Abstract
Background:
The increasing prevalence of Escherichia coli as a foodborne pathogen in poultry poses a high risk to food safety. The resistant strains of E. coli may contribute resistant genes to human endogenous flora, causing fatal diseases. Moreover, these pathogens are serious threats to poultry farming.Objectives:
The current study aimed to investigate the effects of seasonal variations on the bacterial load of E. coli and to evaluate its antibiogram profile.Methods:
All feed samples were evaluated for the identification of E. coli and its serotypes, using conventional culture methods and biochemical characterization. Positive samples were confirmed by polymerase chain reaction (PCR) assay. The bacterial load of E. coli was estimated by measuring the total viable count, and the antibiogram data were calculated using two methods, that is, disc diffusion method and minimum inhibitory concentration (MIC) measurement.Results:
Of 204 feed samples investigated, 38 isolates were positive for E. coli. All positive samples were also confirmed via universal and species-specific PCR assays, and 8/38 were documented as E. coli 0157:H7 strains. The bacterial load of E. coli was also determined by measuring the total viable count, and the results revealed the highest ratio (6.44×108 CFU/g) from June to August and the lowest ratio (2.06×108 CFU/g) from December to February. The multidrug resistance of E. coli O157:H7 was validated by antimicrobial susceptibility tests since all isolates showed high resistance to chloramphenicol, penicillin derivatives, fluoroquinolones, and oxytetracycline, respectively, and were only susceptible to aminoglycosides.Conclusions:
Considering the high bacterial load of E. coli from June to August, the poultry industry needs to establish appropriate and effective hygienic and storage policies, especially during these alarming months. Moreover, surfacing and propagation of resistant strains of these pathogens may obscure future assessments for treatment purposes.Keywords
Poultry Feed Antibiogram Profile Polymerase Chain Reaction Bacterial Load Foodborne Pathogen
1. Background
Microbial food safety is an increasing public health concern worldwide. The primary cause of foodborne diseases is the presence of highly potent pathogens, such as Escherichia coli, in the poultry (1). Concerning its pathogenicity, E. coli is classified into six groups, including enteroinvasive E. coli (EIEC), enterotoxigenic E. coli (ETEC), verotoxin-producing/Shiga toxin-producing E. coli (VTEC/STEC, including its well-known subgroup, enterohemorrhagic E. coli), enteropathogenic E. coli (EPEC), enteroaggregative E. coli (EAEC), and diffusely adherent E. coli (DAEC) (2). Of all these groups, the O157:H7 serotype of EHEC strain is of zoonotic importance (3, 4).
Poultry is the natural reservoir of E. coli O157:H7 serotype, causing hemolytic uremic syndrome and hemorrhagic colitis in humans (5). In the United States, 265,000 diseases due to STEC are recorded annually, 36% of which are caused by the O157:H7 strain (6). Moreover, various poultry diseases are attributed to this strain, including avian colibacillosis, yolk sac infection, and meningitis, leading to heavy economic losses (7). Seasonal fluctuations also have tremendous effects on microbial intensification, and rainy seasons are the most alarming period (8).
Another serious problem regarding the treatment of E. coli O157:H7 is its multiple drug resistance (MDR) due to the irrational use of antibiotics. In veterinary medicine, antibiotics are not only used for treatment and prophylaxis purposes but also as growth promoters (9). Based on the mentioned findings, isolation and determination of the antibiotic resistance of E. coli and its Shiga toxin strain (O157:H7) play an important role in the control of their dissemination.
2. Objectives
This study aimed (1) to isolate potentially dangerous bacteria, such as E. coli, from the poultry feed in Karachi Province, Pakistan, which has never been done before; (2) to assess the effects of seasonal changes on the growth rate of these pathogens; and (3) to determine MDR by using antibiotics.
3. Methods
3.1. Sample Collection
From 2015 to 2016, a total of 204 feed samples were randomly collected. Seventeen poultry farms were selected randomly in Karachi Province for microbiological evaluation of feed samples. All samples were transferred in sterile labeled bags to the Department of Microbiology, University of Karachi, Pakistan.
3.2. Characterization of Isolates
3.2.1. Conventional Culture Method
Each feed sample was mixed with a nutrient broth at a ratio of 1:9 and incubated overnight. Then, 0.1 mL of this dilution was streaked onto the nutrient agar (Oxoid, UK) and kept in an incubator at 37°C for 24 hours (10).
3.3. Determination of Bacterial Load with Seasonal Variations
The total viable count (TVC) of E. coli was determined from 10-1 to 10-8. From each dilution, 1 mL of aliquot was inoculated onto eosin methylene blue agar (Oxoid, UK) and MacConkey agar (Oxoid, UK) plates, using the spread plate method and incubated for 24 hours (11). The TVC was recorded seasonally (September-November, December-February, March-May, and June-August).
3.4. Isolation and Identification of E. coli by the International Organization for Standardization (ISO) Method
The conventional culture method was carried out based on the recommended ISO 16654 method (12) with slight modifications for E. coli serotype identification. This method consists of the following steps.
3.4.1. Pre-Enrichment Stage
Each feed sample (25 g) was placed in 225 mL of modified Tryptone Soya Broth (Oxoid, UK) plus 20 mg/L of Novobiocin (Sigma) and incubated at 41°C for 24 hours.
3.4.2. Concentration Stage
In this stage, 1 mL of each pre-enriched broth was placed in 20 μL of immunomagnetic beads (Thermo Fisher Scientific, USA), coated with antibodies to E. coli O157:H7, and incubated for 24 hours.
3.4.3. Isolation Stage
In this stage, 50 μL of the prepared suspension was streaked on Cefixime Tellurite Sorbitol MacConkey (CT-SMAC) agar (Oxoid, UK) and incubated for 22 hours at 37°C. Clear and colorless colonies, characteristic of E. coli O157:H7, were observed. For further verification, the colonies were streaked again on eosin methylene blue agar (Oxoid, UK) and incubated for the next 24 hours; typical colonies confirmed the presence of E. coli O157:H7.
3.4.4. Confirmatory Stage
Biochemical and molecular characterizations were used for further identification.
3.5. Biochemical Characterization
Biochemical tests, including citrate utilization test, Voges-Proskauer test, indole formation, motility test, carbohydrate fermentation test, hydrogen sulfide production, catalase, oxidase, and urease tests, and methyl red tests, were performed on all E. coli-positive samples for identification (13).
3.6. Molecular Study of Samples
3.6.1. DNA Mining
A method described in the literature (14) with minor changes was used for DNA extraction of all culturally positive E. coli samples. Initially, the bacterial culture (1 mL) was centrifuged at a speed of 13,000 g for 15 minutes, and the supernatant was removed. Then, 80 µL of proteinase K buffer plus enzyme (Thermo Fisher Scientific, USA), 40 µL of sodium dodecyl sulfate (SDS; Thermo Fisher Scientific, USA), and 240 µL of saline water were added and vortexed for two minutes. Next, the mixture was incubated in a water bath for five hours at 55°C. After resting the mixture for 15 minutes, 240 µL of 6 M sodium chloride (NaCl) was added, and incubation was performed at room temperature. Afterward, the mixture was vortexed for two minutes and centrifuged for five minutes at 3000 g. The supernatant was moved to a second tube and centrifuged for 15 minutes at 13,000 g (in duplicate). After adding 460 µL of isopropanol (Longhong Chemical Co., China) to the mixture, centrifugation was carried out for five minutes, and the supernatant was discarded. In the next step, cold ethanol 70% (1000 µL) was added, and after centrifugation for five minutes, the supernatant was discarded. After the tubes were air-dried for two hours, Tris-EDTA (TE) buffer (25 µL) was added, and the extracted DNA was stored at -80°C.
3.6.2. Polymerase Chain Reaction (PCR) Amplification
Universal primers with a target size of 1500 bp were used for confirmation of E. coli at the genus level (15). E. coli O157:H7 (target size, 450 bp) was verified by species-specific PCR assay.
3.6.3. Thermal Cycler Conditions for E. coli O157:H7 Strain
The thermocycling conditions were as follows: initial denaturation for two minutes at 94°C, denaturation for 30 seconds at 94°C, annealing for 30 seconds at 72°C, extension for 30 seconds at 72°C, and a final extension at 72°C for ten minutes. The dideoxy method was used for the sequencing of amplified products, and the NCBI BLAST tool was used for confirmation. The sequences and product sizes are presented in Table 1.
The List of Primers
| Primer sequences | Product size (bp) | Target genes | References | |
|---|---|---|---|---|
| Universal primers | AGAGTTTGATCCTGGCTCAG TACGGTTACCTTGTTACGACTT | 1500 | 16S rRNA | Marchesi et al. (1998), (15) |
| E. coli O157:H7 | AAG CGA CTG AGG TCA CT ACG CTG CTC ACT AGA TGT | 450 | eaeAO157:H7 | Louie et al. (1994) |
3.7. Antimicrobial Susceptibility Testing
The disc diffusion test (DDT) was performed as described by the Clinical and Laboratory Standards Institute (CLSI) (16). The minimum inhibitory concentration (MIC) was also measured according to the CLSI guidelines (17) to evaluate the antimicrobial sensitivity of moxifloxacin, ciprofloxacin, gentamicin, enrofloxacin, chloramphenicol, kanamycin, streptomycin, trimethoprim, tetracycline, oxytetracycline, neomycin, penicillin, ampicillin, and amoxicillin by using a commercially prepared disk (Oxoid, UK) against E. coli O157:H7 strain.
3.8. Statistical Analysis
A one-way ANOVA test was performed in SPSS version 22.0 to determine the relationship between bacterial load and seasonal variations.
4. Results
4.1. Bacterial load with Seasonal Variations
Positive samples of E. coli (n=38) were evaluated by TVC in relation to seasonal variations, using one-way ANOVA, as shown in Table 2 and Table 3. As the P value was less than 0.05 (P = 0.00) and the F value was above one (F = 15.56), the effects of seasonal variations on the bacterial load were significant.
The total Viable Count (TVC) of E. coli in Relation to Seasons
| Seasons | TVC (Log10 CFU/g) | 95% Confidence interval | ||||
|---|---|---|---|---|---|---|
| Positive samples | Minimum | Maximum | Mean ± SE | Lower bound | Upper bound | |
| December to February | 8 | 1 | 3.4 | 2.06 ± 0.29 | 1.357 | 2.767 |
| March to May | 5 | 1.21 | 5.1 | 3.07 ± 0.70 | 1.118 | 5.037 |
| June to August | 12 | 1.98 | 7.9 | 6.44 ± 0.45 | 5.453 | 7.442 |
| September to November | 13 | 1.77 | 6.8 | 4.96 ± 0.47 | 3.932 | 6.000 |
| Total | 38 | 1 | 7.9 | 4.57 ± 0.36 | 3.843 | 5.305 |
ANOVA Results for TVC of E. coli (log10 CFU/g) in Different Seasons
| Df | Mean square | F | P | |
|---|---|---|---|---|
| Between groups | 3 | 35.269 | 15.566 | 0.000 |
| Within groups | 34 | 2.266 | ||
| Total | 37 |
4.2. Isolation of E. coli 0157:H7 Strain by ISO 16654 Method
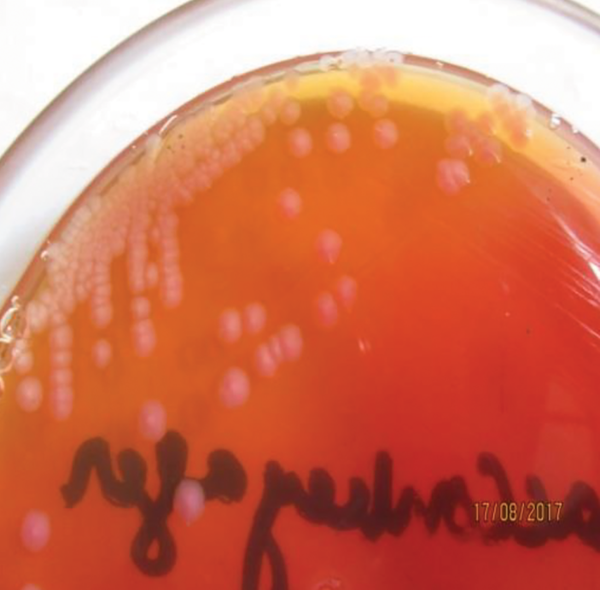
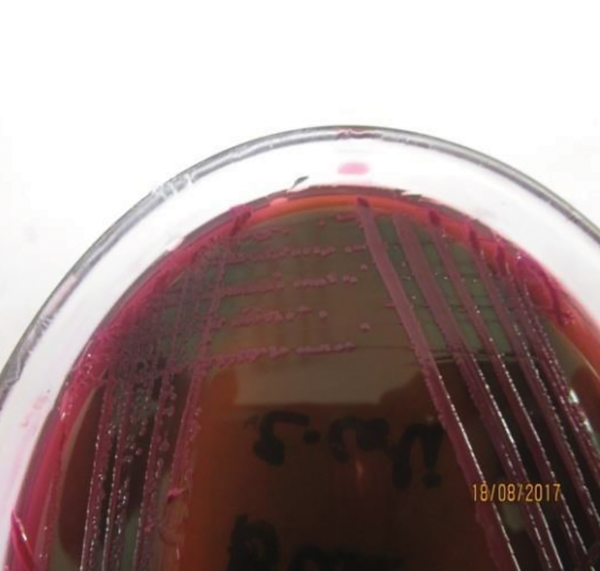
For isolation of the pathogenic strain (E. coli O157:H7), the method recommended by ISO was used for all E. coli-positive culture samples (Figure 1 and Figure 2). Due to colonial variations, some strains of E. coli O157 also fermented sorbitol and did not produce colorless colonies; this was also true for the biochemical properties of this strain. Consequently, a low percentage of profile was produced that may be linked to the genetic evolution and the variety of virulence factors. Therefore, all positive and negative samples for E. coli O157:H7 were subjected to biochemical characterization and further validated by universal and species-specific PCR to differentiate the pathogenic strains of E. coli from non-pathogenic ones.
Colorless colonies on CT-SMAC
Small flat colonies with a metallic green sheen on eosin methylene blue agar (EMB)
4.3. Molecular Characterization
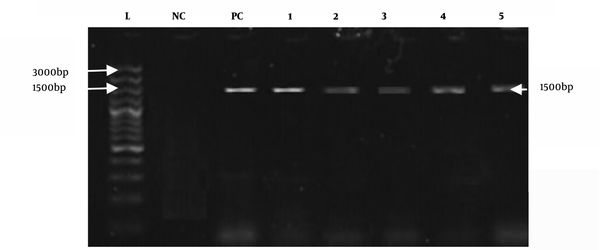
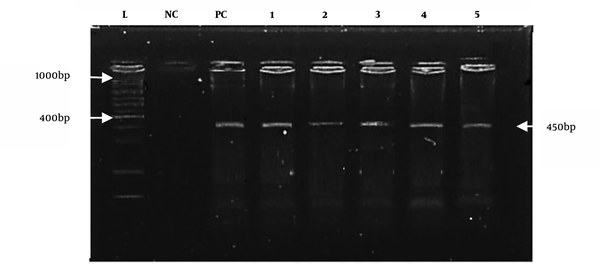
All positive culture samples of E. coli (n = 38) were also positive for the reported universal primers, as shown in Figure 3. However, species-specific primers only confirmed eight positive samples for E. coli O157:H7, as shown in Figure 4.
Amplified products of E. coli with universal primers (1500 bp). L: DNA ladder; NC: negative control; and PC: positive control (positive samples from 1 to 5).
E. coli 0157:H7 amplified with the reported primer (450 bp). M: DNA marker (positive samples from well 2 to well 5). PC is the positive control, and NC is the negative control.
4.4. Antimicrobial Susceptibility Test
The effectiveness of antibiotics against positive samples of E. coli O157:H7 was estimated by antimicrobial susceptibility tests.
4.4.1. DDT
By using DDT, the antibiogram profile of E. coli O157:H7 was calculated (Table 4). The resistance of E. coli O157:H7 to antibiotics was as follows: 100% to enrofloxacin, chloramphenicol, trimethoprim, ampicillin, amoxicillin, and penicillin G; 87.5% to tetracycline and oxytetracycline; 50% to streptomycin; 25% to ciprofloxacin; 12.5% to moxifloxacin; and 0% to gentamicin, neomycin, and kanamycin, respectively.
Disc Diffusion Test (DDT) for E. coli O157:H7
| Antimicrobials | Sensitive, n (%) | Intermediate, n (%) | Resistant, n (%) |
|---|---|---|---|
| Gentamicin (Cn = 30 µg) | 8 (100) | - | - |
| Neomycin (N = 10 µg) | 8 (100) | - | - |
| Kanamycin (K = 30 µg) | 8 (100) | - | - |
| Moxifloxacin (Mxf = 5 µg) | 6 (75) | 1 (12.5) | 1 (12.5) |
| Ciprofloxacin (Cip = 30 µg) | 4 (50) | 1 (12.5) | 2 (25) |
| Streptomycin (S = 10 µg) | 2 (25) | - | 4 (50) |
| Tetracycline (Te = 30 µg) | 1 (12.5) | - | 7 (87.5) |
| Oxytetracycline (Ot = 30 µg) | 1 (12.5) | - | 7 (87.5) |
| Enrofloxacin (Enr = 5 µg) | 0 | - | 8 (100) |
| Chloramphenicol (C = 30 µg) | 0 | - | 8 (100) |
| Trimethoprim (W = 1.25 µg) | 0 | - | 8 (100) |
| Ampicillin (Amp = 10 µg) | 0 | - | 8 (100) |
| Amoxicillin (Aml = 10 µg) | 0 | - | 8 (100) |
| Penicillin G (P10 = 10 µg) | 0 | - | 8 (100) |
4.4.2. MIC Measurements
The effective minimum concentration of antibiotics against isolates was determined by measuring the MIC (Table 5). The significant difference (P < 0.003) between antimicrobial agents indicated the high efficacy of aminoglycosides (gentamicin, kanamycin, neomycin, and streptomycin) with the lowest MICs against E. coli O157:H7. On the other hand, fluoroquinolones (ciprofloxacin and moxifloxacin), oxytetracycline, and tetracycline showed low efficacies with high MICs. Also, trimethoprim, enrofloxacin, chloramphenicol, penicillin, ampicillin, and amoxicillin were completely ineffective; therefore, their MICs were not evaluated.
The Minimum Inhibitory Concentration (MIC) of Antimicrobials Against E. coli O157:H7
| Antimicrobials | Mean ± SEM | F | P Value |
|---|---|---|---|
| Gentamicin | 0.16 ± 0.04 | ||
| Kanamycin | 0.29 ± 0.11 | ||
| Neomycin | 0.25 ± 0.12 | 5.35 | 0.003 |
| Moxifloxacin | 2.33 ± 0.88 | ||
| Ciprofloxacin | 3.33 ± 0.66 | ||
| Streptomycin | 5.33 ± 1.33 | ||
| Oxytetracycline | 9.33 ± 3.52 | ||
| Tetracycline | 6.67 ± 1.33 |
5. Discussion
5.1. High Incidence of E. coli O157:H7 in the Animal Feed
This study was conducted to evaluate E. coli as a major foodborne pathogen, using the conventional culture method and PCR assay. The results of universal PCR revealed that 18.6% (38/204) of samples were positive for E. coli, 21% (8/38) of which were confirmed for E. coli O157:H7 through species-specific PCR. This rate is higher than that reported in Argentina, where 5% of chicken product samples were positive for Shiga-toxigenic E. coli O157:H7 (18). On the other hand, 4% of meat samples in the UK showed the presence of E. coli O157:H7 (19). Overall, the high incidence of foodborne pathogens (E. coli O157:H7) in Pakistan, as compared to other countries, is alarming.
5.2. Effects of Seasonal Fluctuations on Bacterial Load
The effects of climatic fluctuations on the propagation of bacterial contamination are important. In the present study, the highest concentration of E. coli (7.9 × 108) was recorded in summer, while the lowest concentration (1 × 108) was reported in winter. This finding is consistent with a previous study (20), which demonstrated the effects of seasonal variations on microbial growth during feed storage and transportation.
5.3. MDR of E. coli O157:H7
Antimicrobials are used routinely to maintain the health of humans and animals. However, the irrational use of antimicrobials may lead to changes in the intestinal microflora and pathogenic bacteria by creating new resistant microbes that cause public health risks (21, 22). The highest resistance of E. coli O157:H7 (100%) to amoxicillin, ampicillin, penicillin, chloramphenicol, trimethoprim, and enrofloxacin has been recorded in recent research. In this regard, Kyabaggu et al. (23) reported the remarkable resistance of E. coli O157:H7 to penicillin derivatives and chloramphenicol; susceptibility was only found for aminoglycosides (neomycin, gentamicin, and kanamycin). Also, Altaee et al. (24) and Ari et al. (25) reported similar findings. Overall, microbial analysis of poultry feed is incomplete without appropriate treatment and control measures. Also, the MDR of these microbes has posed treatment challenges.
5.4. Conclusion
The dominance of foodborne pathogens in the poultry feed represents a constant health risk to poultry and humans. Therefore, feed safety and sustained inspection of antimicrobial resistance of pathogens should be major concerns in developing countries, such as Pakistan, to promote poultry farming and human health.
References
-
1.
Matthew O, Chiamaka R, Chidinma O. Microbial analysis of poultry feeds produced in Songhai farms, Rivers State, Nigeria. J Microbiol Exp. 2017;4(2):110.
-
2.
Jafari A, Aslani MM, Bouzari S. Escherichia coli: a brief review of diarrheagenic pathotypes and their role in diarrheal diseases in Iran. Iran J Microbiol. 2012;4(3):102-17. [PubMed ID: 23066484]. [PubMed Central ID: PMC3465535].
-
3.
Manning SD, Motiwala AS, Springman AC, Qi W, Lacher DW, Ouellette LM, et al. Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Proc Natl Acad Sci U S A. 2008;105(12):4868-73. [PubMed ID: 18332430]. [PubMed Central ID: PMC2290780]. https://doi.org/10.1073/pnas.0710834105.
-
4.
Friesema IH, Van De Kassteele J, D. E. Jager CM, Heuvelink AE, Van Pelt W. Geographical association between livestock density and human Shiga toxin-producing Escherichia coli O157 infections. Epidemiol Infect. 2011;139(7):1081-7. [PubMed ID: 20822576]. https://doi.org/10.1017/S0950268810002050.
-
5.
Karch H, Tarr PI, Bielaszewska M. Enterohaemorrhagic Escherichia coli in human medicine. Int J Med Microbiol. 2005;295(6-7):405-18. [PubMed ID: 16238016]. https://doi.org/10.1016/j.ijmm.2005.06.009.
-
6.
CDC. Medical illustration of E. coli bacteria. 2016, [cited 06.03.2017]. Available from: www.cdc.gov/ecoli/pdfs/cdc-e.-coli-factsheet.pdf.
-
7.
Maciorowski KG, Herrera P, Jones FT, Pillai SD, Ricke SC. Effects on poultry and livestock of feed contamination with bacteria and fungi. Anim Feed Sci Technol. 2007;133(1-2):109-36.
-
8.
R M, M B, A Y; Aliyu; Abubakar; Adamu, et al. Mycological quality of commercially prepared and self compounded poultry feeds in Sokoto Metropolis, Sokoto, Nigeria. Afr J Microbiol Res. 2012;6(46):7314-8. https://doi.org/10.5897/ajmr12.761.
-
9.
Yoon KB, Song BJ, Shin MY, Lim HC, Yoon YH, Jeon DY, et al. Antibiotic Resistance Patterns and Serotypes of Salmonella spp. Isolated at Jeollanam-do in Korea. Osong Public Health Res Perspect. 2017;8(3):211-9. [PubMed ID: 28781944]. [PubMed Central ID: PMC5525558]. https://doi.org/10.24171/j.phrp.2017.8.3.08.
-
10.
Romanus II, Eze AT, Egwu OA, Ngozi AF, Chidiebube NA. Comparison of matrix-assisted laser desorption ionization-time of flight mass spectrometry (maldi-tof ms) with conventional culture and biochemical method of bacteria identification to species level. J Med Lab Diag. 2011;2(1):1-4.
-
11.
Fawole MO, Oso BA. Laboratory manual of microbiology. Spectrum Book Ltd, Ibadan; 1988.
-
12.
ISO 16654:2001. Microbiology of food and animal feeding stuffs — Horizontal method for the detection of Escherichia coli O157. Geneva, Switzerland: ISO; 2001. Available from: https://www.iso.org/standard/29821.html.
-
13.
Bair WB, Tortora GJ, Funke BR, Case CL, Weber D. Microbiology: an introduction. 13th. Edition ed. Boston: Pearson; 2019.
-
14.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. [PubMed ID: 3344216]. [PubMed Central ID: PMC334765]. https://doi.org/10.1093/nar/16.3.1215.
-
15.
Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, et al. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 1998;64(2):795-9. [PubMed ID: 9464425]. [PubMed Central ID: PMC106123]. https://doi.org/10.1128/AEM.64.2.795-799.1998.
-
16.
CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard. 7th ed ed. 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA: Clinical and Laboratory Standards Institute; 2012.
-
17.
CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically Approved standard. 9th ed ed. 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA: Clinical and Laboratory Standards Institute; 2012.
-
18.
Alonso MZ, Lucchesi PMA, Rodríguez EM, Parma AE, Padola NL. Enteropathogenic (EPEC) and Shigatoxigenic Escherichia coli (STEC) in broiler chickens and derived products at different retail stores. Food Control. 2012;23(2):351-5. https://doi.org/10.1016/j.foodcont.2011.07.030.
-
19.
Little CL, de Louvois J. The microbiological examination of butchery products and butchers' premises in the United Kingdom. J Appl Microbiol. 1998;85(1):177-86. [PubMed ID: 9721668]. https://doi.org/10.1046/j.1365-2672.1998.00538.x.
-
20.
D’Mello JPF. Microbiology of animal feeds. Microbiology of Ensilage. Edinburgh, United Kingdom: Formerly of the Scottish Agricultural College (SAC); 2006.
-
21.
Furtula V, Farrell EG, Diarrassouba F, Rempel H, Pritchard J, Diarra MS. Veterinary pharmaceuticals and antibiotic resistance of Escherichia coli isolates in poultry litter from commercial farms and controlled feeding trials. Poult Sci. 2010;89(1):180-8. [PubMed ID: 20008817]. https://doi.org/10.3382/ps.2009-00198.
-
22.
Romanus II, Chinyere OE, Amobi NE, Anthonia OE, Ngozi AF, Chidiebube NA, et al. Antimicrobial resistance of Escherichia coli isolated from animal and human clinical sample. Global Res J Microbiology. 2012;2(1):85-9.
-
23.
Kyabaggu D, Ejobi F, Olila D. The sensitivities to first-line antibiotic therapy of the common urinary tract bacterial infections detected in urine samples at a hospital in metropolitan Kampala (Uganda). Afr Health Sci. 2007;7(4).
-
24.
Altaee AE. Detection of E. coli Serotyping O157:H7 Isolated from Beef and Poultry Products Using Polymerase Chain Reaction. Mosul University; 2012.
-
25.
İNANÇ A, MUSTAFA AS. Antibiotic Resistance of Escherichia coli O157: H7 Isolated from Chicken Meats. Turk J Agric For. 2018;21(1):7.
