Abstract
Background:
Patients with hepatitis virus C (HCV) infection have declined levels of forced expiratory volume (FEV), which is a prognostic marker for chronic obstructive pulmonary disease (COPD).Objectives:
The current study primarily aimed to investigate the incidence of subclinical HCV infection (clinical signs are absent but positive HCV RNA test performed by polymerase chain reaction) in patients with COPD of Zhejiang province, China, and its secondary aim was to investigate the clinical influence of HCV infection on COPD severity by body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) scoring index and pulmonary function tests (PFT).Methods:
A total of 252 patients with COPD (confirmed by routine lab tests, BODE index, and PFT) were included in a cross-sectional multicenter study. An anti-HCV antibody test was used to diagnose HCV infection. Hepatitis virus C RNA was tested for patients with a HCV antibody-positive test.Results:
Twelve patients had a positive anti-HCV antibody test. Of 12 anti-HCV antibody positive test patients, 10 were positive for the HCV RNA. The prevalence of anti-HCV antibody positivity and HCV RNA positivity was 12/ 252 and 10/ 252, respectively. The partial arterial pressure of oxygen was the same for patients with HCV RNA positive test compared to those with a negative anti-HCV antibody test (59.70 ± 5.50 mmHg vs. 63.84 ± 15.63 mmHg, P = 0.791). Patients with a positive HCV RNA test had a higher partial arterial pressure of carbon dioxide compared to those with a negative anti-HCV antibody test (43.70 ± 1.89 mmHg vs. 49.42 ± 7.33 mmHg, P = 0. 007). BODE index was higher for patients with HCV RNA positive test than those with anti-HCV antibody test negative (6 (3 - 7) vs. 4 (2 - 6), P < 0.0001). Among the variables of BODE index scoring, the values of distance walked in 6 min (P < 0.0001) and % predicted forced expiratory volume in 1 s (P < 0.0001) were fewer for patients with HCV RNA positive test than those with a negative anti-HCV antibody test.Conclusion:
This study demonstrated that subclinical HCV infection may be observed in COPD patients.Keywords
Liver Diseases Chronic Obstructive Pulmonary Disease Hepatitis C Virus Infection
1. Background
Hepatitis virus C (HCV) was first identified in 1989 (1), since then, it has been estimated to infect approximately 71 million people, who are at increased risk of liver cirrhosis and liver cancer (2). The HCV infection indicates the chronic, systemic, and asymptomatic course of the disease (1). The extrahepatic manifestation of HCV is more common, which affects multiple organ systems (3). As a hepatic and lymphotropic agent, HCV not only causes serious liver cirrhosis and liver cancer but also triggers a clonal β-cell activation, which leads to lymph proliferative disorders and an increased risk of mortality and morbidity (4). During the past years, several studies have investigated this issue (4, 5), with emerging clinical data suggested that the complication related to the chronic HCV infection can potentially lead to multiple pulmonary problems. These problems are secondary to HCV complications, with the pulmonary disease may also be related to portal hypertension, autoimmune disorders, and liver cirrhosis, which are frequently associated with chronic HCV infection. It has been reported as a major health burden and the seventh leading cause of mortality globally (5).
According to the latest available data, the prevalence of HCV is relatively high in China, particularly compared to its neighboring countries (4). The most common genotype of HCV infection in China is 1b, followed by 2a (6). Most of the hepatitis virus C infections are recorded in developing countries; Meanwhile, its prevalence is reported to be significantly high in China (7, 8). Although World Health Organization (WHO) has developed and implemented an initiative on community-based opioid substitution therapy (methadone maintenance treatment (MMT)) to deliver interventions for HCV-infected individuals and has defined a target for the elimination of viral hepatitis by 2030 (9, 10), the HCV antibody prevalence among Chinese population is still high and very little is known about the status of HCV treatment and its prognosis status in China mainland (11). Although there are sufficient studies regarding the status of HCV in neighboring developed countries (e.g., India and Vietnam), these studies did not give a mirror image of HCV status in China due to differences in MMT program approaches in the country (12).
Chronic obstructive pulmonary disease (COPD) is a moderate public health burden that is associated with an increased risk of mortality in the Zhejiang province of China (13). The overall prevalence of COPD is 8.2% in the Chinese population (14). Extrahepatic HCV with lung involvement results in loss of lung function, especially in smokers (15). It's also reported that COPD is associated with other systematic consequences and several comorbidities such as forced expiratory volume (FEV) and pulmonary function (14). Patients with HCV infection are observed with declined FEV value, which is a prognostic marker for COPD conditions (16). Some studies (17-19) are available worldwide, but scanty studies are available in the prevalence of HCV with COPD on the Chinese population.
2. Objective
The primary aim of the retrospective analyses of this cross-sectional multicenter observation study was to determine the incidence of subclinical HCV infection (clinical signs are absent but positive HCV RNA test performed by polymerase chain reaction) in patients with COPD living in the Zhejiang province (China). Besides, its secondary aim was to investigate the clinical influence of HCV infection on COPD severity by body-mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) scoring index and pulmonary function tests (PFT).
3. Methods
3.1. Study Population
The study was carried out for two years, from 2 January 2017 to 29 December 2018. Appropriate criteria were followed to include or excluded the patients in the study. Patients (> 18 years) with COPD were included in the study, while patients with organ failure were excluded. Patients with a history of non-HCV related chronic liver diseases and patients with known HCV infection were not included in the study. Organ failure and non-HCV-related chronic liver diseases may cause COPD, and patients may have other comorbidities that might act as confounding variables. Therefore, patients with organ failure and non-HCV related chronic liver diseases were excluded from the study. An author-developed questionnaire was used to collect information on the demographic history of participants.
3.2. Diagnostic Criteria
3.2.1. Chronic Obstructive Pulmonary Disease
It was considered if the patient had dyspnea, sputum production, chronic cough, and/ or history of exposure to risk factors for the disease. Spirometry (Spirometer, Chest MI, Inc., Tokyo, Japan) was used at the second level to confirm the diagnosis; with the presence of a post-bronchodilator forced expiratory volume in 1 second (FEV1)/ the forced vital capacity (FVC) < 0.70 and any persistent airflow limitation after the administration of salbutamol. The severity of the disease was graded as per the global initiative of chronic obstructive lung disease (GOLD) criteria (20).
3.2.2. Laboratory Investigation
All patients included in this study were followed by medical history and clinical examination. Laboratory investigations were performed for complete blood count (CBC), liver function test(s), and kidney function test(s). Routine lab tests were also included, for example, erythrocyte sedimentation rate, and serum C-reactive protein (CRP). Arterial blood gas analysis and electrocardiogram were performed for all participants. BODE index (as per Table 1) (21) and PFT (spirometer, the capacity of diffusion of the lungs for carbon monoxide, which was evaluated by computerized spirometer according to the American thoracic society guidelines (22)) were also evaluated. The reductions in the FVC were graded as mild: 70 - 79 % of predicted, moderate: 60 - 69 % of predicted, moderately severe: 50 - 59 % of predicted, severe: 35 - 49 % of predicted, and very severe: less than 35 % of predicted. The radiological investigations included non-contrast X-rays of the chest (CXR) and high-resolution computed tomography (HRCT; only for those patients with abnormal CXR).
Grading of Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity (BODE) Scoring Index
| Variable | Score of Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity (BODE) Index | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Body mass index (kg/ m2) | > 21 | ≤ 21 | - | - |
| The modified Medical Research Council dyspnea scale a | 0 - 1 | 2 | 3 | 4 |
| Distance walked in 6 min (m) | > 349 | 250 - 349 | 150 - 249 | < 150 |
| % Predicted forced expiratory volume in 1 s b | > 64 | 50 - 64 | 36 - 49 | < 36 |
3.2.3. Hepatitis Virus C Infection Detection
The anti-HCV antibody test was performed using an enzyme-linked immunosorbent assay (ELISA, ORTHO® HCV 3.0, Jonson & Johnson, Raritan, NJ, USA) kit. Patients with anti-HCV > 1 U/ mL were considered positive (18). Hepatitis virus C RNA test was performed by polymerase chain reaction with primers obtained from NS-5’ R (the highly conserved 5’ untranslated region) of the viral genome for the anti-HCV antibody positive test patients. If laboratory investigations were normal but HCV RNA test performed by polymerase chain reaction was positive, then it was considered as subclinical HCV.
3.3. Statistical Analysis
The sample size was calculated based on the assumption that subclinical HCV infection would be observed in 3 ± 2 % of patients suffering from COPD, 80 % power, 5 % two-sided type-I error, and 95 % level of confidence (17). The sample size (minimum number of COPD patients) was 115. InState vWindow, 3.01, GraphPad Software, San Diego, CA, USA was used for statistical analysis. The Fischer exact test was performed for numerical and ordinal data, and the Mann-Whitney test was performed for continuous data. Statistical significance was considered when P-value < 0.05.
4. Results
4.1. Study Population
A total of 257 COPD patients were available during the study period. Among them, two had organ failure and three had non-HCV related chronic liver disease. Therefore, data of these patients (n = 5) were excluded from the analysis. A total of 252 patients with COPD were included in this analysis. The flow diagram of the study is presented in Figure 1.
Flow diagram of the study.
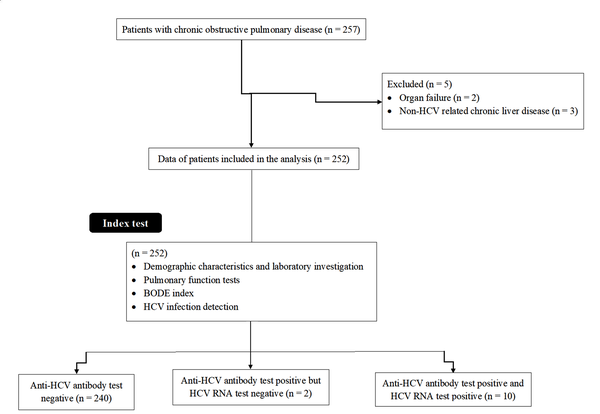
4.2. Demographic Characteristics and Laboratory Investigations
The mean age of the enrolled patients was 47.51 ± 15.22 years, of which 197 (78 %) were male, and 55 (22 %) were female. Other comorbidities of participants were diabetes mellitus (n = 28), rheumatoid arthritis (n = 85), heart failure (n = 7), lupus (n = 31), and gout (n = 21). Demographic characteristics and laboratory investigations of the enrolled patients are reported in Table 2. Descriptive data are reported as frequency (percentages), continuous data are reported as mean ± standard deviation (SD).
Demographic Characteristics and Laboratory Investigation of Enrolled Patients
| Characteristics | Values |
|---|---|
| The numbers of patients included in the study | 252 |
| Age (y) | |
| Minimum | 39 |
| Maximum | 67 |
| Mean ± SD | 47.51 ± 15.22 |
| Gender | |
| Male | 197 (78) |
| Female | 55 (22) |
| Tobacco history | |
| Current smoker | 98 (39) |
| Previous smoker | 114 (45) |
| No smoker | 40 (16) |
| Ethnicity | |
| Han Chinese | 231 (92) |
| Mongolian | 18 (7) |
| Tibetan | 3 (1) |
| Complete blood count (g/ dL) | 16.15 ± 1.85 |
| Liver function test(s) | |
| Serum alanine transaminase (ALT; U/ L) | 32 ± 6 |
| Serum aspartate aminotransferase (AST; U/ L) | 30 ± 3 |
| Serum alkaline phosphatase (ALP; U/ L) | 110 ± 10 |
| Serum albumin (g/ dL) | 3.91 ± 1.12 |
| Serum bilirubin (mg/ dL) | 0.25 ± 0.07 |
| Serum gamma glutamine transferees (GGT; U/ L) | 9.12 ± 1.15 |
| Kidney function test(s) | |
| Urine density (g/ mL) | 1.001 ± 0.015 |
| Urinary proteins (mg/ dL) | 135 ± 12 |
| Serum creatinine (mg/ dL) | 0.81 ± 0.15 |
| Blood urea nitrogen (mg/ dL) | 11 ± 2 |
| Erythrocyte sedimentation rate (mm/ h) | 7 ± 1 |
| Serum C-reactive protein (mg/ L) | 1.01 ± 0.12 |
| Comorbidities | |
| Diabetes mellitus | 28 (11) |
| Rheumatoid arthritis | 85 (34) |
| Heart failure | 7 (3) |
| Lupus | 31 (12) |
| Gout | 21 (8) |
4.3. Pulmonary Function Tests
As per the GOLD criteria, 159 (63 %) patients were included in stages III/ IV of COPD. Data on PFT are included in Table 3. This indicated that FEV1 was ranging from 37.6 % to 73.6 % (55.29 ± 11.79 %), which is considerably lower than the normal range. The normal lower level of FVC was 70 %, in this study case, it was varying from 67.7 % to 107.9 % (87.82 ± 20.13 %). Total lung capacity, in this case, was 121 - 187 % (156 ± 31 %), which was much higher than the normal higher value of 120 %.
| Characteristics | Values |
|---|---|
| The numbers of patients included in the study | 252 |
| Carbon monoxide diffusing capacity of the lung (% predicted) | 49 ± 19 |
| The partial arterial pressure of oxygen (PaO2; mmHg) | 63.74 ± 15.32 |
| The partial arterial pressure of carbon dioxide (PaCO2; mmHg) | 49.17 ± 7.26 |
| FEV1 (Post-bronchodilator forced expiratory volume in 1 s; L) | |
| Minimum | 0.92 |
| Maximum | 1.81 |
| Mean ± SD | 1.35 ± 0.44 |
| FVC (Forced vital capacity; L) | |
| Minimum | 1.84 |
| Maximum | 2.66 |
| Mean ± SD | 2.21 ± 0.51 |
| % FVC (Forced vital capacity (% predicted)) | |
| Minimum | 67.7 |
| Maximum | 107.9 |
| Mean ± SD | 87.82 ± 20.13 |
| % FEV1 / FVC ratio (Tiffeneau-Pinelli index) | |
| Minimum | 0.5 |
| Maximum | 0.68 |
| Mean ± SD | 0.61 ± 0.39 |
| % Functional residual capacity | |
| Minimum | 90 |
| Maximum | 160 |
| Mean ± SD | 120 ± 15 |
| Total lung capacity | |
| Minimum | 121 |
| Maximum | 187 |
| Mean ± SD | 156 ± 36 |
| The severity of COPD (GOLD criteria) | |
| Mild Stage I (FEV1 ≥ 80% predicted; % FEV1 / FVC ratio < 0.7) | 10 (4) |
| Moderate stage II (80% ≥ FEV1 ≤ 50% predicted; % FEV1 / FVC ratio < 0.7) | 83 (33) |
| Severe stage III (30% ≥ FEV1 ≤ 50% predicted; % FEV1 / FVC ratio < 0.7) | 91 (36) |
| Very severe stage IV (FEV1 ≤ 30% predicted; % FEV1 < 30 % predicted) | 68 (27) |
4.4. BODE Index
The median of the BODE index was identified as 4 (ranging from 2 to 7). All parameters of BODE index scoring are presented in Table 4.
Grading of Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity (BODE) Scoring Index of the Enrolled Patients
| Variable | Values | BODE Scoring |
|---|---|---|
| The numbers of patients included in the study | 252 | - |
| Body mass index (kg/ m2) | ||
| Minimum | 20 | 0 |
| Maximum | 29 | 1 |
| Mode | 24 | 0 |
| Mean ± SD | 24.28 ± 1.68 | 0.008 ± 0.089 |
| The modified Medical Research Council dyspnea scale | ||
| Minimum | 0 | 0 |
| Maximum | 4 | 3 |
| Mode | 2 | 1 |
| Mean ± SD | 2.18 ± 0.46 | 1.18 ± 0.44 |
| Distance walked in 6 min (m) | ||
| Minimum | 205 | 1 |
| Maximum | 372 | 2 |
| Mode | 313 | 1 |
| Mean ± SD | 309 ± 34 | 1.06 ± 0.25 |
| % predicted forced expiratory volume in 1 s | ||
| Minimum | 37.6 | 0 |
| Maximum | 73.6 | 3 |
| Mode | 54 | 2 |
| Mean ± SD | 55.29 ± 11.79 | 1.09 ± 0.89 |
| Body-mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) scoring index | ||
| Minimum | 2 | |
| Maximum | 7 | |
| Mode | 4 | |
| Mean ± SD | 3.34 ± 1.03 | |
4.5. Hepatitis Virus C Infection Detection
Twelve patients had a positive anti-HCV antibody test. Hepatitis virus C RNA was tested for all 12 patients with a positive HCV antibody test. Ten were positive for the HCV RNA test. The prevalence of anti-HCV antibody positivity and HCV RNA positivity was 12/ 252 and 10/ 252, respectively. Liver function tests were raised in all these 12 patients. One patient was diagnosed with severe liver cirrhosis, and one had hepatocellular carcinoma. Two patients, who had positive HCV antibody test but negative HCV RNA test, were either false positive or had acute HCV and resolved (not chronic HCV).
4.6. Comparing Patients with a Positive Hepatitis Virus C RNA Test and Those with a Negative Anti- hepatitis Virus C Antibody Test
The partial arterial pressure of oxygen was the same for patients with HCV RNA positive test compared to those with anti-HCV antibody test negative (59.70 ± 5.50 mmHg vs. 63.84 ± 15.63 mmHg, P = 0.791, Figure 2). The partial arterial pressure of carbon dioxide was fewer for patients with HCV RNA positive test than those with anti-HCV antibody test negative (43.70 ± 1.89 mmHg vs. 49.42 ± 7.33 mmHg, P = 0. 007, Figure 3). BODE index was higher for patients with HCV RNA positive test than those with anti-HCV antibody test negative (6 (3 - 7) vs. 4 (2 - 6), P < 0.0001, Table 5). Among variables of the BODE index scoring, the values of distance walked in 6 min (P < 0.0001, Figure 4) and % predicted FEV1 (P < 0.0001, Figure 5) were fewer for patients with HCV RNA positive test compared to those with anti-HCV antibody test negative. The detailed comparisons of demographic characteristics, laboratory investigation, and pulmonary function tests between patients with HCV RNA positive test and those with anti-HCV antibody test negative are reported in Table 6.
The partial arterial pressure of oxygen evaluation.

The partial arterial pressure of carbon dioxide evaluation

Comparisons of Grading of Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity (BODE) Scoring Index Between Patients with HCV RNA Positive Test and Those with Anti-HCV Antibody Test Negative a, b
| Variable | HCV RNA-Positive Patients, N = 10 | Anti-HCV Antibody Test Negative Patients, N = 240 | Comparisons Between Cohorts | |||
|---|---|---|---|---|---|---|
| Value | BODE Scoring | Value | BODE Scoring | Value | BODE Scoring | |
| Body mass index (kg/ m2) | 0.519 | N/A | ||||
| Minimum | 22 | 0 | 20 | 0 | ||
| Maximum | 27 | 0 | 29 | 1 | ||
| Mode | 24 | 0 | 24 | 0 | ||
| Mean ± SD | 24.6 ± 1.65 | 0 ± 0 | 24.25 ± 1.68 | 0.008 ± 0.091 | ||
| The modified Medical Research Council dyspnea scale | 0.4221 | 0.422 | ||||
| Minimum | 0 | 0 | 2 | 1 | ||
| Maximum | 3 | 2 | 4 | 3 | ||
| Mode | 2 | 1 | 2 | 1 | ||
| Mean ± SD | 1.9 ± 0.88 | 1 ± 0.67 | 2.20 ± 0.43 | 1.20 ± 0.43 | ||
| Distance walked in 6 min (m) | < 0.0001 | < 0.0001 | ||||
| Minimum | 205 | 1 | 242 | 1 | ||
| Maximum | 255 | 2 | 372 | 3 | ||
| Mode | N/A | 2 | 313 | 1 | ||
| Mean ± SD | 232 ± 16 | 1.80 ± 0.42 | 312.78 ± 30.24 | 1.03 ± 0.19 | ||
| % Predicted forced expiratory volume in 1 s | < 0.0001 | < 0.0001 | ||||
| Minimum | 33.6 | 2 | 35 | 0 | ||
| Maximum | 41 | 3 | 73.6 | 3 | ||
| Mode | N/A | 2 | 71 | 2 | ||
| Mean ± SD | 36.73 ± 2.70 | 2.40 ± 0.52 | 56.17 ± 11.36 | 1.03 ± 0.86 | ||
| Body-mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) scoring index | < 0.0001 | |||||
| Minimum | 3 | 2 | ||||
| Maximum | 7 | 6 | ||||
| Mode | 6 | 4 | ||||
| Mean ± SD | 5.20 ± 1.23 | 3.26 ± 0.95 | ||||
Distance walk in 6 min test evaluation.

Predicted forced expiratory volume in 1 s evaluation.

Comparisons of Demographic Characteristics, Laboratory Investigation, And Pulmonary Function Tests Between Patients with HCV RNA Positive Test and Those with Anti-HCV Antibody Test Negative a, b, c
| Characteristics | Anti-HCV Antibody Test Negative Patients, N = 240 | HCV RNA-Positive Patients, N = 10 | Comparisons Between Both Cohorts, P-Value |
|---|---|---|---|
| Age (y) | 0.307 | ||
| Minimum | 39 | 45 | |
| Maximum | 62 | 67 | |
| Mean ± SD | 43.52 ± 14.22 | 48.15 ± 7.18 | |
| Gender | 0.696 | ||
| Male | 186 (78) | 9 (90) | |
| Female | 54 (22) | 1 (10) | |
| Tobacco history | 0.091 | ||
| Current smoker | 90 (38) | 7 (70) | |
| Previous smoker | 110 (46) | 3 (30) | |
| No smoker | 40 (17) | 0 (0) | |
| Carbon monoxide diffusing capacity of the lung (% predicted) | 51 ± 20 | 45 ± 18 | 0.352 |
| FEV1 (Post-bronchodilator forced expiratory volume in 1 s; L) | 0.707 | ||
| Minimum | 0.93 | 0.92 | |
| Maximum | 1.85 | 1.81 | |
| Mean ± SD | 1.37 ± 0.41 | 1.32 ± 0.45 | |
| FVC (Forced vital capacity; L) | 0.905 | ||
| Minimum | 1.85 | 1.84 | |
| Maximum | 2.65 | 2.66 | |
| Mean ± SD | 2.20 ± 0.52 | 2.22 ± 0.53 | |
| % FVC (Forced vital capacity (% predicted)) | 0.135 | ||
| Minimum | 71 | 67.7 | |
| Maximum | 107.9 | 88 | |
| Mean ± SD | 90.88 ± 22.15 | 80.24 ± 17.82 | |
| % FEV1 / FVC ratio (Tiffeneau-Pinelli index) | 0.553 | ||
| Minimum | 0.55 | 0.5 | |
| Maximum | 0.68 | 0.64 | |
| Mean ± SD | 0.65 ± 0.31 | 0.59 ± 0.38 | |
| % Functional residual capacity | 0.443 | ||
| Minimum | 90 | 100 | |
| Maximum | 160 | 150 | |
| Mean ± SD | 125 ± 20 | 121 ± 20 | |
| Total lung capacity | 0.156 | ||
| Minimum | 121 | 135 | |
| Maximum | 187 | 150 | |
| Mean ± SD | 161 ± 35 | 145 ± 29 | |
| The severity of COPD (GOLD criteria) | 0.341 | ||
| Mild Stage I (FEV1 ≥ 80% predicted; % FEV1 / FVC ratio < 0.7) | 10 (5) | 0 (0) | |
| Moderate stage II (80% ≥ FEV1 ≤ 50% predicted; % FEV1 / FVC ratio < 0.7) | 82 (34) | 1 (10) | |
| Severe stage III (30% ≥ FEV1 ≤ 50% predicted; % FEV1 / FVC ratio < 0.7) | 85 (35) | 5 (50) | |
| Very severe stage IV (FEV1 ≤ 30% predicted; % FEV1 < 30 % predicted) | 63 (26) | 4 (40) | |
| Complete blood count (g/ dL) | 16.01 ± 2.01 | 16.08 ± 1.81 | 0.914 |
| Liver function test(s) | |||
| Serum alanine transaminase (ALT; U/ L) | 31 ± 7 | 36 ± 6 | 0.027 |
| Serum aspartate aminotransferase (AST; U/ L) | 29 ± 4 | 36 ± 4 | < 0.0001 |
| Serum alkaline phosphatase (ALP; U/ L) | 109 ± 10 | 118 ± 15 | 0.007 |
| Serum albumin (g/ dL) | 3.92 ± 1.09 | 5.15 ± 2.15 | 0.001 |
| Serum bilirubin (mg/ dL) | 0.24 ± 0.06 | 0.36 ± 0.16 | < 0.0001 |
| Serum gamma glutamine transferees (GGT; U/ L) | 9.11 ± 1.11 | 9.91 ± 1.41 | 0.028 |
| Kidney function test(s) | |||
| Urine density (g/ mL) | 1.00 ± 0.01 | 1.002 ± 0.014 | 0.543 |
| Urinary proteins (mg/ dL) | 133 ± 11 | 131 ± 9 | 0.571 |
| Serum creatinine (mg/ dL) | 0.82 ± 0.14 | 0.81 ± 0.14 | 0.825 |
| Blood urea nitrogen (mg/ dL) | 11 ± 3 | 10 ± 1 | 0.295 |
| Erythrocyte sedimentation rate (mm/ h) | 7 ± 1.5 | 6.9 ± 1.1 | 0.788 |
| Serum C-reactive protein (mg/ L) | 1.00 ± 0.11 | 1.02 ± 0.13 | 0.576 |
5. Discussion
Chronic HCV infection seems to have multiple other associated extrahepatic manifestations in organs such as the lung. These sometimes affects lung function, which leads to secondary effects with symptoms of progressive liver disease and drug treatment for HCV. Also, the presence of viremia is associated with a spirometric abnormality due to inflammation of the lungs (23). China is reported to have a varying prevalence of HCV infection compared to the neighboring countries (4, 24). Few studies have estimated the subclinical HCV infection in COPD in China. The study is about the prevalence of HCV–RNA positive in Chinese patients with COPD. In the current study, the prevalence of HCV–RNA positivity was found to be 3.99 %. The subclinical HCV infection among COPD patients is a new finding in China. COPD is an acute worsening condition of the respiratory system that requires additional therapy. It is now well understood that chronic HCV infection forms the base for several extrahepatic manifestations, including pulmonary, rheumatic diseases, etc. HCV infection directly and/or indirectly affects pulmonary function by worsening the lung capacity in asthma and COPD patients. The impact of extrahepatic indexes of HCV on the outcomes of patients is highlighted by observational studies that reported a significant association between cure from HCV (the sustained viral response) and decreased liver-and renal-related complications. Therefore, there is a need for clinical trials to evaluate the efficacy and tolerance of the direct-acting antiviral agents for the treatment of HCV-associated renal diseases.
In the present study, we found a comparatively low prevalence of HCV in COPD patients compared to previous studies worldwide (25, 26). The reports on the association between HCV infection and COPD are exiguous. In the current study, the prevalence of positive HCV antibodies in COPD patients was lower than the values reports by Silva et al. (7.5 %) (17) and Minakata et al. (22 out of 156; 14.10 %) (15). This may be due to the low epidemic prevalence of HCV in such a region (i.e., Zhejiang province of China). In the current study, patients with HCV had lower FEV1 values, increased BODE index, and fewer partial pressure of carbon dioxide compared to patients without HCV infection. This report is in concordance with previous findings (17-19). The BODE index is a prognostic parameter in COPD (21). The higher the BODE index, the more severe will be the outcomes. Chronic HCV infection plays a critical role in pulmonary manifestations by causing inflammation in the lungs, which leads to COPD in HCV patients. In the recent past, autoimmune and inflammatory lung diseases are well-known HCV associated with extrahepatic disorders.
The pathogenesis behind COPD in HCV infection is not well studied but can be attributed to the accelerated loss of lung function due to certain risk factors such as inflammation of lung tissues, smoking, heavy tobacco exposure, occupational hazards, etc. (3). In the present study, HCV infected patients had an advanced stage of COPD, classified according to the GOLD criteria, than that of negative patients. The underlying cause of systemic inflammation in HCV is aggravated lung disease. COPD is associated with increased inflammatory cytokines IL (interleukin)-1β, IL-6, IL-8, and TNF (tumor necrosis factor)-α, which exacerbates the disease (27). Based on the findings of the current study, COPD is newly emerging as a source of morbidity and mortality in HCV-positive patients. Thus, by screening high-risk populations, we can provide early detection of COPD in HCV patients. Reduced FEV1 can be the prognostic marker for the detection of HCV infection. The study results corroborate the prevalence of HCV in patients with more severe COPD. The current research is the first study to investigate the subclinical HCV infection among patients with COPD in Zhejiang province, China. However, additional studies with larger sample sizes are required to deduce the role of HCV in the severity of disease and the underlying pathogenesis of HCV in COPD patients. The evaluation of the association of subclinical HCV and COPD lead to control high treatment costs and treatment-emergent adverse effects. Understanding potential confounders are much more important because patients with comorbidities may have other unfavorable factors, which may underlie the lack of treatment response and may lead to higher mortality rates.
In the present, we found a statistically similar partial pressure of oxygen for patients with HCV RNA positive test than those with anti-HCV antibody test negative. However, the expected results of the partial pressure of oxygen were fewer for patients with HCV RNA positive test than those with an anti-HCV antibody-negative test. These results indicated that lungs were well-ventilated for patients with HCV RNA positive test, which can be attributed to the effectiveness of therapeutic interventions for disease(s) (28). Also, the statistically same values of the partial pressure of oxygen for patients with anti-HCV antibody test negative compared to those with HCV RNA positive test was due to more other comorbidities of the included patients. Further research is required to compare the levels of the partial pressure of oxygen with that of carbon dioxide. According to the findings, patients with a positive HCV RNA had raised liver function tests compared to those with a negative anti-HCV antibody test (23). Subclinical HCV infection increases the risk of deterioration of liver functions.
The current study had limitations, including very limited data and the lack of a control group. Besides, the results are limited to HCV plus prevalence in COPD patients with several systemic diseases. Although this is a study of prevalence, we did not evaluate whether there is a difference between the risk factors to contract HCV (mainly via parenteral), in a group with frequent comorbidities (diabetes (n = 28), rheumatoid arthritis (n = 85), and lupus (n = 31)). Also, we did not compare the differences among these comorbidities between the 10 patients with a positive HCV RNA test and patients with a negative anti-HCV antibody test. In addition, the clinical impact of this study is weakened by the small sample size and largely confirmatory nature of the results. The follow-up procedure considered for the patients and treatment(s) outcomes after diagnosis were not discussed. The pulmonary complication of cirrhosis is well-identified, but the association between subclinical HCV infection and COPD is not mechanistically explained. Patients with HCV have high-risk behaviors and a high prevalence of drug abuse and smoking. The statistical association between a few variables does not establish a causative association. Last but not least, the hepatitis virus C subtype is not reported and discussed.
5.1. Conclusions
This study demonstrated that a subclinical hepatitis C virus infection (clinical signs are absent but positive HCV RNA test performed by polymerase chain reaction) may be observed in patients suffering from COPD living in the Zhejiang province, China.
References
-
1.
Bukh J. The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J Hepatol. 2016;65(1 Suppl):S2-S21. [PubMed ID: 27641985]. https://doi.org/10.1016/j.jhep.2016.07.035.
-
2.
Jefferies M, Rauff B, Rashid H, Lam T, Rafiq S. Update on global epidemiology of viral hepatitis and preventive strategies. World J Clin Cases. 2018;6(13):589-99. [PubMed ID: 30430114]. [PubMed Central ID: PMC6232563]. https://doi.org/10.12998/wjcc.v6.i13.589.
-
3.
Khakban A, Sin DD, FitzGerald JM, McManus BM, Ng R, Hollander Z, et al. The projected epidemic of chronic obstructive pulmonary disease hospitalizations over the next 15 years. A population-based perspective. Am J Respir Crit Care Med. 2017;195(3):287-91. [PubMed ID: 27626508]. https://doi.org/10.1164/rccm.201606-1162PP.
-
4.
Fu Y, Wang Y, Xia W, Pybus OG, Qin W, Lu L, et al. New trends of HCV infection in China revealed by genetic analysis of viral sequences determined from first-time volunteer blood donors. J Viral Hepat. 2011;18(1):42-52. [PubMed ID: 20196805]. [PubMed Central ID: PMC3020328]. https://doi.org/10.1111/j.1365-2893.2010.01280.x.
-
5.
Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081-8. [PubMed ID: 27394647]. [PubMed Central ID: PMC5100695]. https://doi.org/10.1016/S0140-6736(16)30579-7.
-
6.
Xiang Y, Lai XF, Chen P, Yang Y. The correlation of HCV RNA and HCV core antigen in different genotypes of HCV. J Clin Lab Anal. 2019;33(1). e22632. [PubMed ID: 30069909]. [PubMed Central ID: PMC6430366]. https://doi.org/10.1002/jcla.22632.
-
7.
Chiu WC, Tsan YT, Tsai SL, Chang CJ, Wang JD, Chen PC, et al. Hepatitis C viral infection and the risk of dementia. Eur J Neurol. 2014;21(8):1068-e59. [PubMed ID: 24313931]. https://doi.org/10.1111/ene.12317.
-
8.
He Q, He Q, Qin X, Li S, Li T, Xie L, et al. The relationship between inflammatory marker levels and hepatitis C virus severity. Gastroenterol Res Pract. 2016;2016:2978479. [PubMed ID: 28090206]. [PubMed Central ID: PMC5206414 conflict of interests]. https://doi.org/10.1155/2016/2978479.
-
9.
Meng X, Wei G, Chang Q, Peng R, Shi G, Zheng P, et al. The platelet-to-lymphocyte ratio, superior to the neutrophil-to-lymphocyte ratio, correlates with hepatitis C virus infection. Int J Infect Dis. 2016;45:72-7. [PubMed ID: 26948479]. https://doi.org/10.1016/j.ijid.2016.02.025.
-
10.
Waheed Y, Siddiq M, Jamil Z, Najmi MH. Hepatitis elimination by 2030: Progress and challenges. World J Gastroenterol. 2018;24(44):4959-61. [PubMed ID: 30510370]. [PubMed Central ID: PMC6262254]. https://doi.org/10.3748/wjg.v24.i44.4959.
-
11.
Gao Y, Yang J, Sun F, Zhan S, Fang Z, Liu X, et al. Prevalence of anti-HCV antibody among the general population in Mainland China between 1991 and 2015: A systematic review and meta-analysis. Open Forum Infect Dis. 2019;6(3):ofz040. [PubMed ID: 30863789]. [PubMed Central ID: PMC6408870]. https://doi.org/10.1093/ofid/ofz040.
-
12.
Sullivan SG, Wu Z, Rou K, Pang L, Luo W, Wang C, et al. Who uses methadone services in China? Monitoring the world's largest methadone programme. Addiction. 2015;110 Suppl 1:29-39. [PubMed ID: 25533862]. https://doi.org/10.1111/add.12781.
-
13.
Fang L, Gao P, Bao H, Tang X, Wang B, Feng Y, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421-30. [PubMed ID: 29650407]. [PubMed Central ID: PMC7185405]. https://doi.org/10.1016/S2213-2600(18)30103-6.
-
14.
Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): A national cross-sectional study. Lancet. 2018;391(10131):1706-17. [PubMed ID: 29650248]. https://doi.org/10.1016/S0140-6736(18)30841-9.
-
15.
Minakata Y, Ueda H, Akamatsu K, Kanda M, Yanagisawa S, Ichikawa T, et al. High COPD prevalence in patients with liver disease. Intern Med. 2010;49(24):2687-91. [PubMed ID: 21173543]. https://doi.org/10.2169/internalmedicine.49.3948.
-
16.
Mekov EV, Petkov RE, Kostadinov DT, Antonov KA, Jelev DT. Chronic obstructive pulmonary disease and hepatitis C. Folia Med (Plovdiv). 2017;59(2):132-8. [PubMed ID: 28704182]. https://doi.org/10.1515/folmed-2017-0018.
-
17.
Silva DR, Stifft J, Cheinquer H, Knorst MM. Prevalence of hepatitis C virus infection in patients with COPD. Epidemiol Infect. 2010;138(2):167-73. [PubMed ID: 19563696]. https://doi.org/10.1017/S0950268809990276.
-
18.
Muayad A M, AbdulAziz Sulaiman S, Zaki Ali B, Saad K. Hepatitis C virus infection and the risk of chronic obstructive pulmonary diseases in Azadi Teaching Hospital, Duhok, Kurdistan. Res Rev Infect Dis. 2017;1(2). https://doi.org/10.36959/719/558.
-
19.
El-Habashy MM, Eldahdouh SS, Mohamed AA. The impact and effect of liver insufficiency of HCV infection on patients with chronic obstructive pulmonary diseases. Egypt J Chest Dis Tuberc. 2014;63(1):81-5. https://doi.org/10.1016/j.ejcdt.2013.11.003.
-
20.
Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-55. [PubMed ID: 17507545]. https://doi.org/10.1164/rccm.200703-456SO.
-
21.
Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-12. [PubMed ID: 14999112]. https://doi.org/10.1056/NEJMoa021322.
-
22.
Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. An official American thoracic society and european respiratory society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70-88. [PubMed ID: 31613151]. [PubMed Central ID: PMC6794117]. https://doi.org/10.1164/rccm.201908-1590ST.
-
23.
Bal T, Onlen Y, Babayigit C, Yumer Y, Sahin SI. The impact of hepatitis C viremia status on lung functions in chronic hepatitis c patients. Afr Health Sci. 2019;19(2):1988-92. [PubMed ID: 31656481]. [PubMed Central ID: PMC6794516]. https://doi.org/10.4314/ahs.v19i2.21.
-
24.
Tan Y, Wei QH, Chen LJ, Chan PC, Lai WS, He ML, et al. Molecular epidemiology of HCV monoinfection and HIV/HCV coinfection in injection drug users in Liuzhou, Southern China. PLoS One. 2008;3(10). e3608. [PubMed ID: 18974888]. [PubMed Central ID: PMC2571986]. https://doi.org/10.1371/journal.pone.0003608.
-
25.
Carvalho-Louro DM, Soares EB, Trevizoli JE, Marra TMG, da Cunha ALR, Rodrigues MP, et al. Hepatitis C screening, diagnosis, and cascade of care among people aged > 40 years in Brasilia, Brazil. BMC Infect Dis. 2020;20(1):114. [PubMed ID: 32041537]. [PubMed Central ID: PMC7011476]. https://doi.org/10.1186/s12879-020-4809-2.
-
26.
Cooper CL, Galanakis C, Donelle J, Kwong J, Boyd R, Boucher L, et al. HCV-infected individuals have higher prevalence of comorbidity and multimorbidity: a retrospective cohort study. BMC Infect Dis. 2019;19(1):712. [PubMed ID: 31438873]. [PubMed Central ID: PMC6706878]. https://doi.org/10.1186/s12879-019-4315-6.
-
27.
Garth J, Barnes JW, Krick S. Targeting cytokines as evolving treatment strategies in chronic inflammatory airway diseases. Int J Mol Sci. 2018;19(11). [PubMed ID: 30380761]. [PubMed Central ID: PMC6275012]. https://doi.org/10.3390/ijms19113402.
-
28.
Cukic V. The changes of arterial blood gases in COPD during four-year period. Med Arch. 2014;68(1):14-8. [PubMed ID: 24783904]. [PubMed Central ID: PMC4272457]. https://doi.org/10.5455/medarh.2014.68.14-18.