-symptomatic transmission of COVID-19 and the relative incidence of asymptomatic and symptomatic transmission are not clear because of conflicting reports. A study from a nursing home in Washington State highlighted the role of asymptomatic or pre-symptomatic transmission; the results indicated that persons with asymptomatic infection may have been responsible for the significant transmission of the disease (14). Some other recent studies also suggest that asymptomatic persons may spread the virus (15). However, according to previous reports from the WHO, “available evidence from contact tracing reported by countries suggests that asymptomatically infected individuals are much less likely to transmit the virus than those who develop symptoms” (16, 17).
Children are frequent transmitters of seasonal influenza, while data on COVID-19 shows that individuals less than 19 years of age are at low risk of infection, transmission, and morbidity; children usually get infected from adults rather than the other way round. Besides, COVID-19 spreads quickly to a lot of persons, as one infected person may be a super spreader and may transmit the infection to more than 2 - 3 people, who can again spread the infection to more individuals.
2.3. Clinical Manifestations
As both diseases are respiratory illnesses, they share clinical manifestations, but influenza starts with spiking fever, myalgia, and headache, while COVID-19 usually has an insidious onset with headache, some fever, cough, and body ache that may become worse. Some cases may just report a loss of sense of taste and/or smell in the beginning. Both influenza and COVID-19 may present with gastrointestinal symptoms, fatigue, or difficult breathing (18). It is not possible to differentiate the two diseases based on symptoms alone, and testing is necessary for a definite diagnosis (19). Although both COVID-19 and influenza (COV-Flu) may result in severe morbidity and mortality, especially in cases of extreme age, the presence of comorbidities, a compromised immune system, or pregnancy, the proportion of patients developing a severe disease is different in the two illnesses. Individuals considered high risk for influenza, but not COVID-19, are very young children less than five-years-old, especially those under two years of age and people under 19 years of age who have been on aspirin therapy for a long time (16, 20).
With COVID-19, clinical manifestations range from minimal symptoms to respiratory failure and multiple organ damage (21). Studies have shown that 80 - 95% of infected people are asymptomatic or mild, 5 - 15% are severe, requiring oxygen, and 1 - 5% requiring ventilation. Dysregulated immune response to SARS-CoV-2 is the prime cause leading to severe illness or death; in addition, the development of secondary bacterial infection may complicate the path to recovery. As compared to COVID-19, the risk of complications is higher in school-aged children infected with the influenza virus. Besides, COVID-19 is associated with thrombotic complications and may rarely cause multi-system inflammatory syndrome in children (MIS-C); these complications are not seen with influenza (19). Acute infections are correlated with the risk of developing a venous thromboembolic event (22). More than 30% of severe COVID-19 cases are afflicted with hazardously high levels of blood clotting. Studies from the Netherlands and France reported thrombotic events in 20 - 30% of critically ill COVID-19 cases (23-25). Influenza infections have been associated with procoagulant changes in about 5 - 10% of cases in the H1N1 pandemic (25).
2.4. Diagnosis
Epidemiological history, clinical symptoms, and some paraclinical findings such as nucleic acid detection, imaging, enzyme-linked immunosorbent assay (ELISA), and culture can provide a confirmation basis for COVID-19 diagnosis (26).
2.4.1. Testing
A definite diagnosis can only be made based on microbiologic testing. Several tests are performed to diagnose influenza and COVID-19 that include molecular assays, viral culture, rapid antigen tests, serologic testing, and immunofluorescence assays. The reverse transcription-polymerase chain reaction (RT-PCR) test can detect the viral RNA or nucleic acids in respiratory specimens. Among respiratory specimens for viral isolation, nasopharyngeal samples characteristically have higher accuracy than nasal or throat swab samples (27).
A positive RT-PCR from respiratory specimens clinches the diagnosis of either infection; however, false-negative results may be as high as 30 - 40%, especially in the early days of the illness in COVID-19. If the patient is on mechanical ventilation, bronchoalveolar lavage fluid should be collected for RT-PCR or other molecular tests (28). Multiplex assays can be used for the detection of nucleic acids of SARS-CoV-2, as well as influenza A and B viruses, as a single test. Who should be tested for SARS-CoV-2? The control of this pandemic can only be achieved through a rigorous ‘test, trace, and isolate’ strategy. Testing programs, however, differ in different countries, depending on available resources, government policy, and local circumstances, i.e., time, place, and availability of the test. In China, for example, 11 million people were tested in five days.
In any case, all symptomatic individuals must be tested. Asymptomatic individuals, who have been in close contact with persons with COVID-19, including neonates born to mothers with COVID, must be tested. The optimal time for testing contact is not clear, but 5 - 7 days after exposure may be suitable. However, as false-negative results may be as high as 30%, even if the test results are reported as negative, those with close contact should be quarantined as per the local health policies. If COVID-19 cases are identified in a congregate living facility, like care homes, correctional institutions, orphanages, etc., then all residents and employees in the facility must be tested. In addition, the intermittent screening of residents and workers in such facilities is recommended (29).
2.4.2. Imaging
There is a lot of overlaps in CT patterns between COVID-19 and other types of viral pneumonia (30).
- Initial CT findings in COVID-19 cases contain bilateral or multi-lobar ground-glass opacification (GGO), mostly in the lower lobes. Consolidation overlying on GGO is found in a small number of cases. Pleural thickening, septal thickening, bronchiectasis, and sub-pleural involvement are unconventional findings. Pericardial effusion, pleural effusion, lymphadenopathy, cavitation, and pneumothorax are rare findings seen in the course of the disease (31).
- Flu virus infection can cause bilateral GGO, consolidations, and crazy paving that appear similar to the picture seen in COVID-19.
Although both diseases share similar chest CT scan findings, some differences are seen. In COVID-19, the ground-glass lesions are well-defined, peripheral, and involve the lower lobes. In influenza, the distribution of lesions is random, involving all lobes, central distribution around the bronchi, and peri-bronchial cuffing (32). The chest X-ray is insensitive early in the disease but may be more useful in the follow-up of disease progression.
2.4.3. Laboratory Tests
Routine laboratory tests are not diagnostic for either of the two diseases; however, lymphopenia and elevated cytokine levels are associated with poor prognosis in COVID-19 (33, 34). If bacterial pneumonia or sepsis is suspected, a repeated test may be recommended (12).
3. Results
3.1. Management
Supportive treatment is started for all patients admitted with either of the two diseases. Our approach to COVID-19/Influenza-specific therapy in hospitalized patients depends on the severity of the disease. The treatment of influenza is the same in hospitalized patients of any age with the progressive disease of any duration and for children and adults at high risk of influenza complications. Oseltamivir should be initiated without waiting for flu testing results irrespective of suspecting co-infection with SARS-CoV-2. If bacterial pneumonia or sepsis is suspected, antibiotic treatment is given. Despite numerous ongoing clinical trials for COVID-19 in many parts of the world, definitive treatment is not yet available, although various medications to control the disease and/or regulate the immune system have been developed by the National Institute of Health (NIH) that are regularly updated as new evidence emerges (35). Current management is based on patient isolation, supportive care, supplemental oxygen, assisted ventilation as needed, antiviral agents, immunomodulator drugs, and anti-coagulants (36).
High-dose corticosteroids, monoclonal antibody, hemoperfusion, convalescent plasma, cytokine and kinase inhibitors, and other immunomodulatory agents have all been tried to control the so-called cytokine storm in severe COVID. However, the results have been conflicting, except for dexamethasone that was shown to reduce 28-day mortality by about 20% in patients who needed respiratory support, i.e., supplemental oxygen or assisted ventilation (37). The severity of COVID-19 may depend on the magnitude of the “cytokine storm” (38, 39). Several antiviral medications have been tried to treat COVID-19, and most promising reports support the use of remdesivir in hospitalized patients. The final report on the treatment of COVID-19, published in The New England Journal of Medicine in October 2020, states that remdesivir shortened the time to recovery as compared to placebo in hospitalized adults with signs of lower respiratory tract involvement with COVID-19 (40).
On October 22, 2020, the U.S. Food and Drug Administration (FDA) approved the use of remdesivir for the treatment of COVID-19 in adults and pediatrics > 12-years-old (at least 40 kg) who were hospitalized confirmed cases. The Emergency Use Authorization (EUA) for younger patients was issued in May 2020 (41). Favipiravir was initially marketed as an anti-influenza agent in Japan. In a study, favipiravir demonstrated better clinical recovery seven days after beginning treatment in the moderate form of the disease, but no significant difference was reported in the outcome of patients with severe COVID (42, 43). Interferons (IFNs), either alone or in combination with other antiviral agents, have been tried in COVID-19 with varying results, but no reduction in mortality was observed with these agents (44).
3.2. Vaccine
After more than 80 preclinical trials, several vaccines have successfully been tested in phase III clinical trials for efficacy and safety and have been approved for EUA in different countries. ‘Pfizer’ and ‘Moderna’ have been the two leading pharmaceutical companies to develop vaccines using the modern technology of utilizing messenger RNA to form spike protein that induces an immune response against the spike protein of SARS-CoV-2. Logistic problems for general availability include the huge amount of doses that are needed and global transport while maintaining the cold chain, as these vaccines need to be kept and stored at subfreezing temperatures. The production of these two vaccines has been followed by the pharmaceutical giants in other countries, notably the Astra-Zeneca vaccine from Sweden and UK that uses the inactivated virus, the Sputnik vaccine from Russia, several vaccines from China and various other vaccines. Iran is developing a vaccine as a joint project with Cuba that will soon start phase III trials. The efficacy of the approved vaccines is over 80% in most cases and > 90 - 94% in some; safety issues have largely been solved, and very few severe reactions have been reported.
The more recent vaccines are not mRNA vaccines and do not need subfreezing temperatures for storage; thus, global distribution is less problematic. However, even after the vaccines are available for the general public, preventive measures will remain necessary for the foreseeable future to control COVID-19. These include frequent handwashing, social distancing, wearing masks, and avoiding the “3Cs: spaces that are closed, crowded, or involve close contact”. New variants of the virus have been emerging in various parts of the world, which include the UK variant (B.1.1.7), the South Africa variant (B.1.351), the Brazil variants, and others. Some of these are far more transmissible and may be associated with higher morbidity. Present vaccines may have variable efficacy against these new variants, thus underscoring the importance of continuing general preventive measures and following global and local health protocols. Most vaccines that protect from viral illnesses also reduce the transmission of the virus that causes the disease by those who are vaccinated. While it is hoped this will be the case, the scientific community does not yet know if the Pfizer-BioNTech COVID-19 vaccine will reduce such transmission.
3.3. Influenza
Multiple FDA-approved vaccines are produced annually to give coverage against the influenza virus types that researchers expect to spread each year. Four classes of seasonal vaccines currently licensed include inactivated, Live-attenuated Influenza Vaccines (LAIV), recombinant HA vaccines (A & B), and cell-cultured vaccines (45).
3.4. Prognosis
The mortality rate of seasonal influenza is < 0.1% as compared to much higher mortality for COVID-19 at 3 - 4%. According to the WHO, globally, influenza may result in 290,000 to 650,000 deaths every year, while COVID-19 has already taken the lives of more than two million human beings globally (46).
3.5. Co-infection
It is crucial to know that the detection of another pathogen does not rule out infection with SARS-CoV-2. Nasopharyngeal swabs from confirmed cases of COVID-19 are positive for other bacterial or viral pathogens, most commonly, rhinovirus, enterovirus, respiratory syncytial virus, and other coronaviruses (8). Overall, secondary infections do not appear to be common complications of COVID-19, although data are limited (47).
3.6. Isolation Period
Previous guidelines from the WHO advised ending the isolation period of COVID-19 patients after two negative PCR tests that were taken at least 24 hours apart. However, in the light of current information and the lack of feasibility of performing repeated testing on hundreds of thousands of patients worldwide, both the CDC and the WHO have revised these recommendations, and now, clinical criteria for ending the isolation period for symptomatic patients are “10 days after beginning symptoms plus at least 24 hours after the end of fever”, without the use of antipyretics and other symptoms' recovery (48-50). For asymptomatic individuals, it is “10 days after a positive test for SARS-CoV-2”. For patients who are seriously immunocompromised, an infectious diseases specialist can advise for treatment. The management of patients presenting with symptoms suspected of either influenza virus or SARS-CoV-2 infection is given below along with an algorithmic approach to patients presenting with symptoms compatible with either of the two diseases (Figure 1 and 2).
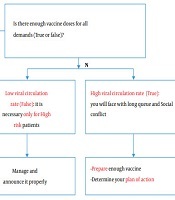
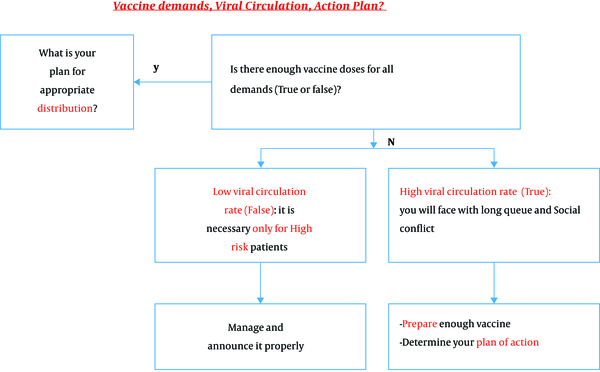
Vaccine demand, viral circulation, and action plan
Viral circulation, comorbidity, testing, and managing
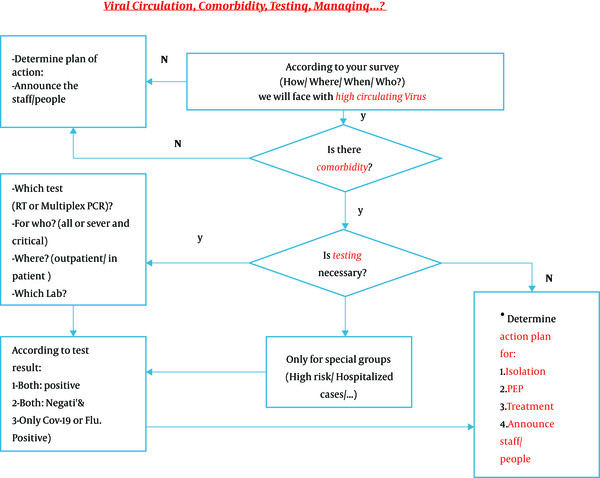
4. Management of Patients with Clinical Manifestations Compatible with Influenza Virus or SARS-Cov-2 Infection if There is a Known Outbreak of Influenza in the Community
1 - If the patient tests positive for both influenza and COVID-19: In addition to taking standard isolation precautions and personal protective equipment (PPE) for health professionals and caretakers, pharmacological treatment must be started for both infections.
2 - If testing is negative for both diseases: As tests are more sensitive for influenza than for COVID-19, patients with compatible symptoms should be managed according to the current standard recommendations for the treatment of COVID-19. Repeat testing for both diseases would be helpful in these patients.
3 - If the patient tests positive for influenza and negative for COVID-19: Standard treatment for influenza should be started, but in critical patients, treatment for both diseases must be commenced, as there seems to be > 30% chance for a false-negative result of COVID-19.
4 - If tests are negative for influenza but positive for COVID-19: Treat the patient for COVID-19 (Figure 2).
5. Conclusions
To save lives and avoid overwhelming health care systems all over the world, it is crucial to implement preventive strategies to control the spread of SARS-CoV-2 until an effective vaccine is approved and available for global distribution. These measures include frequent hand washing, social distancing, wearing masks, and crowd avoiding, but they will only be successful if they are strictly adhered to by more than 90% of the population. Although the influenza vaccine is only 40 - 60% effective, mass coverage of the population with this vaccine, combined with standard operative procedures (SOPs) against respiratory infections, will have a significant impact on decreasing morbidity and mortality in the influenza season (Figure 3).
Approach to a child with symptoms compatible with both COVID-19 and influenza
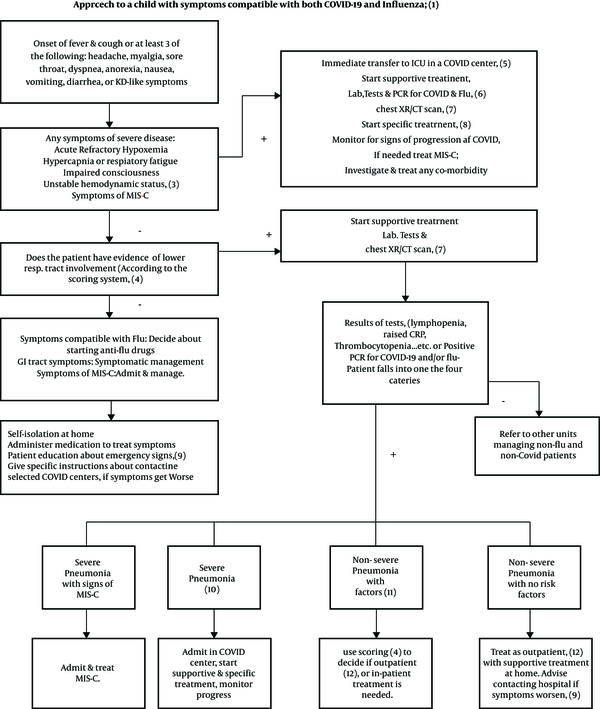
5.1. Approach to a Child with Symptoms Compatible with Both COVID-19 and Influenza
1- Definitions
COVID-19 and influenza share symptomatology.
COVID-19: Acute onset of fever, cough, or at least three of the following symptoms: headache, myalgia, fatigue, weakness, sore throat, breathlessness, GI tract symptoms.
B: Probable infection:
Possible infection plus:
• Chest X-ray/CT scan compatible with COVID-19
• Rapidly progressive pneumonia unresponsive to standard treatment
• PCR results unknown or negative
• Unexplained mortality in a patient with possible COVID-19
C: Definite infection: A patient with signs of illnesses, confirmed by a positive PCR result for either or both illnesses (51).
Influenza:
A: Possible infection
• At least two of the following: Temperature > 38°C, cough, sore throat, rhinorrhea, headache, GI tract symptoms, or unexplained irritability in a child in influenza season with unknown or negative PCR for the influenza virus, with or without
• History of contact with a person with influenza within four days before the onset of symptoms
B: Definite infection: A patient with signs of illnesses, confirmed by a positive PCR result for either or both illnesses
Rejected case of COVID-19 and influenza: A patient with a negative PCR for both illnesses, and symptoms, radiology, and lab tests compatible with a disease other than influenza or COVID-19.
2- Acute refractory hypoxemia:
SpO2 < 93% despite receiving O2 via the following devices:
• Nasal canola 5 L/min.
• Simple mask 8 - 10 L/min
• Reservoir mask 10 - 15 L/min
• Venturi mask 40 - 60%
3- Unstable hemodynamic status:
• Systolic BP < 2 SD or < fifth percentile for age
• Two or three of the following signs: Tachycardia or bradycardia (heart rate > 160/min or < 90/min for infants under one year of age and HR > 150 or < 70 in older children), capillary refill > 2 seconds, weak pulse, tachypnea, cold extremities, petechiae or purpura, hyper- or hypothermia, oliguria, and raised serum lactate.
4- It is recommended to use the Fifth National Guide to Diagnosis and Treatment of COVID-19 to make decisions about home care, continuing outpatient treatment, or referral for hospitalization (32). Undoubtedly, this system does not replace clinical judgment, and the doctor decides on the patient's condition.
5- Admission to PICU: Separate negative pressure ICU isolation rooms are desirable. If not possible, patient beds must be separated by at least two meters with individualized equipment (pulse oximeter, BP apparatus, etc.). Central air conditioning should change the room air 6 - 12 times per hour. The personnel must be protected by PPE at all times, especially when performing aerosol-generating procedures like intubation, tracheal suction, etc.
6- PCR for COVID-19 and flu: Nasopharyngeal and oropharyngeal swabs are collected by sterile Dacron swabs by trained personnel completely protected with PPE. The laboratory is notified, and the specimen is carefully transferred in a sealed protective container labeled with the patient's ID, without the danger of contaminating the personnel or the surroundings.
7- Imaging: For detailed recommendations, see the imaging part in the text.
8- Specific treatment: For detailed treatment recommendations, see the management part in the text.
9- MIS-C: Multisystem Inflammatory Syndrome in Children (MIS-C) is a rare complication associated with COVID-19. The CDC criteria for MIS-C include an individual < 21-years-old positive for exposure to or confirmed SARS-CoV-2 infection with fever, severe illness with multisystem (> 2 systems) organ involvement, and laboratory results compatible with inflammation plus no evidence of another diagnosis. Common clinical manifestations may be similar to those of Kawasaki disease, toxic shock syndrome, thrombosis, cardiac and GI symptoms, or acute kidney injury (52). Symptoms may start weeks after infection from a known case or an asymptomatic carrier of the virus. Treatment strategies are still changing but, at present, are largely based on respiratory support, fluid and inotropic resuscitation, and ECMO in severe cases. Besides, IVIG, steroids, and anti-coagulants are used to suppress the hyper-inflammatory response (16). After recovery, children need close follow-up, including cardiology follow-up for a few weeks. Management guidelines are available at the American Academy of Pediatrics and American College of Rheumatology websites (52, 53).
10- Emergency signs: The patient is advised to contact a hospital if any of the following signs appear:
• Respiratory signs: Tachypnea, respiratory distress, cyanosis
• Inability to eat or drink
• Irritability
• Lethargic when awake
• Symptoms of dehydration
• Fever ≥ 38.5°C or lasting for > 5 days
• Recrudescence of symptoms after getting better
11- Severe pneumonia: Symptoms of severe pneumonia include high fever, altered consciousness, cyanosis, hypoxia with SpO2 < 90% in room air, respiratory distress, tachycardia, delayed capillary refill, dehydration, or pneumonia, along with one of the emergency signs.
12- Non-severe pneumonia with risk factors: If pneumonia exists without severe symptoms but risk factors present. Risk factors for COVID-19 include immunocompromised patients on immunosuppressive medications or with co-morbidities like diabetes, chronic pulmonary disease like CF, or moderate to severe asthma, cardiovascular disease, chronic hepatic or renal disease, hematologic diseases like thalassemia, neurodevelopmental diseases, hypertension, and morbid obesity (BMI > 30). For influenza, very young age (< 5 years) and long-term aspirin use are the additional risk factors.
13- Outpatients: A scoring system is useful in treating outpatients. The time to discharge patients from the hospital depends on the availability of hospital beds and trained personnel in addition to the patient’s general condition. It is recommended that all of the following conditions must be met before the patient is discharged:
• Absence of fever for at least 24 hours before discharge without the use of antipyretics
• Stable hemodynamic status with subsidence of respiratory symptoms like cough
• SpO2 > 93% in room air (in children without preexisting respiratory or cardiac co-morbidity)
• No need for intravenous medication
• CBC normal or improving, CRP decreased by 50% and ESR by 20%
• If chest X-R done, no new lesions on CXR
• Repeat RT-PCR is not needed for discharge, except for special cases like immunocompromised or institutionalized children or those recovering from very severe disease (Figure 3).
References
-
1.
Capone A. Simultaneous circulation of COVID-19 and flu in Italy: Potential combined effects on the risk of death? Int J Infect Dis. 2020;99:393-6. [PubMed ID: 32768696]. [PubMed Central ID: PMC7405819]. https://doi.org/10.1016/j.ijid.2020.07.077.
-
2.
Shatizadeh Malekshahi S, Shafiei-Jandaghi NZ, Yavarian J, Shadab A, Naseri M, Mokhtari Azad T. Detection of respiratory co-infections in children less than five years with adenovirus infection. Arch Pediatr Infect Dis. 2016;5(1). https://doi.org/10.5812/pedinfect.36953.
-
3.
World Health Organization. FluNet Summary. Geneva, Switzerland: WHO; 2020, [updated 15th Mar 2021; cited 28th Sep]. Available from: https://www.who.int/influenza/gisrs_laboratory/updates/summaryreport/en/.
-
4.
Mayo Clinic Staff. Coronavirus vs. flu: Similarities and differences. Rochester, New York State, USA: Mayo Clinic; 2020, [updated 3rd Mar 2021; cited 12th Nov]. Available from: https://www.mayoclinic.org/coronavirus-vs-flu/art-20490339.
-
5.
Liu DX, Liang JQ, Fung TS. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae). In: Bamford DH, Zuckerman M, editors. Encyclopedia of virology. 2. 4 ed. Cambridge, Massachusetts, United States: Academic Press; 2021. p. 428-40. https://doi.org/10.1016/b978-0-12-809633-8.21501-x.
-
6.
Azimi L, Karimi A, Alebouyeh M, Shirvani A, Maham S, Rafiei Tabatabaei S, et al. Current concepts on immunopathology of COVID-19 and emerging therapies. Arch Pediatr Infect Dis. 2020;8(3). e104266. https://doi.org/10.5812/pedinfect.104266.
-
7.
Shah VK, Firmal P, Alam A, Ganguly D, Chattopadhyay S. Overview of immune response during SARS-CoV-2 infection: Lessons from the past. Front Immunol. 2020;11:1949. [PubMed ID: 32849654]. [PubMed Central ID: PMC7426442]. https://doi.org/10.3389/fimmu.2020.01949.
-
8.
European Centre for Disease Prevention and Control. Infection. Latest evidence on COVID-19. Solna, Sweden: European Centre for Disease Prevention and Control; 2020, [updated 30th Jun 2020; cited 27th Oct]. Available from: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/infection.
-
9.
Hien H Nguyen, Robert W Derlet, Christian E Sandrock. Influenza. New York, USA: Medscape; 2020, [updated 24th Mar 2021; cited 7th Aug]. Available from: https://emedicine.medscape.com/article/219557-overview.
-
10.
World Health Organization. Weekly operational update on COVID-19. Geneva, Switzerland: WHO; 2020, [updated 29th Mar 2021; cited 6th Nov]. Available from: https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19---29-march-2021.
-
11.
Kim H, Webster RG, Webby RJ. Influenza virus: Dealing with a drifting and shifting pathogen. Viral Immunol. 2018;31(2):174-83. [PubMed ID: 29373086]. https://doi.org/10.1089/vim.2017.0141.
-
12.
Ohadian Moghadam S, Afshar D. A review on coronavirus disease 2019 (COVID-19) in pediatric patients. Arch Pediatr Infect Dis. 2020;8(3). e104225. https://doi.org/10.5812/pedinfect.104225.
-
13.
Hashemieh M. Blood safety in SARS-CoV-2 infection. Arch Pediatr Infect Dis. 2020;8(3). e104525. https://doi.org/10.5812/pedinfect.104525.
-
14.
Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the achilles' heel of current strategies to control Covid-19. N Engl J Med. 2020;382(22):2158-60. [PubMed ID: 32329972]. [PubMed Central ID: PMC7200054]. https://doi.org/10.1056/NEJMe2009758.
-
15.
Centers for Disease and Preventation. How to protect yourself and others. Atlanta, Georgia, United States: Centers for Disease and Preventation; 2020, [updated 8th Mar 2021; cited 13th Oct]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html.
-
16.
Tabatabaii SA, Soltani P, Khanbabaee G, Sharma D, Valizadeh R, Farahbakhsh N, et al. SARS Coronavirus 2, severe acute respiratory syndrome, and middle east respiratory syndrome in children: A review on epidemiology, clinical presentation, and diagnosis. Arch Pediatr Infect Dis. 2020;8(4). e104860. https://doi.org/10.5812/apid.104860.
-
17.
World Health Organization. Coronavirus disease 2019 (COVID-19) situation report - 73. Geneva, Switzerland: WHO; 2020, [updated 2nd Apr 2020; cited 2nd Apr]. Available from: https://www.who.int/publications/m/item/situation-report---73.
-
18.
Mirahmadizadeh A, Borazjani R, Ebrahimi M, Hashemizadeh Haghighi L, Kamali K, Hamzavi SS, et al. COVID-19 presented with gastrointestinal manifestations in an 11-days-old neonate: A case report and review of the literature. 10.5812/pedinfect.104508. 2020;8(3). e104508. https://doi.org/10.5812/pedinfect.104508.
-
19.
Centers for Disease and Preventation. Similarities and differences between flu and COVID-19. Atlanta, Georgia, United States: Centers for Disease and Preventation; 2020, [updated 27th Jan 2021; cited 6th Oct]. Available from: https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm.
-
20.
Karimi A, Rafiei Tabatabaei S, Pourmoghaddas Z. Applied clinical algorithm of influenza: Management of influenza in young people. J Iran Med Council. 2018;1(1):2-6.
-
21.
Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. [PubMed ID: 32325252]. [PubMed Central ID: PMC7169933]. https://doi.org/10.1016/j.clim.2020.108427.
-
22.
van Wissen M, Keller TT, Ronkes B, Gerdes VE, Zaaijer HL, van Gorp EC, et al. Influenza infection and risk of acute pulmonary embolism. Thromb J. 2007;5:16. [PubMed ID: 17939867]. [PubMed Central ID: PMC2104525]. https://doi.org/10.1186/1477-9560-5-16.
-
23.
Lemke G, Silverman GJ. Blood clots and TAM receptor signalling in COVID-19 pathogenesis. Nat Rev Immunol. 2020;20(7):395-6. [PubMed ID: 32488201]. [PubMed Central ID: PMC7264968]. https://doi.org/10.1038/s41577-020-0354-x.
-
24.
Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-7. [PubMed ID: 32291094]. [PubMed Central ID: PMC7146714]. https://doi.org/10.1016/j.thromres.2020.04.013.
-
25.
Bunce PE, High SM, Nadjafi M, Stanley K, Liles WC, Christian MD. Pandemic H1N1 influenza infection and vascular thrombosis. Clin Infect Dis. 2011;52(2):e14-7. [PubMed ID: 21288835]. https://doi.org/10.1093/cid/ciq125.
-
26.
Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102-8. [PubMed ID: 32282863]. [PubMed Central ID: PMC7104082]. https://doi.org/10.1016/j.jpha.2020.03.001.
-
27.
Shirvani A, Azimi L, Mansour Ghanaie R, Alebouyeh M, Fallah F, Rafiei Tabatabaei S, et al. Utility of available methods for diagnosing SARSCoV-2 in clinical samples. Arch Pediatr Infect Dis. 2020;8(3). e103677. https://doi.org/10.5812/pedinfect.103677.
-
28.
Centers for Disease and Preventation. Influenza signs and symptoms and the role of laboratory diagnostics. Atlanta, Georgia, United States: Centers for Disease and Preventation; 2020, [updated 31st Aug 2020; cited 31st Aug]. Available from: https://www.cdc.gov/flu/professionals/diagnosis/labrolesprocedures.htm.
-
29.
Caliendo AM, Hanson KE. COVID-19: Diagnosis. Waltham, Massachusetts, United States: UpToDate; 2020, [updated 26th Feb 2021; cited 30th Oct]. Available from: https://www.uptodate.com/contents/covid-19-diagnosis?search=coronavirus-disease-2019-covid-19%20diagnosis&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1.
-
30.
Radiology Assistant. COVID-19 differential diagnosis. Netherlands: Radiology Assistant; 2020, [cited 10th Mar]. Available from: https://radiologyassistant.nl/chest/covid-19/covid-19-differential-diagnosis.
-
31.
Radiology Assistant. COVID-19 imaging findings. Netherlands: radiology Assistant; 2020. Available from: https://radiologyassistant.nl/chest/covid-19/covid19-imaging-findings.
-
32.
Vice- Chancellor in Treatment Affairs. [Instructions]. Tehran, Iran: Shahid Beheshti University of Medical Sciences; 2020, [updated 16th Mar 2021; cited 19th Oct]. Persian. Available from: http://treatment.sbmu.ac.ir/index.jsp?pageid=63989&p=1.
-
33.
Tavakolpour S, Rakhshandehroo T, Wei EX, Rashidian M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol Lett. 2020;225:31-2. [PubMed ID: 32569607]. [PubMed Central ID: PMC7305732]. https://doi.org/10.1016/j.imlet.2020.06.013.
-
34.
Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. J Intensive Care. 2020;8:36. [PubMed ID: 32483488]. [PubMed Central ID: PMC7245646]. https://doi.org/10.1186/s40560-020-00453-4.
-
35.
Centers for Disease and Preventation. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Atlanta, Georgia, United States: Centers for Disease and Preventation; 2020, [updated 16th Feb 2021; cited 3rd Nov]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html.
-
36.
World Health Organization. COVID-19 clinical management: Living guidance. Geneva, Switzerland: WHO; 2020.
-
37.
Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19-preliminary report. N Engl J Med. 2020;384(8):693-704. [PubMed ID: 32678530]. [PubMed Central ID: PMC7383595]. https://doi.org/10.1101/2020.06.22.20137273.
-
38.
Heimfarth L, Serafini MR, Martins-Filho PR, Quintans JSS, Quintans-Junior LJ. Drug repurposing and cytokine management in response to COVID-19: A review. Int Immunopharmacol. 2020;88:106947. [PubMed ID: 32919216]. [PubMed Central ID: PMC7457938]. https://doi.org/10.1016/j.intimp.2020.106947.
-
39.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [PubMed ID: 31986264]. [PubMed Central ID: PMC7159299]. https://doi.org/10.1016/S0140-6736(20)30183-5.
-
40.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - Final Report. N Engl J Med. 2020;383(19):1813-26. [PubMed ID: 32445440]. [PubMed Central ID: PMC7262788]. https://doi.org/10.1056/NEJMoa2007764.
-
41.
FDA. FDA approves remdesivir, first treatment for COVID‐19. The Brown University Child & Adolescent Psychopharmacology Update. 2020;22(12):7-8. https://doi.org/10.1002/cpu.30542.
-
42.
Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, et al. Favipiravir versus arbidol for COVID-19: A randomized clinical trial. medRxiv. 2020;Inpress. https://doi.org/10.1101/2020.03.17.20037432.
-
43.
Agrawal U, Raju R, Udwadia ZF. Favipiravir: A new and emerging antiviral option in COVID-19. Med J Armed Forces India. 2020;76(4):370-6. [PubMed ID: 32895599]. [PubMed Central ID: PMC7467067]. https://doi.org/10.1016/j.mjafi.2020.08.004.
-
44.
Haji Abdolvahab M, Moradi-Kalbolandi S, Zarei M, Bose D, Majidzadeh AK, Farahmand L. Potential role of interferons in treating COVID-19 patients. Int Immunopharmacol. 2021;90:107171. [PubMed ID: 33221168]. [PubMed Central ID: PMC7608019]. https://doi.org/10.1016/j.intimp.2020.107171.
-
45.
Centers for Disease and Preventation. Types of seasonal influenza vaccine. Atlanta, Georgia, United States: Centers for Disease and Preventation; 2019, [cited 11th Oct]. Available from: https://www.ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/types-of-seasonal-influenza-vaccine.
-
46.
World Health Organization. Coronavirus disease (COVID-19): Similarities and differences with influenza. Geneva, Switzerland: WHO; 2020, [updated 17th Mar 2020; cited 17th Mar]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19-similarities-and-differences-with-influenza.
-
47.
McIntosh K. COVID-19: Clinical features. Waltham, Massachusetts, United States: UpToDate; 2020, [updated 18th Feb 2021; cited 28th Oct]. Available from: https://www.uptodate.com/contents/covid-19-clinical-features.
-
48.
World Health Organization. Criteria for releasing COVID-19 patients from isolation. Geneva, Switzerland: WHO; 2020, [updated 17th Jun 2020; cited 17th Jun]. Available from: https://www.who.int/news-room/commentaries/detail/criteria-for-releasing-covid-19-patients-from-isolation.
-
49.
Communicable Disease Epidemiology and Immunization Section. Updated guidance regarding discontinuation of transmission-based precautions of patients with COVID-19 in healthcare settings and criteria for return to work for healthcare personnel with COVID-19. Washington State, USA: Public Health Seattle & King County; 2020, [updated 5th Dec 2020; cited 22nd Jul]. Available from: https://www.kingcounty.gov/depts/health/communicable-diseases/health-care-providers/advisories/2020/22-july.aspx.
-
50.
Centers for Disease and Preventation. Interim Guidance on Duration of Isolation and Precautions for Adults with COVID-19. Atlanta, Georgia, United States: Centers for Disease and Preventation; 2020, [updated 13th Feb 2021; cited 19th Oct]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html.
-
51.
Karimi A, Rafiei Tabatabaei S, Rajabnejad M, Pourmoghaddas Z, Rahimi H, Armin S, et al. An algorithmic approach to diagnosis and treatment of coronavirus disease 2019 (COVID-19) in children: Iranian expert’s consensus statement. Arch Pediatr Infect Dis. 2020;8(2). e102400. https://doi.org/10.5812/pedinfect.102400.
-
52.
American Academy of Pediatrics. Multisystem inflammatory syndrome in children (MIS-C) interim guidance. Itasca, Illinois, United States: American Academy of Pediatrics; 2020, [updated 10th Feb 2021; cited 17th Nov]. Available from: https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/multisystem-inflammatory-syndrome-in-children-mis-c-interim-guidance/.
-
53.
Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: Version 1. Arthritis Rheumatol. 2020;72(11):1791-805. [PubMed ID: 32705809]. [PubMed Central ID: PMC7405113]. https://doi.org/10.1002/art.41454.