Abstract
Background:
Ammonia is a commonly used chemical in the process industries. Chemical leakage is one of the main problems threatening the staff, facilities, and the environment in the process industries.Objectives:
The aim of this study was to model the emission of ammonia and its consequences in the petrochemical industry.Methods:
In this study, three accident scenarios of the most probable ones were chosen, including toxic vapor cloud, jet fire, and boiling liquid expanding vapor explosion (BLEVE). Then, the scenario modeling was done using areal locations of hazardous atmospheres (ALOHA) software.Results:
In the first scenario, the total released ammonia is 81,316 kg. The concentration of ammonia toxic vapor is greater than 1,100 ppm (AEGL-3 region) at a distance of 1 km, which might cause death in 60 seconds. The overpressure never exceeds 3.5 psi; thus, there is no possibility of serious injury or destruction of buildings. In the third scenario, the thermal radiation of BLEVE is greater than 10 kW/m2 at a distance of 376 m, which is potentially lethal within 60 seconds.Conclusions:
One of the main risks in petrochemical companies is the leakage of ammonia. The toxicity of ammonia is the most significant threat to people. The overpressure of vapor cloud explosion does not cause serious injury or of building destruction. The thermal radiation from jet fire and fireball has no effect on the city while it may cause death to the staff within 60 seconds. Thus, safety precautions should be considered to prevent the consequences of leakage accidents.Keywords
1. Background
Process industries have long been established as heavy industries to underlie the development of countries. Employees in the petrochemical industries often work under dangerous conditions because there is always the possibility of accidents such as toxic substance leakage (1, 2). The process failure, facility failure, and human error or the integration of the three factors can cause unusual situations (3). The main consequences of a petrochemical accident might be fire, explosion, and toxicity (4). Therefore, the accidents will threaten the existence and development of process industries. Bhopal disaster is probably the world's worst industrial disaster in which about 41 metric tons of methyl isocyanate gas unintentionally leaked from a reservoir of an Indian company in Bhopal in 1984 and killed thousands of people as an immediate consequence (5).
Ammonia is a widely used chemical in the process industries. Exposure to concentrations exceeding 2,500 ppm can be lethal if the duration of exposure exceeds 30 minutes, and is immediately fatal at 5,000 ppm (6). Ammonia is stored in a liquid state under pressure. Any opening in ammonia reservoirs can lead to its leakage. In one of the accidents in an ethanolamine plant in Kentucky, USA, in 1968, an explosion occurred when ammonia of the plant accidentally entered the ethylene oxide feed vessel. One man was killed and 12 were injured (7).
According to the occurred accidents, the approach to hazards and unsafe conditions has changed over the last decades. This approach is named the loss prevention approach that deals with paying more attention to technological measures to control hazards and unsafe conditions (1).
One of the useful methods to reduce the consequences of an accident is its modeling and investigation using simulation software (8). There are different simulators such as PHAST (process hazard analysis software tool) and ALOHA (areal locations of hazardous atmospheres) that can be used to simulate the emission of toxic and dangerous materials (9).
ALOHA software was created by the Environmental Protection Agency to model different scenarios such as toxic gas clouds, flammable gas clouds, and BLEVE (Boiling liquid expanding vapor explosion) (10, 11). The database of ALOHA consists of more than 1,000 substances (12). ALOHA uses AEGLs (acute exposure guideline levels) for airborne chemicals for the modeling of events such as ammonia emission in the petrochemical industry. ALOHA is free for use; thus, it is widely used by experts, organizations, and departments (13). Lee et al. indicated that ALOHA is the best simulator for the determination of ammonia toxicity (14).
Kamali and Mohammadi analyzed the consequences of ammonia leakage in Kermanshah petrochemical company using PHAST software and found that the nearby residential complexes are at risk of ammonia toxicity, especially in winter (15). In another survey, the distribution of ammonia concentration was simulated based on the Gaussian model; the dangerous area was divided into five zones and the severity and scope of ammonia leakage were determined (16). Lim et al. computed the influence ranges of ammonia emission using KORA (Korea off-site risk assessment) supporting tool with four different environmental factors: ground roughness, sealing, operating temperature, pressure, and leakage hole size (17). In another study, the leakage of ammonia in the storage of an industrial area was investigated using ALOHA and the affected population was estimated using the interpolation method in GIS (18). Yilang et al. used the causing factors and the fault trees to propose the theoretical risk control system model of liquid ammonia leakage accidents (19).
2. Objectives
There are scarce data on simulating different probable scenarios of ammonia leakage accidents in the petrochemical industry to estimate the loss of property and determine the population at risk. Thus, the aim of this study was to model the leakage of ammonia and determine its consequences in the petrochemical industry using ALOHA software.
3. Methods
This study dealt with the modeling of ammonia emission in a petrochemical company in Asaluyeh. The selected company is one the largest and most important companies in Iran with the history of several leakage accidents and the available results of HAZOP (hazard and operability) study. In this study, a panel of experts used the results of the HAZOP study to determine the likelihood and severity of the consequences for each scenario and graded the scenarios using the 5 × 5 risk rating matrix. Finally, the worst scenarios (with the maximum risk number) were chosen for modeling.
In this company, ammonia is stored as a liquid in a 60,000-gallon reservoir. The given accident occurred on 8 Aug 2012 at 5:30 AM. The ALOHA simulation was limited to 1 hour after the beginning of the leakage. The tank was vertical with the height of 100 f. The hole was rectangular in shape. Its length and width were 13 in and 0.4 in, respectively. About 80% of the tank was full. The leak was 2 m above the bottom of the tank. According to the annual wind rose data of the region provided by Iran Meteorological Organization in 2012, the wind was blowing to the southwest (20) with the speed of 15 mph. The air temperature was 38°C and the relative humidity was 47%. The sky was partly cloudy. There were no forest or urban areas around the source. The stability class was D and there was no inversion. Different levels of AEGL were as follows:
AEGL-3 is the airborne concentration of a chemical, in ppm or mg/m3, that is potentially lethal to people.
AEGL-2 is the airborne concentration of a chemical, in ppm or mg/m3, that causes irreversible permanent adverse effects on people.
AEGL-1 is the airborne concentration of a chemical, in ppm or mg/m3, that causes pain and irritation in people. However, the consequences are not disabling but transient after the end of the exposure (21).
The scenarios were as follows:
1- The leaking chemical forms an evaporating puddle but it is not burning.
2- The leaking chemical is burning like a jet fire.
3- The tank explodes and the chemical burns in a fireball.
4. Results
4.1. The First Scenario
The evaporation rate is 1,690 kg/min in the beginning, as shown in Figure 1. The total released amount of liquid is 81,316 kg.
The evaporation rate of ammonia

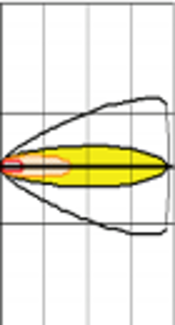
According to Figure 2, the accidental release of ammonia would cause a red zone of 1 km (AEGL-3: 1,100 ppm), an orange zone of 3.3 km (AEGL-2: 160 ppm), and a yellow zone stretching to 7.8 km (AEGL-1: 30 ppm) to downwind from the source.
The toxic area of the vapor cloud

The overpressure threat zone is shown in Figure 3. The pressure is 1 psi at a distance of 64 m from the tank and it never exceeds 3.5 psi.
The blast area of vapor cloud explosion

4.2. The Second Scenario
The maximum flame length is 50 m and the maximum burn rate is 1,760 kg/min. According to Figure 4, the thermal radiation level is greater than 10 kW/m2 (red zone) at a distance of 10 m. Furthermore, there is a yellow zone of 37 m from the source.
Text modeling of the jet fire

4.3. The Third Scenario
The modeling of the BLEVE, as shown in Figure 5, indicates that the fireball diameter is 289 m and the burn duration is 17 seconds. The thermal radiation from the fireball makes three different zones of red, orange, and yellow at distances of 376, 546, and 864 m from the tank, respectively.
The threat zone of thermal radiation from the fireball

5. Discussion
The evaporation rate is the amount of a liquid that turns to the vapor state within a certain time. The exposed surface of the puddle to the air decreases by time; therefore, the evaporation rate of the toxic ammonia vapor shows a descending function and its maximum level occurs in the initial period. Thus, the emergency response at the beginning of the leakage is vital because the release rate is the highest.
The distance from the source to the control room is 90 m and to the nearby companies is 125 m, which are in the red zone of BLEVE and AEGL-3; thus, the ammonia concertation is potentially lethal. Jafari et al. found that the staff of control rooms are at the highest risk of an accident (22). Besides, the nearby companies should consider the results of modeling in locating the facilities. Furthermore, storerooms and transfer lines of the company are at a distance of 70 m in the possible region of fire and explosion; thus, new storerooms should be built up out of this region. It is also suggested that the current storeroom be used for storing inflammable and nonexplosive materials.
The nearest city in the southwest of the source is located at a distance of 4.5 km from the tank (AEGL-1 region). Therefore, the accident may cause citizens to experience pain or irritation. There is no other threat like fire or explosion. In line with this result, Anjana et al. indicated that according to the distribution model of ammonia vapor, the worst scenario would cause a dangerous region of 4 km with a possibility of death for over one-hour exposure although the region is an inhabited area. They also suggested that the weather condition influences the region of hazard; the threat area is greater in winter than in summer and the evening is less dangerous than the morning (18). The main focus of the mentioned study was on assessing the extent of hazard areas under different atmospheric conditions while in the current study, one certain air condition was imagined. Another study assessed an ammonia incident in the industrial area of Matanzas and found that the red zone of ammonia toxic vapor cloud is 3,400 m (23). The mentioned study estimated the affected people using a probit equation, while the current study did not determine the injured people. However, both studies supported the theory that the toxicity of ammonia is the most significant threat to the staff.
In a 2018 survey focused on the modeling of the ammonia leakage from ammonia reservoirs in one of the process industries in the south of Iran (24), it was found that the total amount of ammonia emission is 21,7500 kg within 10 minutes and the overpressure is greater than 8 psi at a distance of 700 m from the source that would destruct the buildings. However, in this study, the total amount of ammonia emission is 81,316 kg and the overpressure does not exceed 3.5 psi; thus, there is no possibility of serious injury or destruction of buildings. However, the amount of overpressure at a distance of 64 m to downward from the tank may shatter the glass.
Kamali and Mohammadi found that the thermal radiation level from jet fire is greater than 12.5 kW/m2 at a distance of 31.85 m in summer (15), while in this study, the thermal radiation level is greater than 10 kW/m2 (red zone) at a distance of 10 m, which is potentially lethal within 60 seconds.
The red and orange zones are both 10 m in the second scenario, the reason is probably the limitation of ALOHA in analyzing these consequences precisely, at very close distances. As the thermal radiation from the jet fire is potentially lethal at a distance of 10 m from the source, visit limitations and safe distance should be considered in this area. Atabi et al. found the safe distance for five commonly used toxic materials in the accidents of road transportation using PHAST, CEI (chemical exposure index), and ALOHA and reported that based on ERPG-3 (emergency response planning guidelines), ammonia had the highest hazard distance (2,800 m) (8).
According to Figure 5, the wind direction has no effect on the dispersion of thermal radiation. However, glass or other weak materials in the company and nearby industries may be shattered; thus, it is suggested that high-quality and pressure-resistant materials be used in buildings. A specific ventilation system should be designed for emergencies to keep the concentration of ammonia below 25% of the lower explosive limit to prevent the explosion accident (25).
The results of the modeling enable us to take precautions against accidents. As changing the location of ammonia tanks may be impossible, the building of protective walls around ammonia reservoirs can prevent the emission of the gas by reducing the effect of the wind (18). In this study, the wind is often blown to the southwest; thus, the muster station must not be designed in the wind direction. The number of at-risk people should be estimated to make the muster station with enough capacity and facilities. Due to the lack of time, Horng et al. suggested that people in the ERPG-2 region should shelter in place instead of escaping (26). The mechanism of fire and explosion is very fast and a delay in human reaction could make the condition worse. Therefore, it is useful to design devices that sense the leakage quickly and give the staff enough time to prepare and react in an emergency. Xibo and Ru-Yyue designed a wireless alarm system for monitoring the concentration of ammonia within the process line and detecting the location of ammonia leakage (27). The reason for many industrial accidents is the corrosion of facilities; thus, technical inspection is an effective preventive measure for accidents (28). Other precautions include holding the fire and explosion maneuvers, regular safety inspections, and supplying enough first-aid kits.
5.1. Limitations and Strengths
The limitations of ALOHA account for part of the limitations of the study. ALOHA cannot model the chemical mixtures; besides, the simulation duration in some sections is limited. Moreover, the accuracy of the results is not acceptable in extremely low velocities of the wind and highly stable weather conditions. Creating a panel of experts to rate and determine the scenarios and using simple, reliable software for modeling the consequences are the strengths of the study. Future research may focus on different factors influencing the dispersion model of ammonia, simulating the release of a mixture of substances using other software, determining the affected people, and providing the emergency preparedness and response (EPR).
5.2. Conclusions
This study showed that ammonia toxic vapor poses the main risk in a leakage accident. It is potentially lethal (at a concentration of 1,100 ppm) to the staff of the company and the nearby factories. Moreover, it can cause pain (at a concentration of 30 ppm) in the citizens of the nearest city. The thermal radiation from the jet fire and BLEVE causes death to people who are very close to the source. The overpressure is not enough to destruct the buildings. ALOHA is a simple software that can simulate the consequences of an accident with an acceptable estimation but it cannot be used for complex scenarios. It is proposed to consider the safety in locating the facility, safety inspections, safety devices, visit limitations, safe distances, safety awareness promotion, and EPR development as some of the precautions that might be effective to prevent the consequences of a leakage accident.
Acknowledgements
References
-
1.
Lees F. Lees' loss prevention in the process industries: Hazard identification, assessment and control. Oxford: Butterworth-Heinemann; 2012.
-
2.
Jafari MJ, Nourai F, Pouyakian M, Torabi SA, Rafiee Miandashti M, Mohammadi H. Barriers to adopting inherently safer design philosophy in Iran. Process Saf Prog. 2018;37(2):221-9. https://doi.org/10.1002/prs.11927.
-
3.
Eljack F, Kazi MK. Process safety and abnormal situation management. Curr Opin Chem Eng. 2016;14:35-41. https://doi.org/10.1016/j.coche.2016.07.004.
-
4.
Chang JI, Lin CC. A study of storage tank accidents. J Loss Prev Process Ind. 2006;19(1):51-9. https://doi.org/10.1016/j.jlp.2005.05.015.
-
5.
Rajkumar S. Safety security and risk management - aftermath bhopal disaster. Int J Biosen Bioelectron. 2017;2(6):180-3. https://doi.org/10.15406/ijbsbe.2017.02.00044.
-
6.
Neghab M, Mirzaei A, Kargar Shouroki F, Jahangiri M, Zare M, Yousefinejad S. Ventilatory disorders associated with occupational inhalation exposure to nitrogen trihydride (ammonia). Ind Health. 2018;56(5):427-35. [PubMed ID: 29887542]. [PubMed Central ID: PMC6172184]. https://doi.org/10.2486/indhealth.2018-0014.
-
7.
Shao H, Duan G. Risk quantitative calculation and ALOHA simulation on the leakage accident of natural gas power plant. Procedia Eng. 2012;45:352-9. https://doi.org/10.1016/j.proeng.2012.08.170.
-
8.
Atabi F, Ghorbani R, Jabbari M. [Assessment of safe distance for five toxic materials commonly in the accidents of chemical road transportation using ALOHA and PHAST software and CEI index (case study: Tehran-Qazvin highway)]. Iran Occup Health J. 2017;14(4):42-35. Persian.
-
9.
Beheshti MH, Hajizadeh R, Mehri A, Jebeli MB. [Modeling the result of hexane leakage from storage tanks and planning an emergency response program in a petrochemical complex]. Iran Occup Health. 2016;13(69-79). Persian.
-
10.
Tseng JM, Su TS, Kuo CY. Consequence evaluation of toxic chemical releases by ALOHA. Procedia Eng. 2012;45:384-9. https://doi.org/10.1016/j.proeng.2012.08.175.
-
11.
Bhattacharya R, Kumar G. Consequence analysis for simulation of hazardous chemicals release using ALOHA software. Int J ChemTech Res. 2015;8(4):2038-46.
-
12.
Poluyan LV, Syutkina EV, Guryev ES. Software systems for prediction and immediate assessment of emergency situations on municipalities territories. IOP Conf Ser Mater Sci Eng. 2017;262:12199. https://doi.org/10.1088/1757-899x/262/1/012199.
-
13.
Timashev SA, Alekhin VN, Poluyan LV, Guryev ES. Innovative masters program "safety of civil engineering critical infrastructures and territories". IOP Conf Ser Mater Sci Eng. 2015:1166-70. https://doi.org/10.1109/icl.2015.7318199.
-
14.
Lee HE, Sohn JR, Byeon SH, Yoon SJ, Moon KW. Alternative risk assessment for dangerous chemicals in south korea regulation: Comparing three modeling programs. Int J Environ Res Public Health. 2018;15(8). [PubMed ID: 30060550]. [PubMed Central ID: PMC6121683]. https://doi.org/10.3390/ijerph15081600.
-
15.
Kamali J, Mohammadi H. Modeling and analysis of the consequences of Kermanshah Petrochemical ammonia tank with using phast7.11 software. 6th National Conference on Safety Engineering & HSE Management. 2016.
-
16.
Wang W, Xu Y, Zhang J. Research of ammonia concentration distribution simulation in road transportation leakage based on the Gaussian model. 1st International Conference on Transportation Infrastructure and Materials. 2016.
-
17.
Lim H, Kwak S, Jung J, Ryu T, Choi W, Lee J, et al. A study on the factors affecting the influence ranges of ammonia leakage by using KORA program. J Korean Inst Gas. 2018;22(3):38-44. Korean.
-
18.
Anjana NS, Amarnath A, Harindranathan Nair MV. Toxic hazards of ammonia release and population vulnerability assessment using geographical information system. J Environ Manage. 2018;210:201-9. [PubMed ID: 29353114]. https://doi.org/10.1016/j.jenvman.2018.01.021.
-
19.
Yilang H, Xiaoli Y, Chao H. Analysis of liquid ammonia leakage accidents based on safety system engineering theory. Int J Bus Soc Sci. 2014;5(1):220-6.
-
20.
Iran Meteorological Organization. Historical and climate data. Tehran: Iran Meteorological Organization; 2019. Available from: http://irimo.ir/far/web_directory/2703.html.
-
21.
Nance P, Patterson J, Willis A, Foronda N, Dourson M. Human health risks from mercury exposure from broken compact fluorescent lamps (CFLs). Regul Toxicol Pharmacol. 2012;62(3):542-52. [PubMed ID: 22142629]. https://doi.org/10.1016/j.yrtph.2011.11.008.
-
22.
Jafari MJ, Zarei E, Dormohammadi A. [Presentation of a method for consequence modeling and quantitative risk assessment of fire and explosion in process industry (case study: Hydrogen production process)]. J Journal of Health and Safety at Work. 2013;3(1):55-68. Persian.
-
23.
Orozco JL, Van Caneghem J, Hens L, González L, Lugo R, Díaz S, et al. Assessment of an ammonia incident in the industrial area of Matanzas. J Clean Prod. 2019;222:934-41. https://doi.org/10.1016/j.jclepro.2019.03.024.
-
24.
Shirali GA, Mosavian Asl Z, Jahani F, Siahi Ahangar A, Etemad S. [Modeling the effect of ammonia leakage from ammonia reservoirs using ALOHA software and developing an emergency response program in one of process industries]. J Occup Hyg Eng. 2018;5(2):12-9. Persian. https://doi.org/10.21859/johe.5.2.12.
-
25.
Gangopadhyay RK, Das SK. Ammonia leakage from refrigeration plant and the management practice. Process Saf Prog. 2008;27(1):15-20. https://doi.org/10.1002/prs.10208.
-
26.
Horng JJ, Lin YS, Shu CM, Tsai E. Using consequence analysis on some chlorine operation hazards and their possible effects on neighborhoods in central Taiwan. J Loss Prevent Proc. 2005;18(4-6):474-80. https://doi.org/10.1016/j.jlp.2005.07.024.
-
27.
Xibo D, Ru-Yue W. Development of ammonia gas leak detection and location method. J TELKOMNIKA. 2017;15(3):1207-14. https://doi.org/10.12928/telkomnika.v15i3.5079.
-
28.
Pourbabaki R, Karimi A, Yazdanirad S. [Modeling the consequences and analyzing the dangers of carbon disulfide emissions using ALOHA software in an oil refinery]. J Health Field. 2019;6(3):1-9. Persian.