Abstract
Background:
Chromium (Cr) is an important heavy metal widely used in industries. It is one of the seventeen chemicals with the highest danger to human health according to the United States environmental protection agency (USEPA).Objectives:
The aim of this study was to isolate and identify Cr (VI)-resistant bacteria from Soltan Abad River sediments (Shiraz-Iran) and evaluate their potential for the detoxification of Cr (VI) to Cr (III) under environmental conditions.Materials and Methods:
Bacterial isolates were identified on the basis of colony morphology, Gram staining, and biochemical tests using standard microbiological methods. The minimum inhibitory concentrations (MICs) of chromium were determined by the broth agar dilution method. Cr (VI) reduction assay was determined by measuring the absorbance of the purple complex of Cr (VI) with 1,5-diphenylcarbazide. The growth of Cr (VI)-resistant bacteria was determined at different concentrations of Cr (VI). Finally, the effects of temperature and pH on the growth of selected bacteria and Cr (VI) reduction were investigated using an Luria-Bertani (LB) medium containing 50 mg/L Cr (VI).Results:
The results indicated that Pseudomonas aeruginosa and Serratia marcescens were the most resistant bacteria with MICs of 200 and 150 mg/L, respectively. Both bacteria completely reduced 25 and 50 mg/L of Cr (VI) in 36 hours and 48 hours, respectively. The growth rate of both bacteria decreased with increases in the Cr (VI) concentration, and the chromate reduction was directly correlated with the growth of the bacteria. These bacteria were capable of reducing Cr (VI) at a wide range of temperatures (25 to 45°C) and pH levels (5 to 9). The optimum medium conditions for Cr (VI) reduction and the growth of the isolates were temperatures between 30 and 40°C and a pH of 7.0 to 8.0.Conclusions:
P. aeruginosa and S. marcescens can be good candidates for the detoxification of Cr (VI) in Soltan Abad River.Keywords
Chromium (VI) Resistant Bacteria Minimum Inhibitory Concentration Pseudomonas aeruginosa Serratia marcescens
1. Background
Chromium (Cr) is an important heavy metal widely used in industries. It is released into the environment by a large number of industrial operations, including chrome plating, petroleum refining, leather tanning, wood preservation, textile manufacturing, and pulp processing (1). Cr is one of the seventeen chemicals with the highest danger to human health according to The United States environmental protection agency (USEPA). According to the world health organization (WHO), the maximum concentration of chromium in drinking water should be no more than 0.05 mg/L (2). Hexavalent Cr (Cr6+) is the most toxic and carcinogenic form of Cr, due to its high solubility in water, rapid permeability through biological membranes, and subsequent interaction with intracellular proteins and nucleic acids (3). There are several methods for Cr (VI) removal, such as chemical precipitation, membrane processing, ion exchange, liquid extraction, and electro dialysis (4). These methods are quite expensive and have many other disadvantages, like the generation of toxic sludge and other wastes, high energy requirements, and incomplete metal removal (5). On the other hand, bioremediation, which uses indigenous microorganisms, is an ecofriendly alternative for the detoxification and removal of Cr-pollutants (6). Microbial treatment of Cr (VI) pollution has the advantages of economy, efficiency, and no secondary pollution. Bacterial and fungal remediation has been used in various circumstances (7). Biosorption, bioaccumulation, and enzymatic oxidation/reduction are the processes by which the microorganisms interact with toxic metals. The enzymatic reduction of Cr (VI) involves membrane-bound chromate reductase during anaerobic respiration or a soluble cytosolic chromate reductase under aerobic conditions, the activity of which is enhanced by NADH or glutathione as enzyme co-factors. Throughout this entire process, chromate acts as the terminal electron acceptor (8). There is a strong correlation between the Cr content and the number of metal-resistant bacteria in the soil (9). A wide variety of bacteria have been reported for reducing/transforming Cr (VI) to Cr (III), under aerobic and anaerobic conditions, e.g., Intrasporangium sp. Q5-1 (10), Bacillus sp. (11), Escherichia coli (12), Pseudochrobactrum asaccharolyticum (13), Pseudomonas sp. (14), Staphylococcus aureus IFR-2, and Pediococcus pentosaceus IFR-3 (15).
Soltan-Abad river is a seasonal river in the south of Shiraz, which after passing the industrial region, will end up in Maharlou Lake. High chromium contamination sources of this river are mainly due to industrial and domestic contamination and agriculture activities resulting from the utilization of fertilizers and mycocides (16).
2. Objectives
The aim of this study was to isolate and identify Cr (VI)-resistant bacteria from Soltan Abad river sediments (Shiraz-Iran) and evaluate their potential for the detoxification of Cr (VI) to Cr (III) under environmental conditions.
3. Materials and Methods
3.1. Sampling
Sediment sampling was done at three Soltan Abad river stations. The first sampling station was a site contaminated with urban waste water, while the second and third stations were contaminated with agriculture and industrial wastes, respectively. GPS was used to determine the geographical situation of each site (Table 1). Triplicate sampling was performed 3 times in the spring and summer of 2013, three times in sterilized glass containers for microbial examinations. The depth of sediment sampling was 3 - 5 cm. The in situ temperature and pH have been measured, and samples were transferred in ice packs to the laboratory within 24 hours.
Location of Sampling Stations
| Station | Region | Coordinate | |
|---|---|---|---|
| North | East | ||
| 1 | River sediments under the Soltan Abad entrance bridge | 29° 32ʹ 11.1ʺ | 52° 33ʹ 12.2ʺ |
| 2 | River sediments under the Soltan Abad town center bridge | 29° 31ʹ 12.7ʺ | 52° 32ʹ 26.2ʺ |
| 3 | River sediments under the bridge of the Fasa Road Traffic Police | 29° 29ʹ 07.6 | 52° 38ʹ 17.4ʺ |
3.2. Counting Bacteria
For the enumeration of bacteria, a viable plate count technique was performed. A tenfold serial dilution was prepared by sequential dilutions of 1 gram of sediment samples. Then, 100 µL of the diluted suspension was transferred to nutrient agar plates containing 1 mM of potassium dichromate and incubated for 72 hours at 30°C. Nutrient agar plates without potassium dichromate were used as controls. Following incubation, the plates were checked for colony growth (17).
3.3. Enrichment and Isolation of Chromium (VI)-resistant Bacteria
A total of 1.0 g of sediment was added into flasks containing 100 mL of Luria Bertani (LB) broth medium (1% tryptophan, 0.5% yeast extract, 1% NaCl, a pH of 7) supplemented with potassium dichromate to reach a 50 mg/L concentration of Cr (VI) and maintained in a shaker incubator with 160 rpm at 30°C for 5 days. The medium was then cultured on LB agar plates supplemented with a similar Cr concentration using the spread plate method and incubated at 30°C for 24 to 48 hours (18). Bacterial isolates were identified on the basis of colony morphology, Gram staining, and biochemical tests using standard microbiological methods (19).
3.4. Determination of Minimum Inhibitory Concentrations (MICs) of Cr (VI)
The minimum inhibitory concentrations (MICs) of Cr at which no colony growth occurred were determined by the broth agar dilution method. The isolates were inoculated individually into 25 mL peptone water consisting of 1.0% (w/v) peptone and 5.0% (w/v) NaCl in 150 mL conical flasks and incubated at 28°C at 150 rpm to achieve a bacterial log phase. Nutrient agar plates containing different concentrations of Cr (VI) (50 - 250 mg/L) were inoculated aseptically from the exponential growing cultures of each bacterial strain. These plates were incubated at 37°C for 48 hours. The MIC was considered to be the lowest concentration of Cr (VI) at which no visible growth occurred (18). According to the results, two bacteria (Pseudomonas aeruginosa and Serratia marcescens) were selected for further analysis.
3.5. Cr (VI) Reduction Assay
The more resistant bacteria (P. aeruginosa and S. marcescens) were inoculated into 100 mL Screw capped glass vials containing 85 mL of broth medium (a pH of 8.0 ± 0.2) containing (g/L): sodium thioglycollate (0.5), L-cysteine (0.25), peptone (5.0), NaCl (2.5), sodium sulphite (0.1), supplemented with sterilized Cr (VI) by a 0.22 μm membrane filter (25, 50, 100, and 150 mg/L), and incubated at 37 ± 1°C for 108 hours. Uninoculated media containing Cr (VI) were used as controls. Samples were collected at regular 12-hour intervals up to 96 hours and centrifuged at 10,000 rpm for 10 minutes at 4°C. The supernatant obtained after centrifugation was used to measure Cr (VI) concentrations. The Cr (VI) content in each supernatant was determined by measuring the absorbance of the purple complex of Cr (VI) with 1,5-diphenylcarbazide (DPC, Sigma-Aldrich) at 540 nm using a UV-Vis spectrophotometer (Shimadzu 1700, Japan). The DPC reagent was prepared by adding 24 mL of 85% H3PO4 to 56 mL of distilled water. This solution was mixed with 0.076 g of DPC previously dissolved in 20 ml of 95% ethanol. The reagent was stored in the dark at 4°C. The reaction mixture was set up in a 10 mL volumetric flask as follows: 200 μL of sample volume was increased to 1 mL using glass distilled water, followed by the addition of 330 μL of 6 M of H2SO4 and 400 μL of DPC, and the final volume was increased to 10 mL using glass distilled water. The Cr (VI) concentration was calculated by the standard curve of K2Cr2O7 (25, 50, 100, and 150 mg/L). The efficiency of chromate reduction was determined in terms of % Cr (VI) reduction by measuring the difference between the Cr (VI) concentration at 0 and 108 hours of growth. Total chromium [Cr (VI) + Cr (III)] was measured using a Perkin-Elmer Analyst 300 atomic absorption spectrophotometer (AAS). Hexavalent Cr reduction was determined from the difference between total Cr and Cr (VI) and Cr (III) concentrations (20, 21).
3.6. Growth of Chromium (VI)-resistant Bacteria
The more Cr-resistant bacterial isolates (P. aeruginosa and S. marcescens) were inoculated into LB broth (a pH of 7.0) containing different concentrations of Cr (VI) (25, 50, 100, and 150 mg/L) and incubated in an orbital shaker (200 rpm) at 30°C for 96 hours. The inoculum was 2% of the total volume of the medium. Samples were drawn at regular intervals of 12 hours. Bacterial cell density (diluted 10-fold with water) was determined by measuring the optical density at 600 nm using a UV-Vis spectrophotometer (Shimadzu 1700, Japan) (22).
3.7. Survey on the Effects of Temperature and pH
The effects of temperature and pH on the growth of selected bacteria (P. aeruginosa and S. marcescens) and Cr (VI) reduction were investigated using an LB medium (5 mL) containing 50 mg/L of Cr (VI). To investigate temperature’s effect, media were inoculated with 25 µL of overnight cultures of the bacterial isolates (OD600) and incubated at various incubation temperatures (25 - 45°C). To survey the effect of pH, a sterilized culture medium was adjusted to a pH of 5.0, 6.0, 7.0, 8.0, 9.0, and 10.0 with predetermined amounts of sterilized 0.1 N of HCl or 1 N of NaOH, and the inoculated tubes were incubated at 37°C in a water bath. Bacterial growth was measured after 72 hours of incubation by measuring the optical density (OD) of cultures at 600 nm. The culture was centrifuged (10,000 rpm, 5 minutes) and the supernatant was used to determine the residual Cr (VI) concentration (23).
3.8. Statistical Analysis
Statistical analysis was performed by SPSS software, and the interpretation of data was done based on ANOVA and Duncan tests.
4. Results
4.1. Counting Bacteria
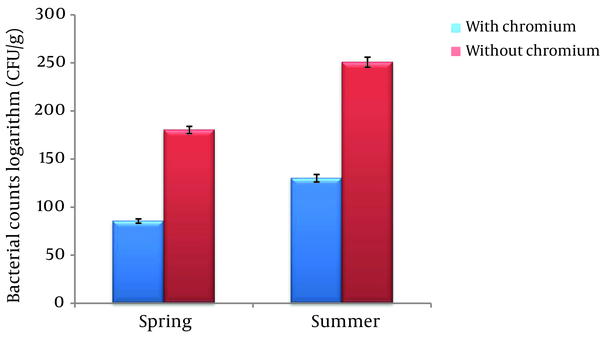
The logarithmic mean of the numbers of bacteria in the medium containing Cr (VI) in the spring (85.532 ± 2.25 (CFU/g)) was significantly lower than the medium without Cr (VI) (180.275 ± 3.72 (CFU/g)) in the same season (P < 0.01). In the summer season, the logarithmic mean of the bacterial count in the medium containing Cr (130.112 ± 3.92 (CFU/g)) was significantly lower than the medium without Cr (250.727 ± 5.23 (CFU/g)) (P < 0.01) (Figure 1).
Bacterial Count Logarithms in Two Seasons, With Cr and Without Cr
4.2. Identification of Cr (VI)-resistant Bacteria
The results obtained from morphological and biochemical tests are shown in Table 2. Six bacteria were identified as Cr (VI)-resistant bacteria, including Pseudomonas aeruginosa, Bacillus cereus, Kelebsiella peneumoniae, Enterobacter cloacae, Serratia marcescens, and Micrococcus luteus.
The Results of Morphological and Biochemical Tests
| Bacteria | Gram Stain | Motility | Spore Formation | Cell Shape | Citrate | KOH | Ph | Urease | Oxidase | Indole | Catalase | VP | H2S | TSI | Nitrate Reductionn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. aeruginosa | - | + | - | Rod | + | + | + | - | + | - | + | - | - | K/K | + |
| B. cereus | + | + | + | Rod | + | - | - | - | + | - | + | + | - | K/A | + |
| K. peneumoniae | - | - | - | Rod | + | - | + | - | - | + | + | - | K/K | + | |
| E. cloacae | - | + | - | Rod | + | - | - | - | - | - | + | + | - | A/A | + |
| S. marcescens | - | + | + | Rod | + | - | - | - | - | - | + | + | - | K/K | + |
| M. luteus | + | - | - | Coccus | - | - | - | - | + | - | + | - | - | K/K | - |
4.3. Minimum Inhibitory Concentration
The results of the MIC experiment indicated that P. aeruginosa has the most resistance to Cr (VI), followed by S. marcescens, B. cereus, K. peneumoniae, E. cloacae, and M. luteus, respectively (Table 3).
The Results of the MIC Test
| Bacteria | Chromium, mg/L | Without Phenanthrene | Without Bacteria | ||||
|---|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | |||
| P. aeruginosa | + | + | + | + | - | + | - |
| S. marcescens | + | + | + | - | - | + | - |
| B. cereus | + | + | - | - | - | + | - |
| K. peneumoniae | + | + | - | - | - | + | - |
| E. cloacae | + | - | - | - | - | + | - |
| M. luteus | + | - | - | - | - | + | - |
4.4. Cr (VI) Reduction Assay
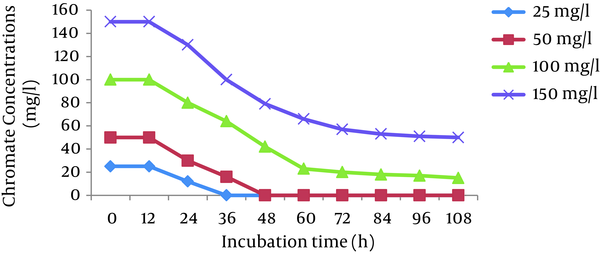
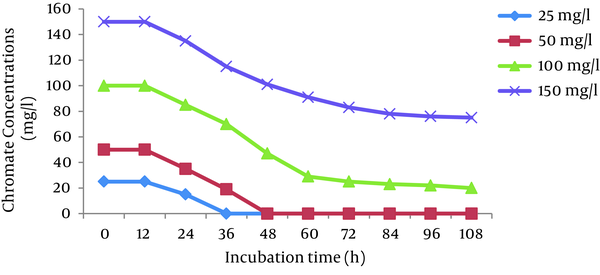
The effects of initial Cr (VI) concentrations (25, 50, 100, and 150 mg/L) were investigated in the Cr (VI)-reducing ability of P. aeruginosa and S. marcescens. The results showed that the cells of both bacteria completely reduced 25 and 50 mg/L Cr (VI) in 36 hours and 48 hours, respectively. The reduction efficiency decreased with increases in the Cr (VI) concentration and reached 66% and 50% for P. aeruginosa and S. marcescens, respectively, at 150 mg/L Cr (VI) (Figures 2 and 3).
Effect of Different Initial Cr (VI) Concentrations on Chromate Reduction by P. aeruginosa
Effect of Different Initial Cr (VI) Concentrations on Chromate Reduction by S. marcescens
4.5. The Rate of Growth
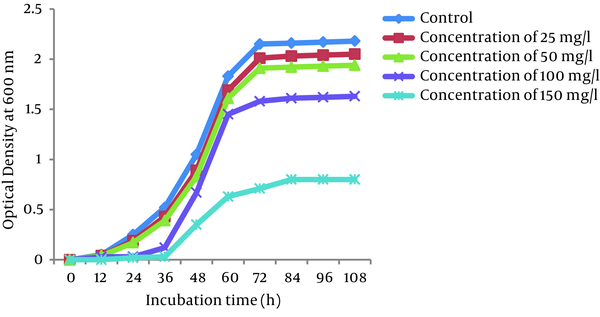
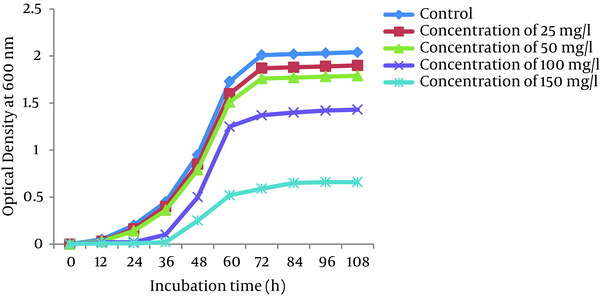
Growth curves of P. auroginosa and S. marcescens in the LB broth medium (a pH of 7.0 and a temperature of 30°C) in the absence and presence of Cr (VI) at different concentrations of Cr (VI) (25, 50, 100, and 150 mg/L) are shown in Figures 4 and 5, respectively. As shown in Figures the the lag phase and optical density of bacteria greatly depend on the concentration of Cr (VI) in the medium. Bacterial growth was significantly influenced by Cr (VI) at the concentration of 150 mg/L, while 25, 50, and 150 mg/L Cr (VI) concentrations had only a slight effect on their growth. At 25 and 50 mg/L concentrations of Cr (VI), the lag phase was 12 hours, whereas in the presence of Cr (VI) concentrations of 100 and 150 mg/L, the lag phase was observed at 24 hours and 36 hours, respectively.
Growth of P. aeruginosa at Different Concentrations of Cr (VI)
Growth of S. marcescens at Different Concentrations of Cr (VI)
4.6. Survey of the Effects of Temperature and pH
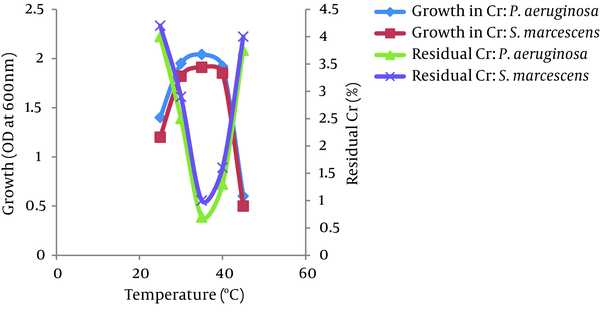
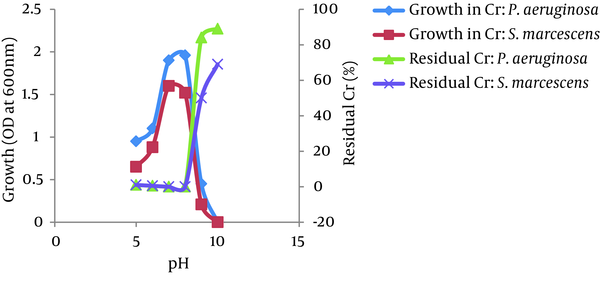
The optimum temperatures for growth and Cr (VI) reduction were between 30 and 40°C for both bacteria, P. aeruginosa and S. marcescens. The growth of both bacteria increased as the temperature increased up to 35°C and then decreased at 45°C. The Cr (VI) reduction by both bacteria also increased as the temperature increased up to 35°C and decreased above 40°C. However, more than 95% of Cr (VI) was reduced by both isolates between 25 - 45°C. No significant difference was observed in the growth of both isolates in the presence or absence of Cr (VI) at different temperatures (Figure 6). The bacterial isolates P. aeruginosa and S. marcescens can grow and reduce more than 95% of Cr (VI) in a wide range of pH levels between 5.0 to 8.0. The optimum pH for bacterial growth and Cr (VI) reduction by both isolates was found to be between 7.0 and 8.0. Growth and Cr (VI) reduction was significantly reduced at a pH greater than 8.0 (Figure 7).
Effect of Temperatures on the Growth and Cr (VI) (50 mg/L) Reduction by P. aeruginosa and S. marcescens
Effect of pH on the Growth and Cr (VI) (50 mg/L) reduction by P. aeruginosa and S. marcescens
5. Discussion
The biotransformation of highly toxic and mutagenic hexavalent Cr to relatively nontoxic trivalent Cr (III) form by chromate-reducing bacteria offers an economical as well as ecofriendly option for Cr bioremediation (6). In the current study, the logarithmic mean numbers of bacteria in the medium containing Cr (VI) in both the spring and the summer were significantly lower than the medium without Cr (VI) in the same seasons. This result showed that hexavalent Cr is a toxic substance for bacteria and inhibits their growth. In the present research, the MIC test was used to determine the most resistant isolated bacteria to Cr (VI). The results indicated that the most resistant bacteria belonged to P. aeruginosa and S. marcescens with MICs of 200 and 150 mg/L, respectively. Moreover, no growth could be observed at a concentration greater than 200 mg/L. It can be concluded that high concentrations of Cr (VI) inhibit the growth of bacteria. Shekhar et al. (2014) showed that Cr (VI) reduction by isolates was considerable and the Pseudomonas sp. was also the most resistant bacterium with an MIC of 200 mg/L (24). In the current study, both P. aeruginosa and S. marcescens completely reduced 25 and 50 mg/L Cr (VI) after 36 h and 48 h, respectively. Dey et al. (2014) showed that cells of Arthrobacter sp. completely reduced 50 and 100 µM of Cr (VI) in 24 hours and 48 hours, respectively (6). Thacker and Madamwar (2005) have shown that the bacterial isolate DM1 reduces 50 mg/L of Cr to 0 mg/L in 54 hours. They added a second aliquot of Cr, which was reduced to 0 mg/L in 99 h, and the third aliquot was reduced to 21.8 mg/L in 126 hours (25). Likewise, chromate reduction by viable cells of different chromate-reducing bacterial isolates was found to be influenced by the initial Cr (VI) concentration (26). The present study showed that the complete reduction by selected isolates failed to occur at the higher initial Cr (VI) concentration (Figures 2 and 3), which also corroborates the findings of several others (23, 27). Such a decrease in bacterial capability for chromium reduction with an increasing concentration of initial Cr (VI) might be due to the toxicity of chromium to viable whole cells.
The growth of P. aeruginosa and S. marcescens decreased as the Cr (VI) concentration increased, and a lag phase of more than 12 hours was observed when they were exposed to a Cr (VI) concentration higher than 50 mg/L. The Cr (VI) concentrations from 25 to 100 mg/L had only a slight effect on the growth of the bacteria. When the Cr (VI) concentration increased to 150 mg/L, a drastic reduction in the bacterial growth rate was observed (Figures 4 and 5). This indicates that the growth of both bacteria was highly inhibited due to the toxicity of Cr (VI) at higher concentrations. Our observations are in agreement with the work reported by Soni et al. (2013) and Singh et al (2013) (28, 29). Bae et al. (2000) reported that the E. coli growth rate decreased and the lag phase increased with increasing Cr (VI) concentrations in their medium. This was basically due to the inhibitory effect of higher Cr (VI) concentrations on the growth of organisms because each organism has a specific resistance at a specified growth condition. As the initial age of the inoculums remains fixed at 24 hours, the acclimatization period at varying Cr (VI) concentrations would not remain the same. Hence, the following behavior was observed (30). Figures 2 - 5 show that the Cr (VI) reduction activity and the growth of P. aeruginosa and S. marcescens in the present study were dependent on each other. The growth of the cells stimulates Cr (VI) reduction, and efficient Cr (VI) reduction subsequently promotes bacterial growth (28, 31). This may be due to the nature of Cr (VI)-resistant bacteria that create a reducing environment to detoxify Cr (VI) for their own growth.
Environmental factors, such as temperature and pH, were found to influence the chromate-reducing potential of the viable cells of P. aeruginosa and S. marcescens (Figures 6 and 7) and regulate their metabolic activities. The optimum temperatures for growth and Cr (VI) reduction was between 30 and 40°C for both bacteria. The optimum temperature was between 35°C - 37°C for Ochrobactrum intermedium Rb-2 (32), Ochrobactrum sp. CSCr-3 (33), O. intermedium SDCr-5 (34), and Nesterenkonia sp. MF2 (35). It is presumed that the deviation of these factors from their optima might alter the chromate reductase activity due to changes in the conformation and/or ionization of the enzyme (36). In the current research, the optimum pH for growth and Cr (VI) reduction by both isolates was between 7.0 and 8.0. More than 95% of Cr (VI) was reduced by both isolates between a pH of 5.0 and 8.0 and temperatures of 25 - 45°C. However, a sharp decline in growth and Cr (VI) reduction was recorded at a pH of greater than 8.0. Thus, the results suggest that a slightly acidic medium (to a pH of 8.0) was the most appropriate range to achieve maximum Cr (VI) reduction. This may be due to the highly stable nature of Cr (VI) reductase enzyme so that changes in pH and temperature are not likely to affect the protein structure as well as the enzyme activity. The maximum growth of Bacillus isolates and Cr (VI) reduction were shown to be directly related to the optimum pH (8.0) (37). McLean et al. (2000) reported an optimum wide pH range between 6.0 and 9.0 for Cr (VI) reduction by P. synxantha (34).
Our results in this study revealed that Cr (VI) concentrations, pH levels, and temperature can be important environmental factors to regulate bacterial metal tolerance. P. aeruginosa and S. marcescens, which can tolerate up to 150 and 200 mg/L of Cr (VI) and have a reduction ability up to 100% at optimized conditions, can be effective in remediation strategies for ecosystems polluted with hexavalent Cr.
Acknowledgements
References
-
1.
Ezaka E, Anyanwu CU. Chromium (VI) tolerance of bacterial strains isolated from sewage oxidation ditch. Int J Environ Sci. 2011;1(7):1725-34.
-
2.
Guidelines for Drinking-water quality. Fourth ed. 2011.
-
3.
Smrithi A, Usha K. Isolation and characterization of chromium removing bacteria from tannery effluent disposal site. Int J Adv Biotechnol Res. 2012;3(3):644-52.
-
4.
Verma A, Chakraborty S, Basu JK. Adsorption study of hexavalent chromium using tamarind hull-based adsorbents. Separat Purific Technol. 2006;50(3):336-41. https://doi.org/10.1016/j.seppur.2005.12.007.
-
5.
Li Q, Zhai J, Zhang W, Wang M, Zhou J. Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk. J Hazard Mater. 2007;141(1):163-7. [PubMed ID: 16930824]. https://doi.org/10.1016/j.jhazmat.2006.06.109.
-
6.
Dey S, Pandit B, Paul AK. Reduction of Hexavalent Chromium by Viable Cells of Chromium Resistant Bacteria Isolated from Chromite Mining Environment. J Mining. 2014;2014. https://doi.org/10.1155/2014/941341.
-
7.
Lu Z, An X, Zhang W. Isolation and Phylogenetic Analysis of Chromium(VI) Reducing Bacteria of a Magnetite Mine Drainage from Hebei China. Mod Appl Sci. 2011;5(2):113. https://doi.org/10.5539/mas.v5n2p113.
-
8.
Mishra R, Sinha V, Kannan A, Upreti RK. Reduction of Chromium-VI by Chromium Resistant Lactobacilli: A Prospective Bacterium for Bioremediation. Toxicol Int. 2012;19(1):25-30. [PubMed ID: 22736899]. https://doi.org/10.4103/0971-6580.94512.
-
9.
Viti C, Giovannetti L. The impact of chromium contamination on soil heterotrophic and photosynthetic microorganisms. Annal microbiol. 2001;51(2):201-14.
-
10.
Yang J, He M, Wang G. Removal of toxic chromate using free and immobilized Cr(VI)-reducing bacterial cells of Intrasporangium sp. Q5-1. World JMicrobiol Biotechnol. 2009;25(9):1579-87. https://doi.org/10.1007/s11274-009-0047-x.
-
11.
Masood F, Malik A. Hexavalent chromium reduction by Bacillus sp. strain FM1 isolated from heavy-metal contaminated soil. Bull Environ Contam Toxicol. 2011;86(1):114-9. [PubMed ID: 21181113]. https://doi.org/10.1007/s00128-010-0181-z.
-
12.
Ackerley DF, Gonzalez CF, Keyhan M, Blake R, Matin A. Mechanism of chromate reduction by the Escherichia coli protein, NfsA, and the role of different chromate reductases in minimizing oxidative stress during chromate reduction. Environ Microbiol. 2004;6(8):851-60. [PubMed ID: 15250887]. https://doi.org/10.1111/j.1462-2920.2004.00639.x.
-
13.
Long D, Tang X, Cai K, Chen G, Shen C, Shi J, et al. Cr(VI) resistance and removal by indigenous bacteria isolated from chromium-contaminated soil. J Microbiol Biotechnol. 2013;23(8):1123-32. [PubMed ID: 23727810].
-
14.
McLean J, Beveridge TJ. Chromate reduction by a pseudomonad isolated from a site contaminated with chromated copper arsenate. Appl Environ Microbiol. 2001;67(3):1076-84. [PubMed ID: 11229894]. https://doi.org/10.1128/AEM.67.3.1076-1084.2001.
-
15.
Ilias M, Rafiqullah IM, Debnath BC, Mannan KS, Mozammel Hoq M. Isolation and Characterization of Chromium(VI)-Reducing Bacteria from Tannery Effluents. Indian J Microbiol. 2011;51(1):76-81. [PubMed ID: 22282632]. https://doi.org/10.1007/s12088-011-0095-4.
-
16.
Kafilzadeh F, Moghtaderi Y, Jahromi Rezaeian A. Isolation and identification of cadmium-resistant bacteria in Soltan Abad river sediments and determination of tolerance of bacteria through MIC and MBC. Europ J Experiment Bio. 2013;3(5):268-73.
-
17.
Standard methods for the examination of water and wastewater. 19th ed. Washington DC, USA: American Public Health Association; 1998.
-
18.
Cappuccino GJ, Sherman N. Microbiology. 10th ed. London: Benjamin Cummings; 2013.
-
19.
Holt GJ, Krieg RN, Sneath AHP, Staley TJ, Williams TS. Bergey's manual of determinative bacteriology. 9th ed. Baltimore, USA: Williams and Wilkins Co; 1994.
-
20.
Bartlett RJ, James BR. Chromium. Sparks DL (ed) Methods of soil analysis. Madison; 1996. p. 683-701.
-
21.
Thacker U, Parikh R, Shouche Y, Madamwar D. Hexavalent chromium reduction by Providencia sp. Process Biochem. 2006;41(6):1332-7. https://doi.org/10.1016/j.procbio.2006.01.006.
-
22.
Farag S, Zaki S. Identification of bacterial strains from tannery effluent and reduction of hexavalent chromium. J Environ Biol. 2010;31(5):877-82.
-
23.
Megharaj M, Avudainayagam S, Naidu R. Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol. 2003;47(1):51-4. [PubMed ID: 12783193]. https://doi.org/10.1007/s00284-002-3889-0.
-
24.
Shekhar S, Sundaramanickam A, Vijayansiva G. Detoxification hexavalent chromium by potential chromate reducing bacteria isolated from turnery effluent. Am J Res Commun. 2014;2(2):205-16.
-
25.
Thacker U, Madamwar D. Reduction of toxic chromium and partial localization of chromium reductase activity in bacterial isolate DM1. World J Microbiol Biotechnol. 2005;21(6-7):891-9.
-
26.
Alam MZ, Ahmad S. Toxic chromate reduction by resistant and sensitive bacteria isolated from tannery effluent contaminated soil. Ann microbiol. 2012;62(1):113-21.
-
27.
Dey S, Paul AK. Optimization of chromate reduction by whole cells of Arthrobacter sp. SUK 1205 isolated from metalliferous chromite mine environment. 2012;4:73–81. https://doi.org/10.4236/gm.2012.24012.
-
28.
Soni SK, Singh R, Awasthi A, Singh M, Kalra A. In vitro Cr(VI) reduction by cell-free extracts of chromate-reducing bacteria isolated from tannery effluent irrigated soil. Environ Sci Pollut Res Int. 2013;20(3):1661-74. [PubMed ID: 22983604]. https://doi.org/10.1007/s11356-012-1178-4.
-
29.
Singh N, Verma T, Gaur R. Detoxification of hexavalent chromium by an indigenous facultative anaerobic Bacillus cereus strain isolated from tannery effluent. African J Biotechnol. 2013;12(10):1091-103.
-
30.
Bae WC, Kang TG, Kang IK, Won YJ. Reduction of hexavalent chromium by Escherichia coli ATCC 33456 in batch and continuous cultures. J Microbiol Biotechnol. 2000;38:36-9.
-
31.
Xu W-H, Liu Y-G, Zeng G-M, Xin L, Song H-X, Peng Q-Q. Characterization of Cr (VI) resistance and reduction by Pseudomonas aeruginosa. Transact Nonferrous Metal SociChina. 2009;19(5):1336-41.
-
32.
Batool R, Yrjala K, Hasnain S. Hexavalent chromium reduction by bacteria from tannery effluent. J Microbiol Biotechnol. 2012;22(4):547-54. [PubMed ID: 22534304].
-
33.
He Z, Gao F, Sha T, Hu Y, He C. Isolation and characterization of a Cr(VI)-reduction Ochrobactrum sp. strain CSCr-3 from chromium landfill. J Hazard Mater. 2009;163(2-3):869-73. [PubMed ID: 18722054]. https://doi.org/10.1016/j.jhazmat.2008.07.041.
-
34.
McLean JS, Beveridge TJ, Phipps D. Isolation and characterization of a chromium-reducing bacterium from a chromated copper arsenate-contaminated site. Environ Microbiol. 2000;2(6):611-9. [PubMed ID: 11214794].
-
35.
Amoozegar MA, Ghasemi A, Razavi MR, Naddaf S. Evaluation of hexavalent chromium reduction by chromate-resistant moderately halophile, Nesterenkonia sp. strain MF2. Process Biochem. 2007;42(10):1475-9.
-
36.
Farrell SO, Ranallo RT. Experiments in Biochemistry. Orlando, Fla, USA: Saunders College Publications; 2000.
-
37.
Gibb HJ, Lees PS, Pinsky PF, Rooney BC. Clinical findings of irritation among chromium chemical production workers. Am J Ind Med. 2000;38(2):127-31. [PubMed ID: 10893505].