Abstract
Background:
Asthma is a chronic inflammatory disorder of lung airways, affecting about 300 million people worldwide. Several risk factors are involved in asthma development, such as environmental allergens, genetic susceptibility, and respiratory viral infections. Viral infections induce NF-kB and inflammatory pathways that lead to the production of cytokines, chemokines, and inflammatory proteins and, finally, a reduction of lung volume and function.Objectives:
The aim of this study was to evaluate viral infections’ prevalence in children with asthma from 2016 to 2017.Methods:
One hundred throat swab samples were collected from asthmatic children. Extraction of RNA and cDNA synthesis were performed to recognize parainfluenza viruses, rhinoviruses, influenza viruses, and respiratory syncytial virus (RSV) using real-time PCR. Also, the associations of age, sex, and other studied factors with asthmatic attacks were evaluated.Results:
In this study, 41 viruses were detected, including 21 cases of rhinoviruses (51.22%), 10 cases of parainfluenza (24.39%), seven cases of respiratory syncytial virus (17.07%), and three cases of the influenza virus (7.32%). Regarding seasonal incidence, the prevalence of the viruses was high in autumn and winter, and there was a significant relationship between seasonal incidence and gender. However, there were no statistically significant relationships between the prevalence of the viruses and age or gender.Conclusions:
The most important viral causes of childhood asthma in this study were found to be rhinoviruses, followed by parainfluenza. The lowest prevalence was related to the RSV and influenza virus, which the two viruses also showed the lowest seasonal outbreaks. Therefore, it can be said that with an increase in the seasonal incidence of respiratory viruses, the effects of these viruses will be greater on asthma.Keywords
Asthma Respiratory Infection Viral Infection Seasonal Outbreak
1. Background
Asthma is a common and severe chronic inflammatory disease of the airways and usually presents with feelings of tightness and pressure on the chest, wheezing, coughing, and sore throat (1). The most common and characteristic clinical symptom is smooth muscle contraction, leading to airway stenosis and obstruction. Allergic reactions, exercises, irritants, non-steroidal anti-inflammatory drugs, inflammation, and respiratory infections have been noted among the most important risk factors of asthma (2). Asthma affects 300 million people around the world, inflicts an annual death of about 250,000 cases globally, and imposes millions of dollars of costs on society every year (3, 4). Asthma symptoms occur in about 11.6% of 6- to 7-year-old children (5). Asthma is the most common chronic pulmonary disease among children, affecting 6.6 million children in the United States alone and 10% of children in the world (6). The prevalence of asthma and severe asthma in pediatric populations in Iran (10.9%) was similar to that of other developing countries in 2018 (7).
Asthma pathogenesis is a complex process, including various clinical endotypes. Complex interactions between genetic, epigenetic, and environmental factors lead to inappropriate and abnormal immunological patterns that bring asthma clinical manifestations to patients (8). Some environmental factors affect the pathogenesis of asthma, and after genetic factors, they have a great influence on the development of asthma in susceptible individuals, especially children who are vulnerable to severe infections of the respiratory system. The association of infections with asthma has been reported in 20 to 38% of cases (9, 10). Childhood infections are among the most important factors contributing to asthma development. Infections within the first months of infancy increase the risk of asthma several times (11). The infectious agents exaggerating the risk of asthma include bacteria, fungi, parasites, and viruses (12). Although all these infectious agents can contribute to asthma development, the role of viruses, in particular respiratory viruses, is more pronounced in the development of asthma, as well as other respiratory diseases such as idiopathic pulmonary fibrosis (IPF).
The inflammation caused by viruses alters lung volume, followed by a decrease in airflow and an increase in airways’ sensitivity (13-15). Moreover, nitric oxide levels rise in viral respiratory infections following a surge in viral titers, sharing a role in the progression of inflammation and development of asthma (16). Various viruses can play a role in asthma pathogenesis and creation. In Iran, several studies have examined the prevalence of various respiratory viruses such as a respiratory syncytial virus (RSV), influenza A and B, coronaviruses, rhinoviruses, metapneumovirus, and the Bocavirus in asthmatic patients using molecular methods, reporting a range of 0 to 46% (17-19).
The rhinovirus is one of the common cold-induced viruses in children and adolescents. The virus has a high reproductive capacity in the bronchial epithelium, causing bronchiolitis and aggravating inflammation and asthma symptoms. In some studies, the virus has been demonstrated an association between viral infection and the severity of asthma symptoms (30% of patients) (20, 21). Parainfluenza is another respiratory virus that causes airway histological changes and increases sensitivity to allergens, which augments susceptibility to asthma. On the other hand, eosinophils, lymphocytes, and neutrophils, which migrate to the site of infection, increase inflammation and facilitate asthma development (22, 23). The respiratory syncytial virus is also one of the most effective viruses for the development of chronic bronchitis in children. This virus shows widespread seasonal outbreaks and increases the risk of asthma in children (30 - 40%). The influenza virus is another important viral agent that, due to its high prevalence and easy transmission, can play a key role in childhood respiratory infections (24, 25).
2. Objectives
In this study, the prevalence and seasonal incidence of influenza viruses, parainfluenza, rhinoviruses, and RSV were investigated. Also, the relationship between these viruses and patients’ age and sex was assessed.
3. Methods
3.1. Patients
In this cross-sectional study, 100 throat swab samples were obtained from children with severe asthma attacks, diagnosed by a specialist, referred to the hospitals affiliated with Kermanshah University of Medical Sciences between April 2015 and December 2016. Of patients, 31% referred from cities other than Kermanshah. The patients were breathless and had chest pain, wheezing, cough, and sputum production. Standard diagnostic criteria for asthma (26, 27), including radiological, clinical, and spirometry findings, were confirmed by a specialized team. All required information (e.g., age, sex, history of viral infections, and consumed drugs) were also recorded. Based on their clinical conditions, the patients were categorized into two groups including exacerbating (n = 26) and stable (n = 74). The two scoring systems of Asthma Control Questionnaire (ACQ) and asthma control test (ACT) were used to monitor the efficacy of treatments.
3.2. Inclusion Criteria and Exclusion Criteria
Inclusion criteria were asthma diagnosis by a specialist, hospitalization due to asthma, and having acute asthma symptoms like wheezing. Patients with connective tissue diseases and chronic hypersensitivity pneumonitis or asbestosis were excluded.
3.3. Sample Collection
Samples were collected under sterile conditions, and the swabs were placed into a virus transport media (Hardy Diagnostics, CA, the USA). The specimens were well-mixed by a vortex before removing the swab and preserved in a freezer (-70°C).
3.4. Nucleic Acid Extraction
RNA extraction was performed by a QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The extracted genomic DNA/RNA was stored at -70˚C.
3.5. Real-Time PCR
For cDNA synthesis, 1 µg of total RNA was reverse transcribed using the QuantiNova Reverse Transcription Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions and stored at -70°C. Real-time PCR was performed with 11 ng of cDNA, 900 nM forward primer, 300 nM reverse primer, 100 nM probe, and 2X master mix in a final volume of 25 µL. Each sample was run in triplicate for 40 cycles at standard real-time PCR cycling conditions. The primers and probes for each virus were designed based on standard guidelines. The sequences of the primers and probes used in this study have been presented in Table 1.
The Sequences of the Probes and Primers Used in Real-Time PCR
| Virus Name | Sequences |
|---|---|
| Parainfluenza | |
| F1 | ATCCAAGAGGRGGAATAGA |
| F2 | ACCCAAGAGGGGGTATAGA |
| R | GTCTCCTTGAACCATTGC |
| Probe | FAM-TCTATAAGTGCAATMCATCTAGCAGCTGTT-TAMRA |
| RSV | |
| F | AACAGATGTAAGCAGCTCCGTTATC |
| R | CGATTTTTATTGGATGCTGTACATTT |
| Probe | FAM-TGCCATAGCATGACACAATGGCTCCT-TAMRA |
| Rhinovirus | |
| F | CGGCCCCTGAATGYGG |
| R | TGGAAACACGGACACCCAA |
| Probe | FAM-YGGGAYGGGACCAACT-BHQ1 |
| Influenza A | |
| F | GACCRATCCTGTCACCTCTGAC |
| R | AGGGCATTYTGGACAAAKCGTCTA |
| Probe | FAM-TGCAGTCCTCGCTCACTGGGCACG-BHQ1 |
| Influenza B | |
| F | TCCTCAAYTCACTCTTCGAGCG |
| R | CGGTGCTCTTGACCAAATTGG |
| Probe | FAM-CCAATTCGAGCAGCTGAAACTGCGGTG-BHQ1 |
3.6. Statistical Analysis
In this study, the frequencies of four types of respiratory viruses including influenza, parainfluenza, rhinovirus, and RSV in asthmatic children under the age of 5 years were investigated. Also, the prevalence of mentioned viruses in different seasons were demonstrated. For data analysis, SPSS v20 and GraphPad Prism were used. The two-way ANOVA statistical method was used to determine the relationships between viruses’ frequencies and children’s age and sex, as well as different seasons. A significant relationship or correlation between these factors was noted at a P value of < 0.05.
4. Results
From a total of 100 participants, 53 (53 %) were males, and the mean age was 4.26 ± 0.80 years. The patients had been diagnosed with asthma based on CT scan findings. The patients’ demographic information has been shown in Table 2.
Patients’ Demographic and Clinical Information
| Variables | Values |
|---|---|
| Gender, No. (%) | |
| Male | 53 (53) |
| Female | 47 (47) |
| Age group (y), No. (%) | |
| < 2 | 63 (63) |
| 2 - 5 | 37 (37) |
| Clinical history | |
| Viral infection | 17 |
| Using interfering drug | 4 |
| Identified viruses, No. (%) | |
| Rhino | 21 (51.22) |
| Parainfluenza | 10 (24.39) |
| RSV | 7 (17.07) |
| Influenza | 3 (7.32) |
| Association of viral infections with age and gender, P-value | |
| Viral infection and Age | |
| Rhino | 0.12 |
| Parainfluenza | 0.7 |
| RSV | 0.1 |
| Influenza | 0.2 |
| Viral infection and Gender | |
| Rhino | 0.2 |
| Parainfluenza | 0.9 |
| RSV | 0.5 |
| Influenza | 0.5 |
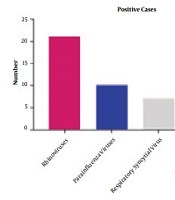
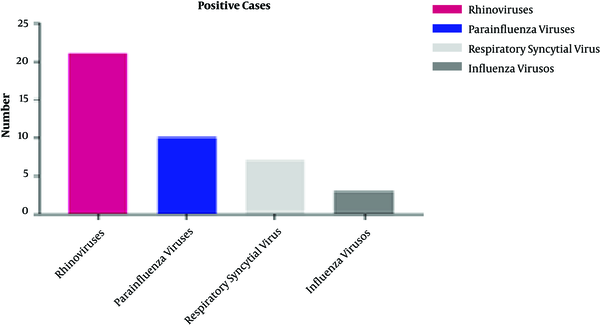
Patients with exacerbating clinical conditions had higher ACQ and ACT scores compared with children with stable conditions (P < 0.001); on the other hand, the rate of inhaled corticosteroid (ICS) maintenance therapy was lower among patients with stable conditions (47.1 vs. 92.3%, P < 0.001). There were no significant differences in the duration of asthma, parents’ smoking status, and rhinitis comorbidity between the two groups of patients (P > 0.05). Forty-one out of 100 samples (41%) were positive for the assessed viral infections. The highest and lowest prevalence of viral infections were related to rhinoviruses (51.21%) and influenza viruses (7.32%), (Figure 1 and Table 2).
Viral infections’ prevalence in children with asthma
No significant correlations were found between the studied viruses and asthmatic children’s age and gender (Table 2). The highest and lowest incidence of the viral infections was in winter and summer, respectively. Rhinoviruses were detected in three out of four seasons, but influenza was detected in winter only. A significant correlation was observed between the seasonal incidence and the gender (P = 0.02) but not age of patients (P = 0.08).
5. Discussion
Several risk factors such as occupational and environmental exposure have been found in epidemiological studies to be involved in asthma pathogenesis. Viral infections, especially respiratory viruses, have been reported as major triggers of asthma exacerbation. In previous studies, viral infections were detected in 25% of asthma patients; however, this rate has changed in recent years, suggesting a more significant role for viral infections in asthma development (28). In fact, severe respiratory infections have been associated with increased sensitivity to common allergens in children, leading to a surge in IgE level and risk of asthma (29). Also, these viruses can induce inflammation that plays an essential role in asthma pathogenesis. The results of the current study showed a frequency of 41% (41/100) for viral infections in asthma patients.
In the present study, the prevalence of influenza viruses, parainfluenza, RSV, and rhinovirus in children with asthma was investigated. We also scrutinized the association of these viral infections with patients’ age and sex, as well as seasonal outbreak. Our results demonstrated that 41 patients were positive for the genomes of these viruses, including 21 (51.21%), 10 (24.39%), 7 (17.07%), and 3 (7.31%) for rhinoviruses, parainfluenza, RSV, and influenza viruses, respectively. Our results were consistent with the results of several previous studies, reporting a positivity rate ranging from 33 to 76.42% for rhinoviruses (30, 31). According to the results of these and other studies around the world, it can be noted that rhinoviruses are among important viral etiologies of asthma (32, 33). Likewise, besides being a major cause of common cold in children and adults, rhinoviruses are also considered to be the leading cause of asthma (6, 34).
The cytopathic effects of the rhinovirus on aerial pathways facilitate asthma development and progression. Specific receptors for different strains of the rhinovirus are expressed on the epithelial cells of airways, largely contributing to the role of the virus in asthma pathologic features (35, 36). On the other hand, previous studies have shown that the virus infects children under two years of age more than the children aged two to five years old, which might seed a background for allergic reactions in early childhood (37).
Another important virus causing infections in children is the parainfluenza virus. In our study, 24.3% of asthmatic children were positive for the parainfluenza virus. In most studies, this virus has been identified as the third most important factor in asthma development, preceded by the rhinovirus and RSV with the respective prevalence of 10 and 21.2% in children with asthma. In this regard, our results were consistent with the findings of similar studies (33, 34). In some studies, the prevalence of the parainfluenza virus was reported to be less than 10%, and the virus showed a lower seasonal incidence and also a lower prevalence in > 5-year-old children with advanced asthma compared with the rhinovirus and RSV (38, 39). In the present study, the seasonal prevalence of the parainfluenza virus was lower than that of the rhinovirus. There was a seasonal outbreak of the RSV and influenza virus, explaining the presence of these viruses in the respiratory tract.
In the current study, the prevalence of RSV was 17.07% that was almost identical to the prevalence of the parainfluenza virus. In terms of the seasonal incidence, the virus was reported only in autumn and winter. This virus infects boys and girls under two years of age. In some investigations, the prevalence of respiratory tract syndrome was in the range of 15 to 22%, which was similar to the ratio observed in our study (28, 40). The RSV has been reported as the dominant virus in asthma patients in some other studies (33, 41). Bronchiolitis due to RSV has been suggested as the main reason for the development and progression of asthma in children younger than two years of age (42).
In this study, the influenza virus showed a prevalence of 7.3%. The seasonal outbreak of this virus was low (exclusively in winter), indicating a relatively low prevalence in children with asthma, which is due to the presence of influenza viruses with different genotypes. In most studies; however, two different genotypes of this virus have been investigated. Also, different seasons in which viruses were isolated, as well as different geographical locations can influence the genotypes of isolated viruses (34, 43). The influenza virus in this study was observed only in children aged 2 to 5 years old and was not observed in children under the age of 2 years. Molecular methods have recently offered an increase in the sensitivity of detecting some viruses up to 10 times, rendering more reliable results (44, 45).
Our study had several limitations including a small sample size and being a single-center research. The assessed pathogens were also specific to the study area. Thus, our results are probably not applicable to other patient populations. Finally, we did not assess other possible microorganisms such as respiratory bacteria that may be involved in asthma pathogenesis. Overall, the association between viral infections and asthma requires further investigations.
5.1. Conclusions
Respiratory viruses were detected in 41% of the studied asthma patients. We here noticed that the patients infected with these viruses had more prominent and persistent cough symptoms, suggesting that respiratory viruses are involved in asthma pathogenesis. Nevertheless, confirming such associations requires further studies with larger sample sizes.
Acknowledgements
References
-
1.
Olin JT, Wechsler ME. Asthma: Pathogenesis and novel drugs for treatment. BMJ. 2014;349:g5517. [PubMed ID: 25420994]. https://doi.org/10.1136/bmj.g5517.
-
2.
Ishmael FT. The inflammatory response in the pathogenesis of asthma. J Am Osteopath Assoc. 2011;111(11 Suppl 7):S11-7. [PubMed ID: 22162373].
-
3.
Masoli M, Fabian D, Holt S, Beasley R; Global Initiative for Asthma Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469-78. [PubMed ID: 15080825]. https://doi.org/10.1111/j.1398-9995.2004.00526.x.
-
4.
World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: A comprehensive approach. Geneva, Switzerland: World Health Organization; 2007.
-
5.
Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International study of asthma and allergies in childhood (ISAAC): Rationale and methods. Eur Respir J. 1995;8(3):483-91. [PubMed ID: 7789502]. https://doi.org/10.1183/09031936.95.08030483.
-
6.
Peebles RSJ, Hartert TV. Respiratory viruses and asthma. Curr Opin Pulm Med. 2000;6(1):10-4. [PubMed ID: 10608419]. https://doi.org/10.1097/00063198-200001000-00003.
-
7.
Fazlollahi MR, Najmi M, Fallahnezhad M, Sabetkish N, Kazemnejad A, Bidad K, et al. Paediatric asthma prevalence: The first national population-based survey in Iran. Clin Respir J. 2019;13(1):14-22. [PubMed ID: 30472812]. https://doi.org/10.1111/crj.12975.
-
8.
Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis. 1990;142(6 Pt 1):1351-8. [PubMed ID: 2252253]. https://doi.org/10.1164/ajrccm/142.6_Pt_1.1351.
-
9.
Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701-9. [PubMed ID: 21345099]. https://doi.org/10.1056/NEJMoa1007302.
-
10.
Szabo SM, Levy AR, Gooch KL, Bradt P, Wijaya H, Mitchell I. Elevated risk of asthma after hospitalization for respiratory syncytial virus infection in infancy. Paediatr Respir Rev. 2013;13 Suppl 2:S9-15. [PubMed ID: 23269182]. https://doi.org/10.1016/S1526-0542(12)70161-6.
-
11.
Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343(8):538-43. [PubMed ID: 10954761]. https://doi.org/10.1056/NEJM200008243430803.
-
12.
Leonardi-Bee J, Pritchard D, Britton J. Asthma and current intestinal parasite infection: Systematic review and meta-analysis. Am J Respir Crit Care Med. 2006;174(5):514-23. [PubMed ID: 16778161]. https://doi.org/10.1164/rccm.200603-331OC.
-
13.
Hansbro NG, Horvat JC, Wark PA, Hansbro PM. Understanding the mechanisms of viral induced asthma: New therapeutic directions. Pharmacol Ther. 2008;117(3):313-53. [PubMed ID: 18234348]. [PubMed Central ID: PMC7112677]. https://doi.org/10.1016/j.pharmthera.2007.11.002.
-
14.
Keyvani H, Moghoofei M, Bokharaei-Salim F, Mostafaei S, Javad Mousavi SA, Monavari SH, et al. Prevalence of respiratory viruses in Iranian patients with idiopathic pulmonary fibrosis. J Med Microbiol. 2017;66(11):1602-6. [PubMed ID: 29068280]. https://doi.org/10.1099/jmm.0.000628.
-
15.
Empey DW, Laitinen LA, Jacobs L, Gold WM, Nadel JA. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976;113(2):131-9. [PubMed ID: 1247226]. https://doi.org/10.1164/arrd.1976.113.2.131.
-
16.
Message SD, Johnston SL. Host defense function of the airway epithelium in health and disease: Clinical background. J Leukoc Biol. 2004;75(1):5-17. [PubMed ID: 12972516]. [PubMed Central ID: PMC7167170]. https://doi.org/10.1189/jlb.0703315.
-
17.
Khalilzadeh S, Boloorsaz MR, Nadji SAR, Mahdaviani SAR, Baghaie N, Hassanzad M, et al. Molecular epidemiology of respiratory viral pathogens in children with asthma exacerbations admitted to Dr. Masih Daneshvari hospital. J Compr Ped. 2010;2(2):58-64.
-
18.
Moattari A, Aleyasin S, Emami A, Fyruzi M, Pirbonyeh N. The prevalence of human metapneumovirus and respiratory syncytial virus and coinfection with both in hospitalized children with acute respiratory infection in south of Iran. Arch Pediatr Infect Dis. 2015;3(3). e21581. https://doi.org/10.5812/pedinfect.21581v2.
-
19.
Nadji SA, Poos-Ashkan L, Khalilzadeh S, Baghaie N, Shiraghaei MJ, Hassanzad M, et al. Phylogenetic analysis of human bocavirus isolated from children with acute respiratory illnesses and gastroenteritis in Iran. Scand J Infect Dis. 2010;42(8):598-603. [PubMed ID: 20166863]. https://doi.org/10.3109/00365540903582442.
-
20.
Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: A longitudinal cohort study. Lancet. 2002;359(9309):831-4. [PubMed ID: 11897281]. https://doi.org/10.1016/S0140-6736(02)07953-9.
-
21.
Leung TF, To MY, Yeung AC, Wong YS, Wong GW, Chan PK. Multiplex molecular detection of respiratory pathogens in children with asthma exacerbation. Chest. 2010;137(2):348-54. [PubMed ID: 19749009]. [PubMed Central ID: PMC7094527]. https://doi.org/10.1378/chest.09-1250.
-
22.
Uhl EW, Castleman WL, Sorkness RL, Busse WW, Lemanske RFJ, McAllister PK. Parainfluenza virus-induced persistence of airway inflammation, fibrosis, and dysfunction associated with TGF-beta 1 expression in brown Norway rats. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1834-42. [PubMed ID: 8970378]. https://doi.org/10.1164/ajrccm.154.6.8970378.
-
23.
Schleich FN, Manise M, Sele J, Henket M, Seidel L, Louis R. Distribution of sputum cellular phenotype in a large asthma cohort: Predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm Med. 2013;13:11. [PubMed ID: 23442497]. [PubMed Central ID: PMC3657295]. https://doi.org/10.1186/1471-2466-13-11.
-
24.
Moghoofei M, Monavari SH, Mostafaei S, Hadifar S, Ghasemi A, Babaei F, et al. Prevalence of influenza A infection in the Middle-East: A systematic review and meta-analysis. Clin Respir J. 2018;12(5):1787-801. [PubMed ID: 29316311]. https://doi.org/10.1111/crj.12758.
-
25.
Esghaei M, Moghoofei M, Keshavarz M, Keyvani H, Bokharaei-Salim F, Farahmand M, et al. Trends in surveillance data of influenza virus in Tehran before decreasing dispatch of Iranian Hajj pilgrims to Mecca. Med J Islam Repub Iran. 2018;32:41. [PubMed ID: 30159292]. [PubMed Central ID: PMC6108279]. https://doi.org/10.14196/mjiri.32.41.
-
26.
Bush A. Diagnosis of asthma in children under five. Prim Care Respir J. 2007;16(1):7-15. [PubMed ID: 17297521]. [PubMed Central ID: PMC6634180]. https://doi.org/10.3132/pcrj.2007.00001.
-
27.
Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Gotz M, et al. Diagnosis and treatment of asthma in childhood: A PRACTALL consensus report. Allergy. 2008;63(1):5-34. [PubMed ID: 18053013]. https://doi.org/10.1111/j.1398-9995.2007.01586.x.
-
28.
Khetsuriani N, Kazerouni NN, Erdman DD, Lu X, Redd SC, Anderson LJ, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119(2):314-21. [PubMed ID: 17140648]. [PubMed Central ID: PMC7112359]. https://doi.org/10.1016/j.jaci.2006.08.041.
-
29.
Kimpen JL. Viral infections and childhood asthma. Am J Respir Crit Care Med. 2000;162(3 Pt 2):S108-12. [PubMed ID: 10988163]. https://doi.org/10.1164/ajrccm.162.supplement_2.ras-11.
-
30.
Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111(1):66-71. [PubMed ID: 12532098]. [PubMed Central ID: PMC7112360]. https://doi.org/10.1067/mai.2003.33.
-
31.
Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119(5):1105-10. [PubMed ID: 17353039]. [PubMed Central ID: PMC7125611]. https://doi.org/10.1016/j.jaci.2006.12.669.
-
32.
Lamson D, Renwick N, Kapoor V, Liu Z, Palacios G, Ju J, et al. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004-2005. J Infect Dis. 2006;194(10):1398-402. [PubMed ID: 17054069]. [PubMed Central ID: PMC7110122]. https://doi.org/10.1086/508551.
-
33.
Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310(6989):1225-9. [PubMed ID: 7767192]. [PubMed Central ID: PMC2549614]. https://doi.org/10.1136/bmj.310.6989.1225.
-
34.
Tan WC. Viruses in asthma exacerbations. Curr Opin Pulm Med. 2005;11(1):21-6. [PubMed ID: 15591884]. https://doi.org/10.1097/01.mcp.0000146781.11092.0d.
-
35.
Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, Marlor CW, et al. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56(5):839-47. https://doi.org/10.1016/0092-8674(89)90688-0.
-
36.
Canonica GW, Ciprandi G, Pesce GP, Buscaglia S, Paolieri F, Bagnasco M. ICAM-1 on epithelial cells in allergic subjects: A hallmark of allergic inflammation. Int Arch Allergy Immunol. 1995;107(1-3):99-102. [PubMed ID: 7613226]. https://doi.org/10.1159/000236943.
-
37.
Edwards MR, Bartlett NW, Hussell T, Openshaw P, Johnston SL. The microbiology of asthma. Nat Rev Microbiol. 2012;10(7):459-71. [PubMed ID: 22669219]. [PubMed Central ID: PMC7097220]. https://doi.org/10.1038/nrmicro2801.
-
38.
Minor TE, Dick EC, DeMeo AN, Ouellette JJ, Cohen M, Reed CE. Viruses as precipitants of asthmatic attacks in children. JAMA. 1974;227(3):292-8. [PubMed ID: 4358170].
-
39.
Horn ME, Brain EA, Gregg I, Inglis JM, Yealland SJ, Taylor P. Respiratory viral infection and wheezy bronchitis in childhood. Thorax. 1979;34(1):23-8. [PubMed ID: 220747]. [PubMed Central ID: PMC471001]. https://doi.org/10.1136/thx.34.1.23.
-
40.
Lemanske RFJ, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571-7. [PubMed ID: 16159626]. https://doi.org/10.1016/j.jaci.2005.06.024.
-
41.
Camara AA, Silva JM, Ferriani VP, Tobias KR, Macedo IS, Padovani MA, et al. Risk factors for wheezing in a subtropical environment: Role of respiratory viruses and allergen sensitization. J Allergy Clin Immunol. 2004;113(3):551-7. [PubMed ID: 15007360]. [PubMed Central ID: PMC7127801]. https://doi.org/10.1016/j.jaci.2003.11.027.
-
42.
Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9(9):731-45. [PubMed ID: 21905783]. [PubMed Central ID: PMC3215509]. https://doi.org/10.1586/eri.11.92.
-
43.
Kim WK. Association between respiratory viruses and asthma exacerbations. Korean J Pediatr. 2014;57(1):26-8. [PubMed ID: 24578713]. [PubMed Central ID: PMC3935109]. https://doi.org/10.3345/kjp.2014.57.1.26.
-
44.
Freymuth F, Vabret A, Galateau-Salle F, Ferey J, Eugene G, Petitjean J, et al. Detection of respiratory syncytial virus, parainfluenzavirus 3, adenovirus and rhinovirus sequences in respiratory tract of infants by polymerase chain reaction and hybridization. Clin Diagn Virol. 1997;8(1):31-40. [PubMed ID: 9248656]. https://doi.org/10.1016/s0928-0197(97)00060-3.
-
45.
Ellis JS, Fleming DM, Zambon MC. Multiplex reverse transcription-PCR for surveillance of influenza A and B viruses in England and Wales in 1995 and 1996. J Clin Microbiol. 1997;35(8):2076-82. [PubMed ID: 9230385]. [PubMed Central ID: PMC229906]. https://doi.org/10.1128/JCM.35.8.2076-2082.1997.