Abstract
Context:
Evaluating the prevalence of Staphylococcus aureus nasal carriage and methicillin-resistant S. aureus (MRSA) that are sources of nosocomial infection among medical students.Evidence Acquisition:
Electronic databases were searched by preferred subject headings and free-text keywords. After omitting duplicates, retrieved articles were screened by two independent reviewers in a three-step process based on inclusion criteria. Then, reviewers critically appraised the selected studies by JBI checklists and extracted the required data. Finally, the pooled prevalence rates of S. aureus nasal carriage and MRSA were meta-analyzed by Stata V.16 software. The heterogeneity of included studies was calculated by I2 and chi-square. Subgroup analysis was carried out according to study designs, as well as the continent origin of clinical and preclinical students.Results:
Of 858 retrieved studies, 15 were included in the meta-analysis. The results showed that the pooled prevalence of nasal S. aureus carriage was 28% [prevalence rate: 0.028, 95% CI: 0.21 - 0.34, P < 0.001, I2: 96.40%, chi2: 360.98 (df = 14)]. The prevalence of S. aureus among clinical students was 33% (pooled prevalence rate: 0.33, 95% CI: 0.18 - 0.47) whereas, in preclinical students, it was 25% (pooled prevalence rate: 0.25, 95% CI: 0.23 - 0.28). Also, in a subgroup analysis of continents, Australia (Oceania) had the highest prevalence rate. According to an evaluation of publication bias, the distribution of studies was very high. Moreover, pooled MRSA prevalence among medical students was 2% (prevalence rate: 0.02 95% CI: 0.01 - 0.03, P < 0.001).Conclusions:
In this meta-analysis, S. aureus and MRSA prevalence rates among medical students were estimated at 28% and 2%, respectively. More attention should be given to the prevention of MRSA colonization and screening strategies among medical students across the world.Keywords
Meta-analysis Staphylococcus aureus Methicillin-resistant Prevalence Systematic Review
1. Context
Staphylococcus aureus is an important human bacterial pathogen that is often found in the skin and the upper respiratory tract (1). It is known to be one of the main bacterial agents responsible for nosocomial and community infections (2). Methicillin-resistant S. aureus (MRSA) was reported for the first time in the 1960s and spread rapidly in the 1980s (3). Over the past 45 years, hospital-related MRSA clones and community-acquired MRSA (CA-MRSA) spread around the world. Without taking any specific control measures, the risk of occurrence of an epidemic with these strains is high (4). The first cases of CA-MRSA among children were reported in the late 1990s (5). It has been documented that this infection is more prevalent in gyms, military bases, and newborn nurseries. Moreover, it has been reported in homosexuals (6).
Carrying S. aureus pathogens in the nose increases the risk of infection, especially in the hospital setting (7), and is the most important risk factor for the transmission of this pathogen (8). Studies on the transmission of S. aureus by hospitalized patients in Madagascar showed that the prevalence of MRSA was between 4 and 13%, and the prevalence of S. aureus in outpatients was reported to be 38%, of which 15% were MRSA cases (9). Another study in Brazil revealed that the nasal carriage of S. aureus is 40.8% of which, 5.8% were MRSA (10). Medical staff members, including medical students, act as a bridge between the hospital and the community. Nasal carriage of S. aureus, especially CA-MRSA, has recently been proposed by medical students as a possible mechanism for increasing the transmission of these species between hospitals and the community (11). The prevalence of MRSA carriage among hospital staff is associated with the length of stay in the ward. Several studies on the prevalence of S. aureus nasal carriage have been published by medical students in recent decades (7, 10-12).
Several studies have also been conducted to estimate the prevalence of S. aureus in nasal carriage worldwide, including some national studies about the estimation of S. aureus nasal carriage by medical students, which shows the need for a comprehensive study for pooling their reported data in this field. In this approach, several systematic reviews and meta-analyses have been published; however, different health-related populations have been investigated in them, except for medical students. For example, Emaneini et al. (13) carried out a systematic review and meta-analysis on nasal carriage rates of S. aureus and MRSA among Iranian healthcare workers. Their study showed the prevalence of S. aureus to be 22.7% and MRSA to be 32.8% among Iranian healthcare workers (13).
In addition, in another systematic review and meta-analysis, the prevalence of community-associated methicillin-resistant S. aureus carriage in the Asia-Pacific region from 2000 to 2016 has been investigated, which shows a prevalence of 0% to 23.5% in the general public and 0.7% to 10.4% in hospital settings (14). The pooled data in S. aureus and MRSA nasal carriage will help the world’s policymakers and health authorities to conduct more useful strategies to reduce the burden of the infection and reach the ultimate goal of healthier medical staff and students. For this purpose, the current systematic review evaluated studies and pooled their data on the prevalence of S. aureus and MRSA nasal carriage among medical students.
2. Evidence Acquisition
This systematic review was performed based on the JBI methodology for a systematic review of prevalence evidence (15). In addition, the method of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was utilized in this project (16).
2.1. Review Questions
The questions of this review were as follows:
What is the prevalence of S. aureus among medical students?
What is the prevalence of MRSA among medical students?
2.2. Inclusion Criteria
2.2.1. Participants
Studies including medical students (preclinical or clinical) as the population were considered in this review.
2.2.2. Condition
Studies in which the prevalence of S. aureus and MRSA nasal carriage were evaluated. We excluded all studies evaluating only the skin or pharyngeal samples.
2.2.3. Context
Studies performed in the medical education setting, including the hospital or university campus, were included in this review.
2.2.4. Types of Studies
All analytical-observational studies, including prospective and retrospective cohorts, as well as case-control, analytical, and descriptive cross-sectional studies, were included in this review. Moreover, studies published in English from 1967 were considered for inclusion in the current review.
2.2.5. Search Strategy
To find the studies (published and unpublished) on the subject, a three-stage method was used. In the first stage, the PubMed database was searched limitedly. In the next stage, the words were searched in the titles and abstracts, as well as the index terms used to describe the articles. The final search was conducted electronically using all detected keywords and index terms. This step was implemented on January 26, 2020, through the following databases: Medline (PubMed), Embase, Scopus, and Web of Science. The unpublished studies and gray literature such as ProQuest (dissertations and theses) and google scholar were searched, as well. In the final stage, the lists of references of all reports and studies of the review were investigated to find any other articles. The strategy by which the full search was performed in PubMed and Embase databases is provided in Appendix 1 in Supplementary File.
2.2.6. Study Selection
After searching, the detected citations were entered in Endnote software version X7.1.3, and the duplicate titles were omitted. Then, two independent critics reviewed and screened the titles and abstracts to make sure of the qualification of the studies concerning the inclusion criteria for the review. The full-texts of the selected studies were obtained and investigated in full detail by two reviewers, and the inclusion criteria were assessed for them. The full-text articles not having the inclusion criteria were omitted from the research. Any disagreements between the two reviewers were resolved using sessions of discussion.
2.2.7. Assessment of Methodological Quality
Two independent reviewers critically reviewed the possible articles for the study using standard critical reviewing tools obtained from the Joanna Briggs Institute Studies Reporting Prevalence Data (17). Any disagreements between the two reviewers were resolved by holding discussion sessions. All studies that were assessed as moderate or high level in terms of quality were included in this review.
2.2.8. Data Extraction
Two independent reviewers were asked to extract the needed information from the studies with the inclusion criteria using a modified standardized JBI data extraction tool (15). The extracted data included information on populations, sample size, study methods, and publication year, as well as the region of the study, mean age, gender, and measurement of outcomes and prevalence of S. aureus and MRSA among students of medicine. Any disagreements that arose between the critics were resolved using discussion sessions. In addition, the corresponding authors of the articles were contacted for missing data and additional information.
2.2.9. Data Synthesis
Data were pooled using statistical meta-analysis with Stata software version 16. The effect size was reported using the event rate (pooled prevalence rate). In addition, a confidence level of 95% was reached to begin the meta-analysis. The heterogeneity of the studies was calculated using the standard chi-square test, as well as the I2 test for heterogeneity. Statistical analyses were conducted by the random-effects method (18). Moreover, subgroup analyses were performed based on study designs and continents in which the study was performed. A sensitivity analysis was performed to locate heterogeneous studies. A funnel plot was generated in Stata software version 16 to assess publication bias.
3. Results
3.1. Study Inclusion
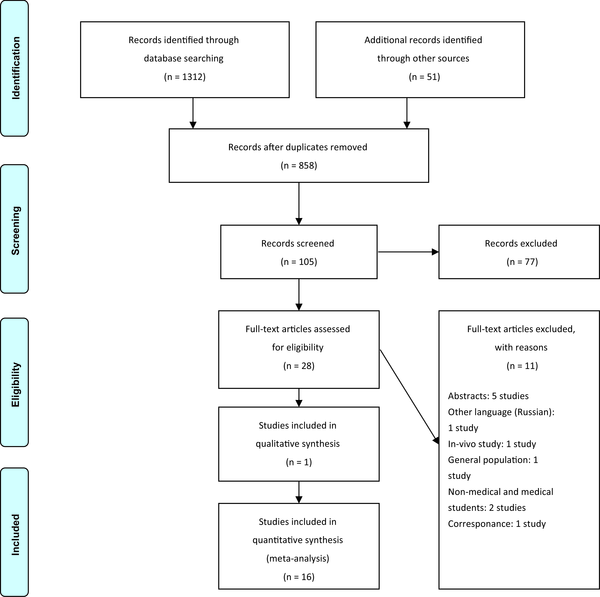
From comprehensive searching, 1,312 studies were retrieved in the electronic search and 51 studies with additional methods. Then, they were imported in Endnote X7.1.3, and duplicated records were removed. In the three steps of screening (title, abstract, and full-text), two expert reviewers selected 28 studies, of which 17 studies remained finally for the critical appraising process. You can find this selection process in PRISMA flowchart 1. Also, the reasons for excluding the articles in the full-text step are presented in Figure 1.
PRISMA flowchart of the selection process (16)
3.2. Methodological Quality
Totally, 16 studies were critically appraised by JBI appraisal tools for prevalence and cohort studies to evaluate the risk of biases. On that account, the quality of nine studies was assessed as high (19-27), six studies as moderate (28-33), and one study as low (34). The study with the lowest quality was excluded from this systematic review and meta-analysis (34) (Appendix 2 in Supplementary File; Table 1). Fifty percent of these studies had not appropriately sampled their populations. In most of them, they had collected participants voluntarily.
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Bhatta et al. (19) | Y | U | Y | Y | Y | Y | Y | Y | Y | Highb |
| Ansari et al. (20) | Y | Y | Y | Y | Y | Y | Y | Y | Y | High |
| Collazos Marin et al. (21) | Y | Y | Y | Y | Y | Y | Y | Y | Y | High |
| Manipura et al. (22) | N | Y | Y | Y | Y | Y | Y | Y | Y | High |
| Hogan et al. (23) | Y | Y | Y | Y | Y | N | Y | Y | Y | High |
| Bettin et al. (24) | Y | Y | Y | Y | Y | Y | Y | Y | Y | High |
| Santhosh et al. (28) | N | U | Y | N | Y | Y | Y | Y | Y | Moderatec |
| Abroo et al. (29) | N | N | Y | N | Y | Y | Y | Y | Y | Moderate |
| Conceicao et al. (34) | N | N | N | N | U | Y | U | Y | Y | Lowd |
| Syafinaz et al. (25) | Y | Y | Y | N | Y | Y | Y | Y | Y | High |
| Zakai (26) | Y | U | Y | Y | Y | Y | Y | U | Y | High |
| Szymanek-Majchrzak et al. (30) | N | Y | Y | N | Y | Y | Y | N | Y | Moderate |
| Stubbs et al. (31) | N | N | Y | N | Y | Y | Y | Y | Y | Moderate |
| Shreyas et al. (27) | Y | Y | Y | N | Y | Y | Y | Y | Y | High |
| Gualdoni et al. (32) | N | N | N | Y | Y | Y | Y | U | Y | Moderate |
| Trepanier et al. (33) | Y | N | Y | Y | N | Y | Y | Y | N | Moderate |
| Total, % | 56.25 | 50 | 87.5 | 56.25 | 87.5 | 93.75 | 93.75 | 81.25 | 93.75 |
3.3. Characteristics of Included Studies
Finally, after critical appraising of 16 studies, 15 studies were included. The study designs were observational, in which two studies were cohort (28, 32) and the others were cross-sectional (19, 26, 28-30, 33, 34). The characteristics of the included studies are presented in Table 2.
Characteristics of Included Studies
| Number | Author | Year | Study Design | Country | Study Population | Sample Size | Subject Characteristics | Study Duration | Methods for Outcome Measurement | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Males | Number of Females | Age | |||||||||
| 1 | Bhatta et al. (19) | 2018 | Cross-sectional | Nepal | Clinical and preclinical (first year, interns) | 200 (100 preclinical, 100 clinical) | A: 59; B: 61 | A: 41; B: 39 | A: 18 - 25; B: 22 - 30 | 5 | Nasal and pharyngeal swabs |
| 2 | Ansari et al. (20) | 2016 | Cross-sectional | Nepal | Clinical and preclinical (first year, interns) | 200 (100 preclinical, 100 clinical) | 105 | 95 | - | 1 | Nasal swabs |
| 3 | Collazos Marin et al. (21) | 2015 | Cross-sectional | Colombia | Clinical and preclinical (first year, interns) in hospital practices | 216 | 97 | 119 | 3 | Skin and nasal swabs | |
| 4 | Manipura et al. (22) | 2016 | Cross-sectional | India | Medical students (second year) | 148 | 63 | 85 | 19 - 22 | Nasal swabs | |
| 5 | Hogan et al. (23) | 2016 | Cross-sectional | Madagascar | Nonmedical students (5 different hospitals) | 1548 (685 students) | 245 | 440 | - | - | Nasal swabs |
| 6 | Bettin et al. (24) | 2012 | Cross-sectional | Colombia | Medical student | 372 | - | - | 15 - 26 (19 ± 2.21) | 6 | Nasal swabs |
| 7 | Santhosh et al. (28) | 2008 | Cohort study | India | Preclinical students | 157 | 65 | 92 | 18 - 22 | Nasal swabs | |
| 8 | Abroo et al. (29) | 2017 | Cross-sectional | Iran | Medical students (basic medical science course) | 350 | 225 | 125 | 18 - 46 | 36 | Nasal swabs |
| 9 | Conceicao et al. (34) | 2017 | Cohort study | Portugal | Nursing student | 47 | - | - | - | 48 | Nasal swabs |
| 10 | Suhaili et al. (35) | 2012 | Cross-sectional | Malaysia | Preclinical and clinical students | 209 | 81 | 128 | - | 6 | Nasal swabs |
| 11 | Zakai (26) | 2015 | Cross-sectional | Saudi Arabia | Clinical students | 150 (intern) and 32 (preclinical) | 77 | 73 | - | 6 | Nasal swabs |
| 12 | Szymanek-Majchrzak et al. (30) | 2019 | Cross-sectional | Poland | Preclinical students | 955 | 377 | 578 | 22 | 24 | Nasal swabs |
| 13 | Stubbs et al. (31) | 1994 | Cross-sectional | Australia | Preclinical and clinical | 808 | A: 124; B: 132; C: 109; D: 142; E: 30 | A: 69; B: 63; C: 60; D: 64; E: 15 | - | 1994 | Nasal swabs |
| 14 | Shreyas et al. (27) | 2017 | Cross-sectional | India | Interns | 150 | 78 | 72 | - | 2 | Nasal swabs |
| 15 | Gualdoni et al. (32) | 2012 | Cohort study | Austria | Medical students (clinical) | 79 | - | - | - | - | Nasal swabs |
| 16 | Trepanier et al. (33) | 2013 | Cross-sectional | Canada | Medical students and residents | 250 | 155 (medical stu: 68 (27.5%), resident: 87 (34.8%) | 95 (medical stu: 72.5%, resident: 65.2%) | Medical students: 21; residents: 26 | 3 | Nasal swabs |
3.4. Staphylococcus aureus Prevalence
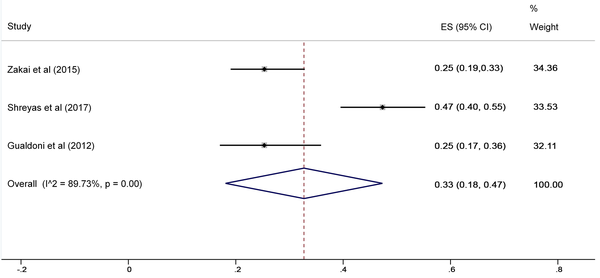
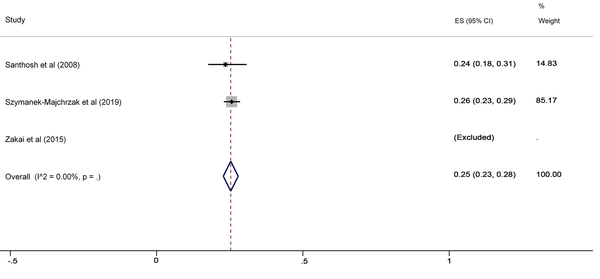
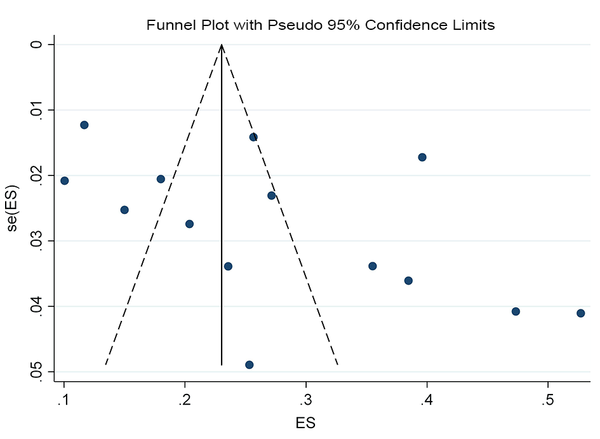
The SA prevalence was reported in 15 studies that were included in the meta-analysis. According to the results, the pooled prevalence of nasal S. aureus was 28% (prevalence: 0.028, 95% CI: 0.21 - 0.34, P < 0.001), which was varied from 10 to 72%. Moreover, the calculated heterogeneity was very high (I2: 96.40%, chi2: 360.98 (df = 14) P < 0.001) (Figure 2). Subgroup analysis based on study design was also performed in this analysis, which demonstrated 28% in cross-sectional studies and 24% in cohort studies. Furthermore, the prevalence of S. aureus among clinical students was 33% [pooled prevalence: 0.33, 95% CI: 0.18 - 0.47]; however, it was 25% among preclinical students [pooled prevalence: 0.25, 95% CI: 0.23 - 0.28] (Figure 3). In addition, subgroup analysis based on continents showed that Oceania had the highest prevalence rate of S. aureus nasal carriage (40%), and Asia (30%), Europe (26%), America (24%), and Africa (12%) had lower prevalence rates of S. aureus nasal carriage (Figure 4). Moreover, the results of the meta-analysis showed that this rate was 33% among clinical students (Figure 5) and 25% among preclinical students (Figure 6). Furthermore, to evaluate publication bias, a funnel plot was drawn. It showed that the distribution rate of studies was very high (Figure 7).
Staphylococcus aureus prevalence among medical students
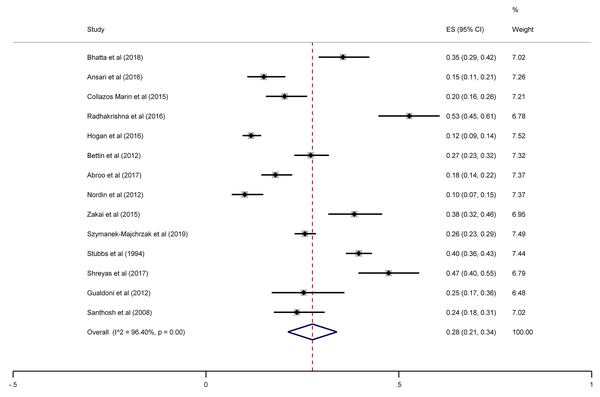
Staphylococcus aureus prevalence among medical students, sub-grouped by study design
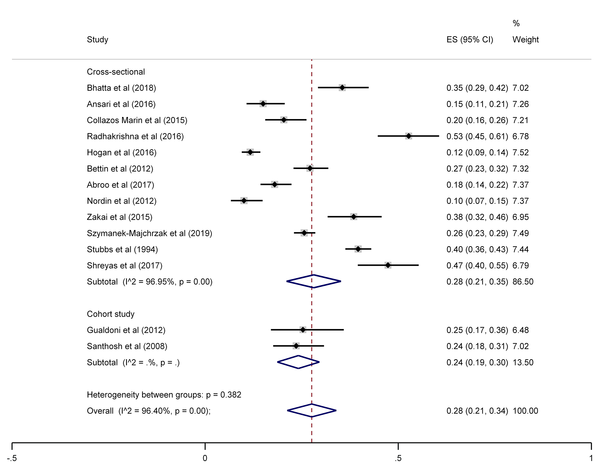
Staphylococcus aureus prevalence among medical students, sub-grouped by continent
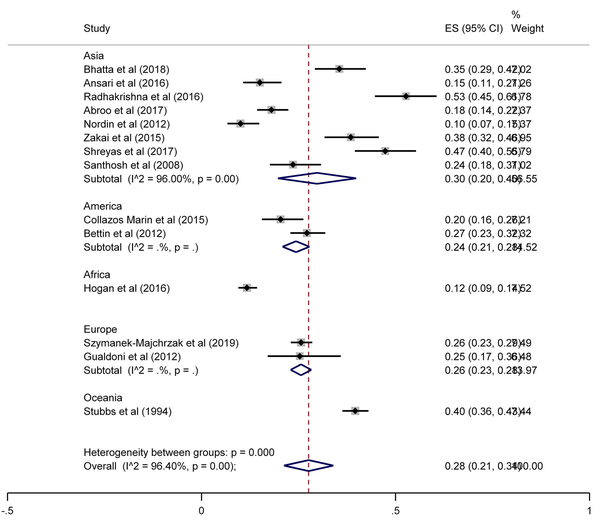
Staphylococcus aureus prevalence among clinical students
Staphylococcus aureus prevalence among preclinical students
Distribution of included studies
3.5. Methicillin-resistant Staphylococcus aureus Prevalence
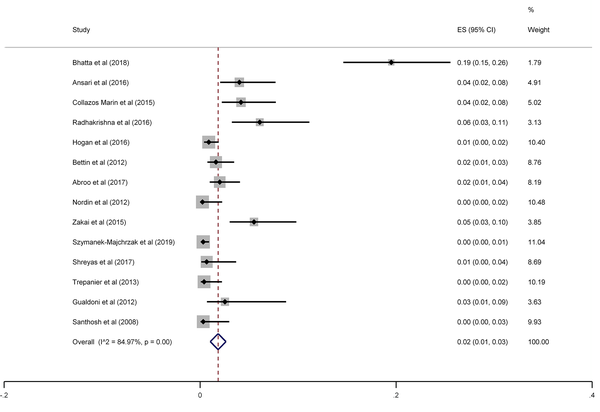
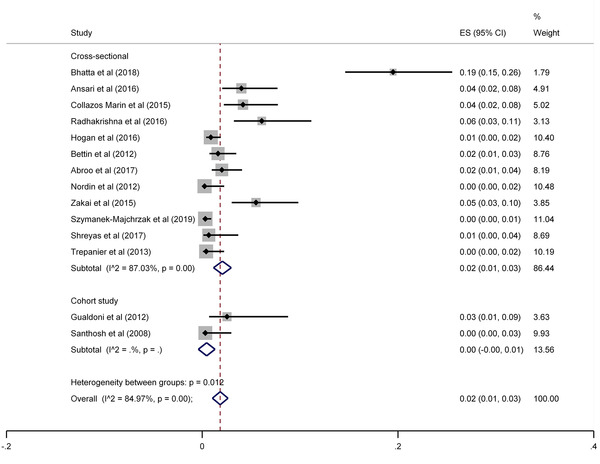
The results of the meta-analysis of 15 studies that had reported nasal MRSA showed that the total prevalence among medical students was 2% (prevalence rate: 0.02, 95% CI: 0.01 - 0.03, P < 0.001) (Figure 8). This rate varied from 0 to 26% in these studies. In addition, the heterogeneity of the included studies was very high (I2: 84.97%, chi2: 86.48 (df = 14) P < 0.001). Therefore, a subgroup analysis was performed according to the study design. This meta-analysis disclosed that the pooled prevalence of MRSA was 2% and 0% in cross-sectional and cohort studies, respectively (Figure 9).
MRSA prevalence among medical students
MRSA prevalence among medical students, sub-grouped by study design
4. Discussion and Conclusions
In this meta-analysis, 15 studies on S. aureus prevalence among medical students in different countries were analyzed. The prevalence rates varied widely over a range between 10 and 72%. The pooled prevalence of nasal S. aureus was 28%, which is comparable with the Kluytmans study. In this study, a mean carriage rate of 26.6% was found among Health Care Workers (HCWs) (36). However, the range of carriage rate was large (16.8 - 56.1%). This may be due to differences in the quality of sampling methods and culture techniques used in these studies (37-42). The range of carriage prevalence rate of S. aureus was higher among clinical students (33%) than in preclinical students (28%). This could be due to the increment in the possibility of exposure with S. aureus because of frequent visits to wards and clinics in the hospital setting (43).
This review incorporated 15 studies on MRSA prevalence among medical students around the world. The pooled prevalence of nasal MRSA colonization among medical students was estimated to be 2%. The estimations of the present study are more than the results of a previous review to a certain extent. That study estimated the average rate of MRSA among HCWs to be 1.8% in Europe and the United States (44). Two other reviews reported the prevalence of MRSA colonization among HCWs to be around 5% (45, 46). In both reviews, the data belonged to endemic situations and outbreaks, which was a different aspect of our review.
Despite that most of the articles were evaluated as high-quality and moderate-quality (in addition to one study evaluated as poor), the methodological assessment of the studies indicated that the quality of the articles was somehow inconsistent with the sample size and bias resolving method. Moreover, nine articles were evaluated to be of high quality. The high-quality studies showed a higher pooled MRSA involvement rate among medical students than moderate-quality studies. Possible explanations include differences in human populations, predominant strain(s), study design, and laboratory testing methods for determining resistance (47).
The major cause of classification bias was seen in the sampling location, as well as the time of evaluation of medical students (45). In most studies, sampling sites were anterior nares, and it might have led to an underestimation of the accuracy of the results of MRSA rate. The nasal samples were taken by different staff members instead of one educated person, and this could affect the reliability. Another factor was the timing of the screening test that would impact the findings of cohort studies, indicating that MRSA colonization is basically momentary (12).
Our study showed that the MRSA rate in medical students had a large variation in a range from 0% to 26%. Carriage rates among HCWs are higher than in normal populations that have no recognized risk factors (circa 0.2%) (48). This is of high importance because colonized HCWs give service to high-risk patients, including those with infections of the surgery site, neonates, and patients admitted to the intensive care unit.
In addition, it was shown that several factors can affect the design and implementation of primary studies. These factors are also effective in calculating the prevalence. It was found during this meta-analysis that cohort studies reported a lower prevalence of MRSA carriage. Although the final prevalence of MRSA carriage was reported to be circa 2%, which is similar to previous articles, a high rate of heterogeneity was seen in study populations, as well as study designs. These estimations might help find more precise estimations of the global phenomenon incidents. We might also be able to consider different factors affecting the prevalence rates.
It can be stated that the results from the present meta-analysis provide an estimation of SA and MRSA prevalence rates among medical students. This estimation comes from several performed studies among different populations. Regarding the fact that medical students are at the highest exposure risk for MRSA colonization, the prevention of MRSA colonization in this group should be considered more seriously. The results of this meta-analysis indicate that decision-makers and officials need to focus more on this public health matter and develop more accurate screening strategies for medical students in all countries.
Health care workers who are at the interface between the hospital and community may serve as specialists of cross-contamination of hospital-acquired MRSA and community-acquired MRSA. The identification of HCWs in outbreak settings colonized with MRSA is valuable in reducing the transmission and controlling the spread of MRSA. Since reducing the carrier rate of SA, especially methicillin-resistant cases, can be effective in reducing infections caused by this organism, studying and knowing the number of carriers, especially in the medical staff, can reduce nosocomial infections caused by this organism. It is suggested that the prevalence of nasal S. aureus carriage of all health professionals, including medical doctors and specialists, nurses, and other medical staff and patients at risk of infections, be evaluated in the next studies. In addition, the carrier rate of MRSA may be considered.
Acknowledgements
References
-
1.
Pidwill GR, Gibson JF, Cole J, Renshaw SA, Foster SJ. The role of macrophages in Staphylococcus aureus infection. Front Immunol. 2020;11:620339. [PubMed ID: 33542723]. [PubMed Central ID: PMC7850989]. https://doi.org/10.3389/fimmu.2020.620339.
-
2.
Aghamali M, Taghizadeh S, Mehramuz B, Gholizadeh P, Tanomand A, Ganbarov K, et al. Nasal carriage of methicillin-resistant Staphylococci among healthcare workers of a university teaching hospital, Iran. Gms Hygiene Infect Control. 2020;15:6.
-
3.
Lee AS, de Lencastre H, Garau J, Kluytmans J, Malhotra-Kumar S, Peschel A, et al. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers. 2018;4:18033. [PubMed ID: 29849094]. https://doi.org/10.1038/nrdp.2018.33.
-
4.
Plata K, Rosato AE, Wegrzyn G. Staphylococcus aureus as an infectious agent: overview of biochemistry and molecular genetics of its pathogenicity. Acta Biochim Pol. 2009;56(4):597-612. [PubMed ID: 20011685].
-
5.
Chua K, Laurent F, Coombs G, Grayson ML, Howden BP. Antimicrobial resistance: Not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician's guide to community MRSA - its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis. 2011;52(1):99-114. [PubMed ID: 21148528]. https://doi.org/10.1093/cid/ciq067.
-
6.
Maree CL, Eells SJ, Tan J, Bancroft EA, Malek M, Harawa NT, et al. Risk factors for infection and colonization with community-associated methicillin-resistant Staphylococcus aureus in the Los Angeles County jail: a case-control study. Clin Infect Dis. 2010;51(11):1248-57. [PubMed ID: 21034197]. [PubMed Central ID: PMC3697429]. https://doi.org/10.1086/657067.
-
7.
Kluytmans JA, Wertheim HF. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection. 2005;33(1):3-8. [PubMed ID: 15750752]. https://doi.org/10.1007/s15010-005-4012-9.
-
8.
Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5(12):751-62. [PubMed ID: 16310147]. https://doi.org/10.1016/S1473-3099(05)70295-4.
-
9.
Rasamiravaka T, Rasoanandrasana S, Zafindraibe NJ, Rakoto Alson AO, Rasamindrakotroka A. Evaluation of methicillin-resistant Staphylococcus aureus nasal carriage in Malagasy patients. J Infect Dev Ctries. 2013;7(4):318-22. [PubMed ID: 23592641]. https://doi.org/10.3855/jidc.2460.
-
10.
Prates KA, Torres AM, Garcia LB, Ogatta SF, Cardoso CL, Tognim MC. Nasal carriage of methicillin-resistant Staphylococcus aureus in university students. Braz J Infect Dis. 2010;14(3):316-8. [PubMed ID: 20835520].
-
11.
Ma XX, Sun DD, Wang S, Wang ML, Li M, Shang H, et al. Nasal carriage of methicillin-resistant Staphylococcus aureus among preclinical medical students: epidemiologic and molecular characteristics of methicillin-resistant S. aureus clones. Diagn Microbiol Infect Dis. 2011;70(1):22-30. [PubMed ID: 21513841]. https://doi.org/10.1016/j.diagmicrobio.2010.12.004.
-
12.
Eveillard M, Martin Y, Hidri N, Boussougant Y, Joly-Guillou ML. Carriage of methicillin-resistant Staphylococcus aureus among hospital employees: prevalence, duration, and transmission to households. Infect Control Hosp Epidemiol. 2004;25(2):114-20. [PubMed ID: 14994935]. https://doi.org/10.1086/502360.
-
13.
Emaneini M, Jabalameli F, Rahdar H, Leeuwen WBV, Beigverdi R. Nasal carriage rate of methicillin resistant Staphylococcus aureus among Iranian healthcare workers: a systematic review and meta-analysis. Rev Soc Bras Med Trop. 2017;50(5):590-7. [PubMed ID: 29160504]. https://doi.org/10.1590/0037-8682-0534-2016.
-
14.
Wong JW, Ip M, Tang A, Wei VW, Wong SY, Riley S, et al. Prevalence and risk factors of community-associated methicillin-resistant Staphylococcus aureus carriage in Asia-Pacific region from 2000 to 2016: a systematic review and meta-analysis. Clin Epidemiol. 2018;10:1489-501. [PubMed ID: 30349396]. [PubMed Central ID: PMC6190640]. https://doi.org/10.2147/CLEP.S160595.
-
15.
Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Systematic reviews of prevalence and incidence. Adelaide, South Australia: Joanna Briggs Institute reviewer’s manual. The Joanna Briggs Institute; 2017. 0.4 p.
-
16.
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7). e1000097. [PubMed ID: 19621072]. [PubMed Central ID: PMC2707599]. https://doi.org/10.1371/journal.pmed.1000097.
-
17.
Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147-53. [PubMed ID: 26317388]. https://doi.org/10.1097/XEB.0000000000000054.
-
18.
Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196-207. [PubMed ID: 26355603]. https://doi.org/10.1097/XEB.0000000000000065.
-
19.
Bhatta DR, Hamal D, Shrestha R, Parajuli R, Baral N, Subramanya SH, et al. Nasal and pharyngeal colonization by bacterial pathogens: a comparative study between preclinical and clinical sciences medical students. Can J Infect Dis Med Microbiol. 2018;2018:7258672. [PubMed ID: 29983836]. [PubMed Central ID: PMC6011148]. https://doi.org/10.1155/2018/7258672.
-
20.
Ansari S, Gautam R, Shrestha S, Ansari SR, Subedi SN, Chhetri MR. Risk factors assessment for nasal colonization of Staphylococcus aureus and its methicillin resistant strains among pre-clinical medical students of Nepal. BMC Res Notes. 2016;9:214. [PubMed ID: 27068121]. [PubMed Central ID: PMC4828777]. https://doi.org/10.1186/s13104-016-2021-7.
-
21.
Collazos Marin LF, Estupinan Arciniegas G, Chavez Vivas M. Characterization of Staphylococcus aureus isolates that colonize medical students in a hospital of the city of Cali, Colombia. Int J Microbiol. 2015;2015:358489. [PubMed ID: 26495001]. [PubMed Central ID: PMC4606190]. https://doi.org/10.1155/2015/358489.
-
22.
Manipura R, Taneja A, Rao P. Nasal carriage of Staphylococcus aureus with special emphasis on methicillin resistant Staphylococcus aureus among students of a south Indian medical college – prevalence and antibiogram pattern. Asian J Pharm Clin Res. 2016. https://doi.org/10.22159/ajpcr.2016.v9s2.13274.
-
23.
Hogan B, Rakotozandrindrainy R, Al-Emran H, Dekker D, Hahn A, Jaeger A, et al. Prevalence of nasal colonisation by methicillin-sensitive and methicillin-resistant Staphylococcus aureus among healthcare workers and students in Madagascar. BMC Infect Dis. 2016;16(1):420. [PubMed ID: 27526771]. [PubMed Central ID: PMC4986198]. https://doi.org/10.1186/s12879-016-1733-6.
-
24.
Bettin A, Causil C, Reyes N. Molecular identification and antimicrobial susceptibility of Staphylococcus aureus nasal isolates from medical students in Cartagena, Colombia. Braz J Infect Dis. 2012;16(4):329-34. [PubMed ID: 22846119]. https://doi.org/10.1016/j.bjid.2012.06.017.
-
25.
Syafinaz AM, Nur Ain NZ, Nadzirahi SN, Fatimah JS, Shahram A, Nasir MD. Staphylococcus aureus Nasal Carriers Among Medical Students in A Medical School. Med J Malaysia. 2012;67(6):636-8. [PubMed ID: 23770966].
-
26.
Zakai SA. Prevalence of methicillin-resistant Staphylococcus aureus nasal colonization among medical students in Jeddah, Saudi Arabia. Saudi Med J. 2015;36(7):807-12. [PubMed ID: 26108584]. [PubMed Central ID: PMC4503899]. https://doi.org/10.15537/smj.2015.7.11609.
-
27.
Shreyas K, Radhakrishna M, Hegde A, Rao P. Is Acquisition of Methicillin-Resistant Staphylococcus Aureus - an Occupational Hazard for Medical Students in India? Asian J Pharm Clin Res. 2017;10(12). https://doi.org/10.22159/ajpcr.2017.v10i12.20865.
-
28.
Santhosh DV, Shobha KL, Bairy I, Gowrish R, D’Souza J. Phenotypic detection and rate of nasal carriage of heterotypic borderline oxacillin resistant Staphylococcus aureus in pre-clinical medical students from Malaysia. J Clin Diagn Res. 2008;2(4):985-90.
-
29.
Abroo S, Hosseini Jazani N, Sharifi Y. Methicillin-resistant Staphylococcus aureus nasal carriage between healthy students of medical and nonmedical universities. Am J Infect Control. 2017;45(7):709-12. [PubMed ID: 28359610]. https://doi.org/10.1016/j.ajic.2017.02.034.
-
30.
Szymanek-Majchrzak K, Kosinski J, Zak K, Sulek K, Mlynarczyk A, Mlynarczyk G. Prevalence of methicillin resistant and mupirocin-resistant Staphylococcus aureus strains among medical students of Medical University in Warsaw. Przegl Epidemiol. 2019;73(1):39-48. [PubMed ID: 31134773]. https://doi.org/10.32394/pe.73.05.
-
31.
Stubbs E, Pegler M, Vickery A, Harbour C. Nasal carriage of Staphylococcus aureus in Australian (pre-clinical and clinical) medical students. J Hosp Infect. 1994;27(2):127-34. [PubMed ID: 7930539]. https://doi.org/10.1016/0195-6701(94)90005-1.
-
32.
Gualdoni GA, Lingscheid T, Tobudic S, Burgmann H. Low nasal carriage of drug-resistant bacteria among medical students in Vienna. GMS Krankenhhyg Interdiszip. 2012;7(1):Doc04. [PubMed ID: 22558038]. [PubMed Central ID: PMC3334956]. https://doi.org/10.3205/dgkh000188.
-
33.
Trepanier P, Tremblay C, Ruest A. Methicillin-resistant Staphylococcus aureus colonization among medical residents. Can J Infect Dis Med Microbiol. 2013;24(2):e39-41. [PubMed ID: 24421816]. [PubMed Central ID: PMC3720012]. https://doi.org/10.1155/2013/148937.
-
34.
Conceicao T, de Lencastre H, Aires-de-Sousa M. Carriage of Staphylococcus aureus among Portuguese nursing students: A longitudinal cohort study over four years of education. PLoS One. 2017;12(11). e0188855. [PubMed ID: 29190721]. [PubMed Central ID: PMC5708729]. https://doi.org/10.1371/journal.pone.0188855.
-
35.
Suhaili Z, Rafee P, Mat Azis N, Yeo CC, Nordin SA, Abdul Rahim AR, et al. Characterization of resistance to selected antibiotics and Panton-Valentine leukocidin-positive Staphylococcus aureus in a healthy student population at a Malaysian University. Germs. 2018;8(1):21-30. [PubMed ID: 29564245]. [PubMed Central ID: PMC5845972]. https://doi.org/10.18683/germs.2018.1129.
-
36.
Liu S, Tang Y, Lin Y, Chen K, Chen C, Huang Y, et al. Temporal changes in the nasal microbiota and host antimicrobial responses to intranasal mupirocin decolonisation: Observations in healthy staphylococcal carriers. BioRxiv. 2017:126375. https://doi.org/10.1101/126375.
-
37.
Dan M, Moses Y, Poch F, Asherov J, Gutman R. Carriage of methicillin-resistant Staphylococcus aureus by non-hospitalized subjects in Israel. Infection. 1992;20(6):332-5. [PubMed ID: 1293052]. https://doi.org/10.1007/BF01710678.
-
38.
Leedom JM, Kennedy RP, Lepper MH, Jackson GG, Dowling HF. Observations of the staphylococcal nasal carrier state. Ann N Y Acad Sci. 1965;128(1):381-403. [PubMed ID: 4221873]. https://doi.org/10.1111/j.1749-6632.1965.tb11650.x.
-
39.
Troeman DPR, Van Hout D, Kluytmans J. Antimicrobial approaches in the prevention of Staphylococcus aureus infections: a review. J Antimicrob Chemother. 2019;74(2):281-94. [PubMed ID: 30376041]. [PubMed Central ID: PMC6337897]. https://doi.org/10.1093/jac/dky421.
-
40.
Efa F, Alemu Y, Beyene G, Gudina EK, Kebede W. Methicillin-resistant Staphylococcus aureus carriage among medical students of Jimma University, Southwest Ethiopia. Heliyon. 2019;5(1). e01191. [PubMed ID: 30775580]. [PubMed Central ID: PMC6360348]. https://doi.org/10.1016/j.heliyon.2019.e01191.
-
41.
Milliken J, Tait GA, Ford-Jones EL, Mindorff CM, Gold R, Mullins G. Nosocomial infections in a pediatric intensive care unit. Crit Care Med. 1988;16(3):233-7. [PubMed ID: 3277780]. https://doi.org/10.1097/00003246-198803000-00005.
-
42.
Sakr A, Bregeon F, Rolain JM, Blin O. Staphylococcus aureus nasal decolonization strategies: a review. Expert Rev Anti Infect Ther. 2019;17(5):327-40. [PubMed ID: 31012332]. https://doi.org/10.1080/14787210.2019.1604220.
-
43.
Okamo B, Moremi N, Seni J, Mirambo MM, Kidenya BR, Mshana SE. Prevalence and antimicrobial susceptibility profiles of Staphylococcus aureus nasal carriage among pre-clinical and clinical medical students in a Tanzanian University. BMC Res Notes. 2016;9:47. [PubMed ID: 26817605]. [PubMed Central ID: PMC4728816]. https://doi.org/10.1186/s13104-016-1858-0.
-
44.
Dulon M, Peters C, Schablon A, Nienhaus A. MRSA carriage among healthcare workers in non-outbreak settings in Europe and the United States: a systematic review. BMC Infect Dis. 2014;14:363. [PubMed ID: 24996225]. [PubMed Central ID: PMC4094410]. https://doi.org/10.1186/1471-2334-14-363.
-
45.
Albrich WC, Harbarth S. Health-care workers: source, vector, or victim of MRSA? Lancet Infect Dis. 2008;8(5):289-301. [PubMed ID: 18471774]. https://doi.org/10.1016/S1473-3099(08)70097-5.
-
46.
Hawkins G, Stewart S, Blatchford O, Reilly J. Should healthcare workers be screened routinely for meticillin-resistant Staphylococcus aureus? A review of the evidence. J Hosp Infect. 2011;77(4):285-9. [PubMed ID: 21292349]. https://doi.org/10.1016/j.jhin.2010.09.038.
-
47.
Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368(9538):874-85. [PubMed ID: 16950365]. https://doi.org/10.1016/S0140-6736(06)68853-3.
-
48.
Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36(2):131-9. [PubMed ID: 12522744]. https://doi.org/10.1086/345436.
