Abstract
Background:
Lung cancer is one of the most common leading causes of mortality and morbidity worldwide. Despite recent advances in therapeutic approaches, common methods are not fully effective. Thus, researchers are looking for some novel complementary agents to improve the effectiveness of therapies. Emerging evidence has shown the antitumor activity of several natural components such as quinoa seed extracts in various types of cancer.Objectives:
Hence, this study was conducted to evaluate the antiproliferation and anti-apoptotic activity of quinoa on the A549 lung cancer cell line.Methods:
The cell viability of A549 cells treated with quinoa was detected using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The expression levels of BAX and BCL2 as apoptosis-related genes were assessed using real-time polymerase chain reaction (PCR). Finally, the statistical analysis was performed using GraphPad Prism version 7.Results:
Our findings demonstrated that the cell viability decreased in a concentration and time-dependent manner. Also, treating A549 cells with doses of 1.60 and 1.92 mg/mL of quinoa seed extracts could increase BAX and decrease BCL2 expression levels (P < 0.05). However, the higher dose (1.92 mg/mL) was significantly effective.Conclusions:
According to this study, quinoa seed extract could induce apoptosis in lung cancer cells (A549) throughout the increased ratio of BAX/BCL2. However, further investigations are required to confirm the results.Keywords
1. Background
Lung cancer has been recognized as the first leading cause of cancer-related death globally. According to the World Health Organization (WHO) statistics in 2018, this cancer had the highest incidence (11.6%) and mortality (18.4%) rate compared to other cancers (1). The 5-year survival rate in this cancer is poor; it even reaches 13.3% in some areas such as the United Kingdom (2).
Although chemotherapy is the most effective therapeutic strategy against cancer, several anticancer drugs cause serious side effects, including anorexia, nausea, vomiting, diarrhea, gastrointestinal mucositis, constipation, malabsorption, weight loss, fatigue, and anemia (3). Accumulating evidence has demonstrated that using various components of plant extracts with antioxidant, antiproliferative, anti-inflammatory, and anti-angiogenic characteristics can be suggested as complementary anticancer agents, which reduce the required dose of chemotherapy drugs and improve the efficiency of therapy (4).
Quinoa (Chenopodium quinoa Willd.) is a pseudocereal crop that belongs to the Chenopodiaceae family. Although this multipurpose agricultural crop has been traditionally cultivated in the Andes of South America for thousands of years, it has recently been the focus of attention worldwide. Quinoa is rich not only in macronutrients (including bioactive protein, high-quality fatty acids, polysaccharides, and dietary fiber), but also in multiple micronutrients as well as vitamins, essential amino acids, minerals, saponins, phytoecdysteroids, phytosterols, and polyphenolic compounds (5). Polyphenols such as phenolic acids, flavonoids, and tannins contribute to biological functions, including anticarcinogenic, antioxidant, antimicrobial, and anti-inflammatory effects. Hence, quinoa can play a critical role in preventing multiple oxidative stress-associated diseases such as cancer, diabetes, osteoporosis, cardiovascular, gastrointestinal, and neurodegenerative diseases (6).
Inducing apoptosis is one of the main mechanisms of action for anticancer agents. Apoptosis is a critical dominant defense mechanism against cancer, which has extrinsic (death receptor) and intrinsic (mitochondrial) pathways. BCL2 family proteins are considered critical apoptotic regulators through the mitochondrial-mediated pathway, which act as pro-apoptotic (BAX, BAD,BAK, BIK, BLK, BIM, BID) or anti-apoptotic (BCL2, BCL-W, BCL-X, BCL-XS, BCL-XL) proteins. BAX and BCL2 are the main pro-apoptotic and anti-apoptotic proteins, respectively. The balance of these proteins determines cellular fate (7). In the lack of apoptotic stress, BCL2 and BCL-XL interact with BAX and BAK to maintain the outer mitochondrial membrane integrity and prevent mitochondrial apoptosis. On the other hand, apoptotic stress can change the ratios between anti-apoptotic and pro-apoptotic proteins and cause alteration in the construction of BAX/BAK complexes through punching the mitochondria membrane (8). Therefore, dysregulation of the BCL2 family can be a promising strategy for cancer treatment (9).
2. Objectives
In this study, we aimed to evaluate the potential anticancer activity and molecular mechanisms of quinoa seed extract on lung cancer cells through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and real-time polymerase chain reaction (PCR).
3. Methods
3.1. Materials
Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin, trypsin/EDTA solution, dimethyl sulfoxide (DMSO), and phosphate-buffered saline (PBS) were provided from Gibco BRL (Grand Island, NY, USA). MTT was also purchased from Sigma-Aldrich (M2128-1G, USA).
3.2. Extract Preparation
Quinoa (C. quinoa Willd.) seeds were prepared from a seed technology laboratory (Mashhad, Iran). To prepare the quinoa extract, 20 g of finely ground quinoa seeds were mixed with 200 mL of 98.0% (v/v) ethanol. The mixtures were then left in a shaking incubator at room temperature for 24 hours before filtration. The clear extract was collected and then evaporated using a rotary evaporator at 70°C (10).
3.3. Cell Cultivation
To investigate the anticancer activity of quinoa, the A549 cell line was used. A549 cells belong to human lung squamous cell carcinoma. The cells were cultured in DMEM supplemented with 10% FBS, penicillin/streptomycin (100 IU/mL) at 37°C in a humidified atmosphere of 5% CO2 (11).
3.4. Cell Viability Assays
The assessment of A549 cell viability was performed using the MTT assay. For this method, cells were incubated for 24 hours at 37°C and 5% CO2 in a humidified atmosphere. Then, the supernatant was replaced with 0.1 mL of a new culture medium containing a series of quinoa seed extract diluted in various concentrations (0, 0.4, 0.8, 1.2, 1.6, and 2 mg/mL). After 24, 48, and 72 hours of treatment, cell viability was investigated by the MTT assay based on our previous reports (11). The anticancer effects of quinoa were expressed with half maximal inhibitory concentration (IC50), calculated using concentration and time-dependent curves.
3.5. RNA Extraction and Gene Expression Analysis
A549 cells were seeded in 6-well plates and allowed to reach confluence. Cells were treated by adding quinoa seed extract at final concentrations of 1.60 and 1.92 mg/mL. After treatments, total RNA from A549 cells was extracted via the phenol-guanidinium thiocyanate procedure using the RNX PLUS kit (SinaClon, Iran). RNA was quantified at 230, 260, and 280 nm by a NanoDrop device (BioTek, Epoch, USA). Total RNA was reverse transcribed into complementary DNA (cDNA) using the PARSGENOME kit (Tehran, Iran). Primers for real-time PCR analysis were designed using National Center for Biotechnology Information (NCBI) websites and OligoAnalyzer online software. Primers for amplification of BAX were as follows: F: GGTTGTCGCCCTTTTCTA, R: CGGAGGAAGTCCAATGTC, and they were F: GATGTGATGCCTCTGCGAAG, and R: CATGCTGATGTCTCTGGAATCT for amplification of BCL2. GAPDH was used and amplified as a control using the following primers: F: GGTCGGAGTCAACGGA and R: CCAGCATCGCCCCACTT (12). Cycling conditions in a real-time PCR system (Applied Biosystems, Foster City, CA) were as follows: 10 minutes at 95°C, 40 cycles of 15 seconds at 95°C, 30 seconds at 60°C, followed by a melting curve analysis step (15 seconds at 95°C, 60 seconds at 60°C, and 15 seconds at 95°C). Each sample was analyzed in triplicate. The experiments were repeated twice with consistent results. Finally, the ∆∆Ct method was used to analyze gene expression.
3.6. Statistical Analyses
Data were analyzed using GraphPad Prism version 7 (GraphPad Software, San Diego, CA). All the reported data in histograms are expressed as mean ± SD. P values < 0.05 were considered statistically significant.
4. Results
4.1. In Vitro Cytotoxic Activity
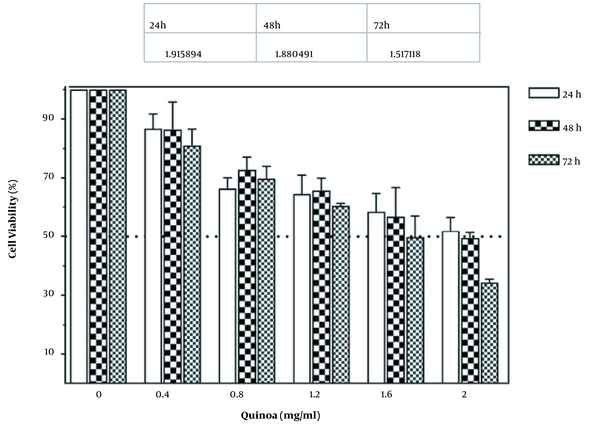
The cytotoxic effects of various doses of quinoa seed extract (0 - 2 mg/mL) for 24, 48, and 72 hours on A549 cells were investigated using the MTT assay. The results showed a potent antiproliferative effect of the quinoa extract against lung cancer. As illustrated in Figure 1, quinoa prohibits the proliferation of treated A549 cells in a concentration and time-dependent manner compared to the control. Cell viability decreased by increasing the time and dose of quinoa. For more investigations on quinoa extracts against the A549 cell line, IC50 values were determined. The results of quinoa IC50 were reported 1.9, 1.8, and 1.5 after 24, 48, and 72 hours of treatment, respectively.
The effect of quinoa ethanol extract (0-2 mg/mL) concentrations on cell viability of human lung cancer cell line A549 after 24, 48, and 72 hours of treatments.
4.2. Gene Expression
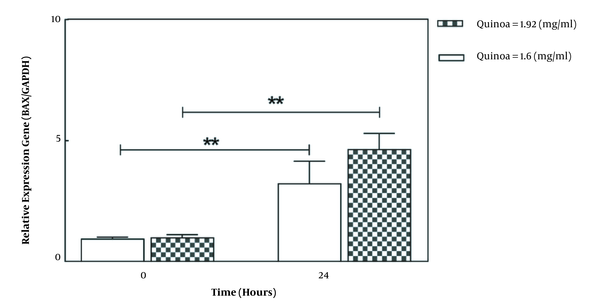
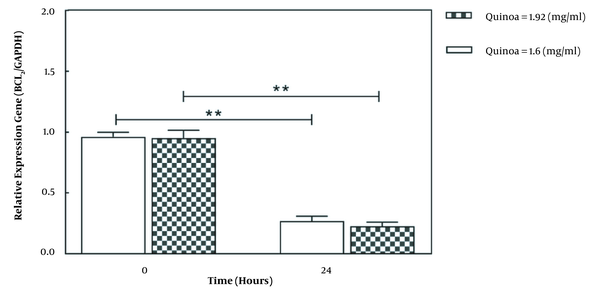
As shown in Figure 2, the selected doses of quinoa seed extract increased BAX expression in A549 after 24 hours (P < 0.05). The gene expression of BAX after 24 hours was significantly higher in the dose of 1.92 mg/mL compared to 1.60 mg/mL. Also, as illustrated in Figure 3, treatment of A549 cells with selected doses of quinoa decreased BCL2 expression (P < 0.05). However, gene expression after 24 hours was lower in higher concentrations (1.92 mg/mL) than in lower concentrations (1.60 mg/mL).
The effect of quinoa extract on messenger RNA expression of BAX and GAPDH in the A549 cell line. Data are expressed as mean ± SD. **P < 0.05 vs controls
The effect of quinoa extract on messenger RNA expression of BCL2 and GAPDH in the A549 cell line. Data are expressed as mean ± SD. **P < 0.05 vs controls
5. Discussion
Over recent years, natural compound-based drugs have attracted much attention (13). Several studies have proposed quinoa as a useful health-promoting product (14). Antitumor activity of quinoa has been reported in various cancers, including breast (15, 16), colorectal (15, 17), liver (16, 18), cervix (19), and prostate (20). However, its molecular mechanisms are not precisely understood. Although lung cancer is the most common cancer globally, only few studies have investigated the anticancer effects of quinoa on this cancer. Thus, we decided to evaluate the impact of quinoa seed extract on this cancer to assess apoptosis-related gene expression levels.
The MTT assay results indicated an antiproliferative property of quinoa in a concentration and time-dependent manner. Moreover, the IC50 values showed that the cytotoxic activity after 72 hours (IC50 = 1.5) of treatment was more effective compared to 24 hours (IC50 = 1.9) and/or 48 hours (IC50 = 1.8).
In line with our findings, the antiproliferative and antioxidant activities of quinoa were also reported in colorectal and breast cancer cell lines (21). Another in vitro study on prostate cancer proposed that phenolic compounds of quinoa leaves had antioxidant and anticancer activities through oxidative stress and reactive oxygen species (ROS)-dependent intracellular signaling (20). Similarly, Ren et al. demonstrated antioxidant and anti-inflammatory activities of quinoa due to the presence of lunasin (22). A study conducted by Mohamed et al. also reported that quinoa was not only a proper treatment to afford hepatoprotection against non-alcoholic fatty liver disease (NAFLD), but also it could be effective in hepatocarcinoma. They also found that quinoa promoted liver functions and significantly controlled triglycerides and total cholesterol (18).
Several studies have investigated the effects of quinoa on the immune system. Hu et al. (16) investigated human liver and breast cancer and reported that quinoa has antioxidant, anticancer, and immune-regulating activities due to a unique bioactive polysaccharide called the C. quinoa polysaccharide (CQP). Furthermore, Franceschelli et al. found that quinoa could prohibit oxidative and inflammatory activities in colorectal cancer based on the analysis of antioxidant enzymes, pro-inflammatory components, and intermediary metabolism products (17). Another research indicated that the structural characteristics of quinoa, such as protease inhibitors, were potentially being used to control hepatocarcinoma, affecting innate immunity in the tumor microenvironment (23).
Additionally, our findings showed that apoptosis-related genes such as BAX and BCL2 were upregulated and downregulated, respectively. The alteration in gene expression was more remarkable for a dose of 1.92 mg/mL compared to other doses. As apoptosis is one of the main cancer-related mechanisms, using such components (which target this process) could be beneficial.
5.1. Conclusions
In conclusion, our findings propose quinoa as a potential complementary agent for lung cancer therapy. However, further evaluations, especially in vivo and clinical trial studies, are necessary to confirm our results.
Acknowledgements
References
-
1.
Miranda-Filho A, Pineros M, Bray F. The descriptive epidemiology of lung cancer and tobacco control: a global overview 2018. Salud Publica Mex. 2019;61(3):219-29. [PubMed ID: 31276337]. https://doi.org/10.21149/10140.
-
2.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023-75.
-
3.
Wang H, Li X, Zhangsun D, Yu G, Su R, Luo S. The alpha9alpha10 Nicotinic Acetylcholine Receptor Antagonist alphaO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuropathic Pain. Mar Drugs. 2019;17(5). [PubMed ID: 31060282]. [PubMed Central ID: PMC6562493]. https://doi.org/10.3390/md17050265.
-
4.
Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, et al. Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12(10):1281-305. [PubMed ID: 22583408]. [PubMed Central ID: PMC4017674]. https://doi.org/10.2174/187152012803833026.
-
5.
Vilcacundo R, Hernández-Ledesma B. Nutritional and biological value of quinoa ( Chenopodium quinoa Willd.). Current Opinion in Food Science. 2017;14:1-6. https://doi.org/10.1016/j.cofs.2016.11.007.
-
6.
Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front Nutr. 2018;5:87. [PubMed ID: 30298133]. [PubMed Central ID: PMC6160559]. https://doi.org/10.3389/fnut.2018.00087.
-
7.
Edlich F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem Biophys Res Commun. 2018;500(1):26-34. [PubMed ID: 28676391]. https://doi.org/10.1016/j.bbrc.2017.06.190.
-
8.
Xiong S, Mu T, Wang G, Jiang X. Mitochondria-mediated apoptosis in mammals. Protein Cell. 2014;5(10):737-49. [PubMed ID: 25073422]. [PubMed Central ID: PMC4180462]. https://doi.org/10.1007/s13238-014-0089-1.
-
9.
Kulsoom B, Shamsi TS, Afsar NA, Memon Z, Ahmed N, Hasnain SN. Bax, Bcl-2, and Bax/Bcl-2 as prognostic markers in acute myeloid leukemia: are we ready for Bcl-2-directed therapy? Cancer Manag Res. 2018;10:403-16. [PubMed ID: 29535553]. [PubMed Central ID: PMC5841349]. https://doi.org/10.2147/CMAR.S154608.
-
10.
Segura R, Vasquez G, Colson E, Gerbaux P, Frischmon C, Nesic A, et al. Phytostimulant properties of highly stable silver nanoparticles obtained with saponin extract from Chenopodium quinoa. J Sci Food Agric. 2020;100(13):4987-94. [PubMed ID: 32597512]. https://doi.org/10.1002/jsfa.10529.
-
11.
Mollaei H, Safaralizadeh R, Babaei E, Abedini MR, Hoshyar R. The anti-proliferative and apoptotic effects of crocin on chemosensitive and chemoresistant cervical cancer cells. Biomed Pharmacother. 2017;94:307-16. [PubMed ID: 28763753]. https://doi.org/10.1016/j.biopha.2017.07.052.
-
12.
Atwan ZW. GAPDH spike RNA as an alternative for housekeeping genes in relative gene expression assay using real-time PCR. Bull Natl Res Cent. 2020;44(1). https://doi.org/10.1186/s42269-020-00284-1.
-
13.
Genovese S, Fiorito S, Epifano F, Taddeo VA. A Novel Class of Emerging Anticancer Compounds: Oxyprenylated Secondary Metabolites from Plants and Fungi. Curr Med Chem. 2015;22(30):3426-33. [PubMed ID: 26180000]. https://doi.org/10.2174/0929867322666150716114758.
-
14.
Hernández-Ledesma B. Quinoa (Chenopodium quinoa Willd.) as source of bioactive compounds: a review. Bioact Compd Health Dis. 2019;2(3). https://doi.org/10.31989/bchd.v2i3.556.
-
15.
Ayyash M, Johnson SK, Liu S, Al-Mheiri A, Abushelaibi A. Cytotoxicity, antihypertensive, antidiabetic and antioxidant activities of solid-state fermented lupin, quinoa and wheat by Bifidobacterium species: In-vitro investigations. Lwt. 2018;95:295-302. https://doi.org/10.1016/j.lwt.2018.04.099.
-
16.
Hu Y, Zhang J, Zou L, Fu C, Li P, Zhao G. Chemical characterization, antioxidant, immune-regulating and anticancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int J Biol Macromol. 2017;99:622-9. [PubMed ID: 28274868]. https://doi.org/10.1016/j.ijbiomac.2017.03.019.
-
17.
Franceschelli S, Gatta DMP, Pesce M, Ferrone A, Quiles JL, Genovese S, et al. Modulation of CAT-2B-Mediated l-Arginine Uptake and Nitric Oxide Biosynthesis in HCT116 Cell Line Through Biological Activity of 4'-Geranyloxyferulic Acid Extract from Quinoa Seeds. Int J Mol Sci. 2019;20(13). [PubMed ID: 31269760]. [PubMed Central ID: PMC6650945]. https://doi.org/10.3390/ijms20133262.
-
18.
Mohamed DA, Fouda KA, Mohamed RS. In vitro Anticancer Activity of Quinoa and Safflower Seeds and Their Preventive Effects on Non-alcoholic Fatty Liver. Pak J Biol Sci. 2019;22(8):383-92. [PubMed ID: 31930826]. https://doi.org/10.3923/pjbs.2019.383.392.
-
19.
Paśko P, Tyszka-Czochara M, Namieśnik J, Jastrzębski Z, Leontowicz H, Drzewiecki J, et al. Cytotoxic, antioxidant and binding properties of polyphenols from the selected gluten-free pseudocereals and their by-products: In vitro model. J Cereal Sci. 2019;87:325-33. https://doi.org/10.1016/j.jcs.2019.04.009.
-
20.
Gawlik-Dziki U, Swieca M, Sulkowski M, Dziki D, Baraniak B, Czyz J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts - in vitro study. Food Chem Toxicol. 2013;57:154-60. [PubMed ID: 23537598]. https://doi.org/10.1016/j.fct.2013.03.023.
-
21.
Ayyash M, Johnson SK, Liu SQ, Mesmari N, Dahmani S, Al Dhaheri AS, et al. In vitro investigation of bioactivities of solid-state fermented lupin, quinoa and wheat using Lactobacillus spp. Food Chem. 2019;275:50-8. [PubMed ID: 30724226]. https://doi.org/10.1016/j.foodchem.2018.09.031.
-
22.
Ren G, Zhu Y, Shi Z, Li J. Detection of lunasin in quinoa (Chenopodium quinoa Willd.) and the in vitro evaluation of its antioxidant and anti-inflammatory activities. J Sci Food Agric. 2017;97(12):4110-6. [PubMed ID: 28218804]. https://doi.org/10.1002/jsfa.8278.
-
23.
Laparra JM, Haros CM. Plant seed protease inhibitors differentially affect innate immunity in a tumor microenvironment to control hepatocarcinoma. Food Funct. 2019;10(7):4210-9. [PubMed ID: 31257391]. https://doi.org/10.1039/c9fo00795d.