Abstract
Keywords
MSC-derived Exosomes Kidney Disease Diagnosis Therapy Urinary Exosomes
1. Context
Kidney disease is a severe problem worldwide, and its prevalence has been increasing in recent years. Kidney disease is divided into two main categories: Acute kidney injury (AKI) and chronic kidney disease (CKD) (1). Acute kidney injury is a condition in which kidney function declines abruptly, lasting for seven to 90 days, resulting in decreased glomerular filtration rate and urine output, as well as increased serum creatinine (2, 3). Chronic kidney disease is a broad term that refers to a group of diseases that impair the structure and function of the kidneys for more than three months and progress to end-stage renal disease (ESRD) (4, 5). Disease expression variation is influenced by the etiology, pathophysiology, severity, and progression rate (6).
Kidney transplantation and dialysis are the two main treatments currently available for kidney regeneration. Both are unsatisfactory, and the increase in survival rates after treatment is insufficient. Dialysis has several drawbacks, including high mortality rate, hospitalization, loss of independence, depression, and high drug costs (7). The limitations of kidney transplantation are the shortage of kidney donors, the risk of infection or cancer transmission, the high cost of the surgeon, and severe immune rejection (8). As a result, the development of practical therapeutic approaches has opened up new opportunities for kidney regeneration (9, 10).
Over the last few decades, many studies have shown that transplanting stem cells to patients with kidney disease improves their kidney function (11). Among all stem cell types, mesenchymal stem cells (MSCs) have been identified as one of the most effective cell types for inducing kidney regeneration due to their ease of isolation and expansion and lack of teratoma risk, immunosuppressive properties, and absence of ethical problems (12, 13). Despite the benefits of MSC-based therapy, there are some drawbacks, including the possibility of tumorigenesis, prion, and viral transmission, loss of differentiation and morphological changes after long-term culture, and the possibility of antibody production in the host body after repeated administration of MSCs (14, 15). Cell-free approaches have been used in recent years to reduce the adverse effects of MSC-based cell therapy. Exosomes are a group of extracellular vesicles with sizes ranging from 30 to 100 nm produced inside multivesicular bodies (MVBs) in almost all types of cells and secreted from the original cell to the target recipient cell, which is one of the tools used in cell-free approaches (16, 17). As known, MSC-exosomes contain pro-regenerative molecules from their origin cell and mimic MSC functions in tissue regeneration in cell therapy, mediating intercellular communication; thus, they are a popular substitute for cell therapy and have the potential to regenerate tissues and be used in tissue engineering without the risk of tumorigenesis or high immune rejection (18, 19).
Aside from the availability of diagnostic markers for AKI and CKD, such as serum creatinine and urine output, urinary exosomes have aided in making the diagnostic process more sensitive and faster. Changes in the expression of specific molecules in urinary exosomes derived from the kidney, prostate, and bladder organs can be used as biomarkers to assess kidney health. The lipid bilayer structure of exosomes protects their cargo from degradation, allowing them to be isolated and analyzed for differences in disease biomarker expression levels (20). For example, in ischemia/reperfusion (I/R) models, miRNAs of urinary exosomes revealed the state of kidney injury or fibrosis (21).
This review article will concentrate on the role of MSC-derived exosomes in treating kidney disease and summarize the latest findings on the diagnosis and application of urinary exosomes.
2. Evidence Acquisition
2.1. Acute Kidney Injury
Acute kidney injury is a sudden impairment of renal function and structure associated with a high morbidity and mortality rate in hospitalized patients. Clinical signs of AKI include a sudden rise in serum creatinine, a decrease in urine volume, and a fall in glomerular filtration rate (GFR) (2, 3, 22). The most important definitions of AKI are based on RIFLE (risk, injury, failure, loss, and end-stage renal disease) classification, AKIN (AKI network), and KDIGO (kidney disease improving global outcomes) (Table 1) (2, 23, 24).
Acute Kidney Injury Definition Based on RLIFE, AKIN, and KDIGO
| Class | RIFLE SCr or GFR | Stage | AKINSCr | Stage | KDIGO SCr |
|---|---|---|---|---|---|
| Risk | Increased SCr × 1.5 or GFR decrease > 25% (within 7 days) | 1 | Increase in SCr ≥ 0.3 mg/dL or ≥ 150% to 200% (1.5 to 2-fold) from baseline (within 48 hours) | 1 | Increase in SCr by ≥ 0.3 mg/dL within 48 hours or increase in SCr 1.5 to 1.9 times the baseline which is known or presumed to have occurred within the prior 7 days |
| Injury | Increased SCr × 2.0 or GFR decrease > 50% | 2 | Increase in SCr to more than 200% to 300% (> 2 to 3-fold) from baseline | 2 | Increase in SCr to 2.0 to 2.9 times the baseline |
| Failure | Increased Scr × 3.0 or GFR decrease > 75% or SCr ≥ 4.0mg/dL or acute increase ≥ 0.5 mg/dL | 3 | Increase in SCr to more than 300% (> 3-fold) from baseline or SCr ≥ 4.0 mg/dL with an acute increase of at least 0.5 mg/dL or initiation of renal replacement therapy | 3 | Increase in SCr to 3.0 times the baseline increase in SCr to ≥ 4.0 mg/dL or initiation of renal replacement therapy |
| Loss | Persistent acute renal failure = Complete loss of kidney function > 4 weeks | ||||
| End-stage kidney disease | End-stage of kidney disease (> 3 months) |
The area, number of patients, and definition of AKI play a role in AKI epidemiology (25, 26). In affluent countries, hospital-acquired AKI is more common in older and severely ill patients (27-29). The leading causes of AKI in developing countries, depending on patient accommodation, are healthcare-related conditions such as nephrotoxic drugs and sepsis in urban areas and community-acquired conditions such as infectious disease and diarrhea in rural areas (30-33). A meta-analysis of 154 studies based on the KDIGO definition of AKI was conducted to estimate the global incidence of AKI in developed countries in North America, Northern Europe, and Eastern Asia. They discovered that one in every five adults and one in every three children in the world suffer from AKI during a hospital stay (34). The etiology of AKI is divided into three categories: Pre-renal, post-renal, and intrinsic (35, 36). Glomerular filtration rate decreases in the pre-renal category without impairment of the renal parenchyma; the leading causes in this category are renal hypoperfusion, cardiac failure, intravascular depletion, sepsis, hypotension, pancreatitis and liver diseases (cirrhosis), bleeding, and burns (37-39). Acute obstruction of the urine flow, such as ureteric calculus, causes post-renal AKI, which causes an increase in intra-tubular pressure, impairment of renal blood flow, and inflammations lowering GFR, eventually leading to renal failure (40, 41). Intrinsic AKI, also known as acute renal failure, is when the kidney suffers from various direct and sudden damages. The most common causes of intrinsic AKI are Acute Tubular Necrosis (ATN), nephrotoxins, vasculitis, bacterial or viral infections, allergic interstitial nephritis, hepatorenal syndrome, and glomerulonephritis (2, 36, 42).
2.2. Chronic Kidney Disease
Chronic kidney disease is defined as persistent abnormalities in the structure and function of the kidney lasting for more than three months or a reduction in GFR of less than 60 mL/min per 1.73 m2 (4). The CKD global incidence is increasing, with a global prevalence of 13.4%, and the number of ESRD people ranges from 4.902 to 7.083 million (43). The CKD etiologies include AKI, hypertension, toxic insults, diabetes mellitus, age, obesity, and nephrectomy (6, 44). Besides, CKD has a wide range of characteristics and mechanisms, some of which are associated with AKI pathologic mechanisms. Also, CKD is characterized by pericyte migration, pericyte phenotype change, microvascular loss, chronic tubular hypoxia, cellular senescence, collagen deposition, myofibroblast proliferation, tubular loss, and replacement with collagen scars, chronic leukocyte infiltration, and end-stage renal failure (45-48). End-stage renal failure (ESRD) is the final stage of CKD in which the kidney fails, and dialysis or kidney transplantation is required to survive (49). Diabetes mellitus (DM) is one of the major causes of CKD, in which the inflammation and oxidative stress of the kidney, resulting from hyperglycemia, lead to the production of inflammatory cytokines and the development of diabetic nephropathy. Diabetic nephropathy (DN) is a diabetic kidney disease that results in ESRD by causing structural (glomerular basement membrane thickening and glomerular sclerosis) and functional (GFR reduction, albuminuria, proteinuria, and hyperfiltration) changes in the kidney (50, 51). Because of the difficulty of diagnosing CKD in the early stages due to the asymptomatic condition, disease diagnosis is delayed, and available treatments for CKD regeneration are limited.
2.3. Exosomes
2.3.1. Discovery and Definition of Exosomes
For the first time, scientists observed 50 nm extracellular membrane vesicles carrying transferrin receptors releasing from reticulocytes in 1938. Multivesicular endosomes holding vesicles with transferrin receptors were observed fusing to the membrane of a sheep reticulocyte and releasing those vesicles into the extracellular environment; and in 1987, the term “exosome” was coined to describe this type of extracellular vesicle (52, 53). Exosomes are cup-shaped lipid bilayer vesicles with a size of 30 - 100 nm that are secreted by a variety of cells, including MSCs, neuronal cells, cytotoxic T cells, and platelets, and found in various body fluids, including sperm, blood, urine, and amniotic fluid (16, 54, 55).
2.3.2. Biogenesis of Exosomes
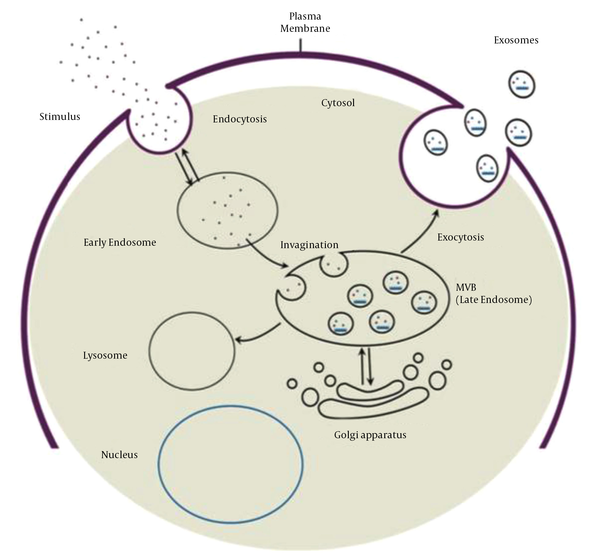
Exosome biogenesis begins with the maturation of an early endosome to a late endosome or MVB. Invagination of the endosomal membrane during late endosome formation results in the absorption of intracellular content and the formation of intraluminal vesicles. Multivesicular bodies have two fusion pathways: Fusion to the lysosome and degradation of the cargo, and fusion to the plasma membrane and release of intraluminal vesicles called exosomes into the extracellular space (Figure 1). Evidence suggests that two distinct mechanisms can form intraluminal vesicles: Endosomal sorting complex required for transport (ESCRT)-independent and ESCRT-dependent mechanisms. ESCRT is composed of four complexes and accessory proteins: ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III. These complexes cooperate in an orderly manner to identify ubiquitinated proteins in the endosomal membrane and form intraluminal vesicles via inward budding. The ESCRT-independent mechanism, on the other hand, is dependent on lipid raft microdomains enriched with sphingomyelinases and tetraspanin-enriched microdomains (56-58).
Exosome biogenesis process and two fusion pathways of MVB (59)
2.3.3. Components of Exosome
Exosomes are complex entities whose contents depend on the parent cell. Exosomes contain various elements, including lipids, proteins, and nucleic acids. Tetraspanins (CD63, CD81, CD82, and CD9) as surface markers, heat shock proteins (HSP70, HSP90), MVB formation and release proteins (ESCRT complex, Alix, and TSG101), and MVB membrane transport and fusion (GTPases and annexins, phospholipase and lipid-related proteins) are all common proteins found in exosomes. Exosomes contain a variety of RNA patterns, including lncRNA, tRNA, mRNA, miRNA, and rRNA, which are involved in several biological functions in the recipient cell. According to the data, microRNA is the most abundant RNA pattern in exosomes. Sphingomyelin, prostaglandins, cholesterol, phosphatidylserine, various fatty acids, and leukotrienes make up the lipid content of exosomes, which play significant roles in structural stiffness and the protection of the inner cargo of exosomes from degradation (58, 60-62).
2.3.4. Isolation of Exosomes
Exosome purification from bodily fluids and conditioned culture media necessitates tools and established procedures. There are several suggested approaches, each with its advantages and disadvantages. Differential ultracentrifugation is the most commonly used method due to advantages such as low cost, short processing time, and density-based exosome purification. However, the lack of specificity of this method is a significant drawback, as it prevents complete purification and permits contamination with other extracellular vesicles, protein aggregates, and genetic elements. Furthermore, due to the presence of complex elements in urine and serum, differential ultracentrifugation is ineffective for purifying their exosomes. Low yield and purification necessitate the use of differential ultracentrifugation in conjunction with a sucrose cushion to increase specificity (63-67). Exosomes are filtered from smaller contaminants using the ultrafiltration purification technique in a quick, easy, and high-yield resolution process; however, there is a risk of contamination fragmentation depending on the pore size. As a result, some particles and fragments of the same size as exosomes can pass through the pores using this mechanism (68, 69). Another method for exosome purification is high-pressure liquid chromatography (HPLC), which creates a homogeneous environment for exosomes while preserving their biological properties. Although this method is highly purifying, it is expensive and has limited scalability (70, 71). Immunoaffinity capture uses antibodies that bind to surface markers on exosomes; however, it produces highly purified exosomes, is expensive, and has a low yield (72). ExoQuick precipitation methods enable the production of highly purified exosomes from a small sample volume in less than two hours (73). Polymer-based precipitation, Microfluidic technology, field-flow fractionation, and a combination of these techniques are used for isolation; therefore, given the impossibility of removing all contaminants, selecting an appropriate approach to provide the most purified exosomes is critical (64, 74).
2.3.5. Identification of Exosomes
Following exosome isolation, it is critical to confirm their characteristics and features. Exosome surface markers are identified using immunocytochemical analysis, western blot, and flow cytometry (CD81, CD63, CD82, and CD9). Atomic force microscopy (AFM), Scanning Electron Microscopy (SEM), and transmission electron microscopy (TEM) can all be used to examine morphology and size (75, 76). Exosome concentration and size distribution can be determined using dynamic light scattering (DLS) and nanoparticle tracking analysis (NTA) (77-79). However, the quick and inexpensive ELISA method is the gold standard for quantifying exosomes and their markers (80). In recent years, surface-enhanced raman spectroscopy (SERS) has been introduced as a precise and sensitive method for identifying and analyzing exosome-like vesicles (81) (Table 2).
Summary of Exosome Features, Isolation, and Identification Methods
| Variables | Exosome Features |
|---|---|
| Diameter and shape | 30 - 100 nm, cup shape |
| Origin | Release of exosomes to extracellular matrix after fusion of multivesicular body with plasma membrane |
| Protein content | CD63, CD81, CD82, CD9, HSP70, HSP90, ESCRT complex, Alix, TSG101, GTPases, annexins, phospholipase, and lipid-related proteins |
| Lipid content | Phosphatidylserine, various fatty acids, leukotrienes sphingomyelin, prostaglandins, and cholesterol |
| Nucleic acids | miRNA, mRNA, DNA, and non-coding RNA |
| Isolation techniques | Ultracentrifugation, ultrafiltration, immunoaffinity capture, high-pressure liquid chromatography, and precipitation |
| Identification techniques | Immunocytochemical analysis, western blot, flow cytometry, microscopic analysis, ELISA, dynamic light scattering, nanoparticle tracking analysis, surface-enhanced Raman spectroscopy |
2.3.6. Function of Exosomes
Exosomes have a variety of functions depending on their origin cells. They participate in immune responses, inflammation, angiogenesis, coagulation, cell-to-cell communication, and spreading pathogens such as prions and viruses. They also play essential roles in disease diagnosis, cell-free therapy, and delivery (proteins, genes, and chemicals) (82-84). This review article concentrates on the diagnosis and regenerative applications of MSC-derived exosomes in kidney disease.
2.4. MSC-derived Exosomes
In 2010, Lai et al. found the paracrine influence of MSCs on tissue regeneration and demonstrated for the first-time particles with a size of 50 - 100 nm called exosomes secreting from MSCs in ischemic/reperfusion injury mice (85). As known, MSC-exosomes have biological functions similar to their origin cells, and depending on the content; they can repair cells and tissues, regulate immune and inflammatory responses, suppress apoptosis, and modulate homeostasis, cell growth, proliferation, survival, migration, tumor progression, and inhibition (86, 87).
There are numerous cargos in the structure of MSC-exosomes that differ depending on the source cell. Tetraspanins, adhesion proteins, antigen-presenting proteins, cytokine receptors, heat shock proteins, lipoproteins, fatty acid-binding proteins, trophic factors, cytokines, chemokines, membrane fusion proteins, translation and transcription proteins, motility proteins, structure proteins, and enzymes are among the nearly 2000 proteins identified in MSC-exosomes. The nucleic acid content of exosomes is enriched with miRNAs, which are involved in various regulatory and pathological states such as angiogenesis, inflammation, cell growth, tumorigenesis, and tumor progression. Besides, MSC-exosome lipid content includes various types of fatty acids, prostaglandins, lysophosphatidylcholine, leukotrienes, and other lipids found in all exosomes (88, 89).
The widespread availability of MSCs and large-scale production of exosomes for cell-free therapy are some of the benefits of using MSC exosomes as a therapeutic tool (90, 91). Also, MSC-exosome isolation is more straightforward, less time-consuming, and less expensive than MSC isolation (92). Furthermore, exosome therapy is safer than MSC-based therapies because exosomes lack the potential to multiply and are free of a substantial quantity of markers that can be shown as antigens by the host body, resulting in reduced immunological rejection (93). In addition, there are no concerns about cell survival in exosome-based regeneration, and structural stability for an extended time at a storage temperature of 20°C makes them an excellent choice for cell-free therapy (94) (Table 3).
Summary of Roles of Urinary Exosomes in Diagnosis and MSC-derived Exosomes in Regeneration of Acute Kidney Injury and Chronic Kidney Disease
| Kidney Disease | Role of Exosome | Main Results | Reference |
|---|---|---|---|
| AKI | Biomarker | Upregulation of miR-16, miR-24, miR-200c, miR-125, miR-351, miR-9a, miR-141, miR-200a, miR-200c, and miR-429 | (21) |
| Biomarker | Downregulation of AQP-2 and AQP-1 after 168 hrs, upregulation of AQP-1 after 24 hrs | (21, 95) | |
| Biomarker | Upregulation of uATF3 | (96) | |
| Biomarker | Upregulation of miR-30c-5p and miR-192-5p | (97) | |
| CKD | Biomarker | Upregulation of miR-21 and downregulation of miR‑29c | (98) |
| Biomarker | Downregulation of miR-29c-5p and miR-15b-5p, upregulation of let-7c-5p | (99) | |
| Biomarker | Upregulation of miR-30a, miR-133b, and miR-342 | (100) | |
| Biomarker | Upregulation of miR-21 | (101) | |
| Biomarker | Downregulation of miR-200b | (102) | |
| Biomarker | Upregulation of miR-451 | (103) | |
| AKI | Therapeutic | Proliferation of injured PTECs | (104) |
| Therapeutic | Enhancement of cell proliferation, anti-apoptotic and antioxidant effects | (105) | |
| Therapeutic | Reduction of Sema3A, cleaved caspase 3, and pro-apoptotic protein Bax., upregulation of Bcl-2 | (106) | |
| Therapeutic | Upregulation of autophagosome marker LC3B, reduction of inflammation and apoptosis, inhabitation of mTOR pathway | (107) | |
| Therapeutic | Downregulation of genes related to hypoxia, apoptosis, and cytoskeleton reorganization | (108) | |
| Therapeutic | Antioxidant effect by upregulation of HO-1 and Nrf2/anti-oxidant response element | (109) | |
| Therapeutic | Enhancement of autophagy and prevention of nephrotoxicity | (110) | |
| Therapeutic | Reduction of CCL2 concentration and recruitment of macrophages/monocytes for inflammation | (111) | |
| Therapeutic | Decreasing oxidative stress, inflammation, fibrotic, and apoptotic biomarkers, increasing anti-apoptotic and angiogenesis biomarkers | (112) | |
| CKD | Therapeutic | Decreasing pro-inflammatory cytokines, medullary oxygenation, and fibrosis | (113) |
| Therapeutic | Reducing fibrosis and downregulating fibrotic genes | (114) | |
| Therapeutic | Reduction of EndoMT and apoptosis, enhancement of endothelial cell proliferation, inhabitation of kidney fibrosis | (115) | |
| Therapeutic | Promoting angiogenesis, reducing microvascular architecture, and endothelial cell apoptosis | (116) | |
| Therapeutic | Reduction of renal ischemia, hypoxia, subsequent fibrosis, and infiltration of inflammatory cells | (117) | |
| Therapeutic | No regenerative effects | (118) | |
| Therapeutic | Suppressing renal fibrosis and EMT and protecting tubular endothelial cells | (119) | |
| Therapeutic | Downregulating of mTOR and fibrotic marker expression Improvement of renal function and morphology | (120) | |
| Therapeutic | Promoting vascular regeneration and cell survival, reduction of urine volume and albumin excretion, increasing glomerular endothelial cell proliferation | (121) |
2.5. Diagnosis Application of Urinary Exosomes in Renal Disease
The complex and unique structure of exosomes, which resembles the constitution of their parent cell, is a tool for demonstrating cellular mechanisms in the body and can thus be used as a biomarker for disease diagnosis. Recent research has shown that exosomes derived from luminal epithelial renal cells reflect renal function. Consequently, urinary exosomal biomarkers can be used to diagnose AKI and CKD.
2.5.1. AKI Diagnosis
Urine analysis, urine output measurement, and blood tests are standard AKI diagnostic tools. On the other hand, Urinary exosomes are valuable sources for diagnosing AKI in a precise and timely manner. According to one study, increased levels of urinary exosomes indicated the injury phase. In contrast, the upregulation of miR-9a, miR-141, miR-200a, miR-200c, and miR-429 indicated the early recovery phase and increased expression level of miR-125 and miR-351 indicated the late stage of the fibrotic phase of ischemia/reperfusion-induced AKI in rat models (21). In another study, researchers assessed the expression levels of urinary exosomal aquaporin-1 (AQP-1) and aquaporin-2 (AQP-2) to see if these assessments can be applied to detect early and late stages of cisplatin-induced AKI in rat models. They observed that a decrease in AQP-2 level of the inner medulla after 168 h, an increase in AQP-1 level of the outer medulla after 24 h, and a reduction after 168 h are the markers of renal impairment in rat models (21, 95). In sepsis-induced AKI mice models, the upregulation of urinary exosomes' activation transcriptional factor 3 (uATF3) was a biomarker for disease diagnosis (96). Besides, miR-30c-5p and miR-192-5p upregulations have been identified as promising biomarkers for ischemia/reperfusion-induced kidney injury (97). 2.5.2. CKD Diagnosis
Many researchers who have looked into biomarker microRNAs in urinary exosomes have relied on databases or profiling to identify CKD markers. Lv et al. investigated the expression levels of miR-29c and miR-21 isolated from urinary exosomes of renal fibrosis (RF) patients to see if urinary exosomes could be used to diagnose RF. They realized that isolating exosomes and observing the upregulation of miR-21 and the downregulation of miR29c is a low-cost and highly sensitive method for diagnosing RF (98). In an investigation on the use of urine exosomes to diagnose Type 2 Diabetic Nephropathy (T2DN), a decrease in urinary exosomal miR-29c-5p and miR-15b-5p and an increase in let-7c-5p were the predictors of type 2 DN (99). Furthermore, bioinformatics analysis has revealed that the upregulation of miR-30a, miR-133b, and miR-342 in urine exosomes is a marker of T2DN (100). The elevation of urine exosomal miR-21, a non-invasive biomarker of CKD, has a deleterious influence on renal function (101). Another example is the reduction of miR-200b in non-proximal renal tubule-derived urinary exosomes as a biomarker for the diagnosis of renal fibrosis in CKD patients (102). Moreover, miR-451 increases as an early response to renal cell injury in CKD patients (103).
2.6. Therapeutic Utilization of MSC-derived Exosomes in Kidney Disease
Anti-apoptotic, antioxidant, anti-fibrotic, anti-inflammatory, and immunomodulatory properties of MSCs highlight their therapeutic potential. As a result, MSC-derived exosomes mimic immunomodulatory and cytoprotective functions of their parent cell and transfer regenerative information to injured cells or tissues (55).
2.6.1. MSC-derived Exosomes in AKI Treatment
Tomasoni et al. used cisplatin to induce AKI in mouse proximal tubular epithelial cells (PTECs). They observed the proliferation of injured PTECs after transferring human bone marrow MSC-derived exosomes containing insulin-like growth factor-1 (IGF-1) (104). In cisplatin-induced AKI mice, Zhou et al. reported that human umbilical cord MSC-derived exosomes increased cell proliferation by activating the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway, reducing blood urea nitrogen (BUN) and creatinine (Cr) levels, tubular protein casts, and proximal epithelium necrosis via anti-apoptotic actions, and acted as an antioxidant (105). Zhu et al. found that exosomes produced by human bone marrow-derived MSC exosomes enriched with miR-199a-3p had an anti-apoptotic effect on renal ischemia/reperfusion injury, one of the most common causes of AKI, in rat models. By activating the ERK and AKT pathways, miR-199a-3p decreased the expression of Semaphorin 3A (Sema3A), cleaved caspase 3, and pro-apoptotic Bcl-2-associated X (Bax) protein, and increased the expression of B-cell lymphoma-2 (Bcl-2) (106). Wang et al. investigated the effects of human umbilical cord MSC-derived exosomes (hucMSC-Ex) on preventing cisplatin-induced nephrotoxicity. They found that pretreatment with hucMSC-Ex increased the light chain 3B (LC3B) autophagosome marker expression in renal proximal tubule epithelial cells by inhibiting the mammalian target of rapamycin (mTOR) pathway. As a result, inflammation and apoptosis reduced, and renal regeneration improved (107). Lindoso et al. used a renal ischemia/reperfusion injury induced by ATP depletion model and incubated them with MSC-extracellular vesicles to investigate the role of MSC-derived extracellular vesicles in changing the miRNAs expression of renal PTECs. They discovered that by modulating the miRNA profile of PTECs, MSC-extracellular vesicles reduced the expression of genes involved in hypoxia, apoptosis, and cytoskeleton reorganization, such as caspase 7 (CASP7), caspase 3 (CASP3), SHC (Src homology 2 domain-containing) transforming protein 1 (SHC1), and SMAD4 (108). Zhang et al. investigated the anti-oxidative role of extracellular vesicles derived from human Wharton's Jelly mesenchymal stromal cells (hWJMSC) in AKI rat models. Cell apoptosis, serum neutrophil gelatinase-associated lipocalin (sNGAL) level, and oxidative stress decreased after hWJMSC-derived extracellular vesicles were treated. Antioxidant activity increased by the augmentation of the Nrf2/antioxidant response element (ARE) pathway and heme oxygenase-1 (HO-1) expression (109). Jia et al. demonstrated that 14-3-3 ζ containing hucMSC-ex enhanced autophagy via binding to autophagy-related protein 16L (ATG16L) and prevented nephrotoxicity in cisplatin-induced AKI rat models (110). Shen et al. investigated the protective impact of MSC-derived exosomes in ischemia/reperfusion-induced kidney damage animal models by administering C-C motif chemokine receptor-2 (CCR2)-enriched BMMSC-exosomes. It has been demonstrated that binding CCR2 to the C-C motif chemokine ligand-2 (CCL2) protein reduces CCL2 concentration and recruitment of macrophages/monocytes for inflammation in renal injury (111). Lin et al. investigated the synergistic effect of adipose-derived mesenchymal stem cell (ADMSC) and ADMSC-derived exosome in acute ischemia/reperfusion injury models. They concluded that ADMSC-derived exosomes protect the kidney by decreasing oxidative stress, inflammation, fibrotic, and apoptotic biomarker levels while increasing anti-apoptotic and angiogenesis biomarker levels (112).
2.6.2. MSC-derived Exosomes in CKD Treatment
The regenerative effect of MSC-derived exosomes was studied in pigs with renal artery stenosis due to CKD. According to the findings, MSC exosomes enriched with interleukin-10 (IL-10) reduced inflammation by lowering the pro-inflammatory cytokines interleukin-1 (IL-1) alpha, IL-1 beta, and tumor necrosis factor-alpha (TNF-alpha), as well as medullary oxygenation and fibrosis (113). Furthermore, researchers used genetically engineered MSCs that overexpressed miRNA-let7c and delivered its exosomes to mice with unilateral ureteral obstruction. Exosome miR-let7c reduced fibrosis and downregulated fibrotic genes such as collagen IV1, α-SMA, transforming growth factor-beta receptor I (TGF-βR1), and (TGF)-β1 (114). Choi et al. investigated the efficacy of kidney mesenchymal stem cell-derived exosomes in unilateral ureteral obstruction (UUO) mouse models with peritubular capillary (PTC) rarefaction. They observed that endothelial-to-mesenchymal transition (EndoMT) and apoptosis reduced while endothelial cell proliferation increased. Furthermore, exosomes inhibited macrophage infiltration (F4/80 positive) and kidney fibrosis (115). Another study found that MSC-derived exosomes, which contain pro-angiogenic genes and proteins, improve renal function and recovery by promoting angiogenesis, reducing microvascular architecture, and endothelial cell apoptosis (116). Furthermore, human adipose-derived MSC (hAD-MSC) exosomes prevented AKI progression to CKD by activating renal tubular Sox9. In C57BL/6 mice, treatment with hAD-MSCs-secreting exosomes increased tubular epithelial cell (TEC) Sox9 and inhibited TEC transformation to a pro-fibrotic phenotype induced by TGF-1. There was also TEC proliferation, reduction of renal ischemia, hypoxia, subsequent fibrosis, and infiltration of inflammatory cells (117). Despite the numerous benefits of MSC-derived exosomes in CKD regeneration, exosomes derived from human embryonic MSCs had no regenerative effects on in vitro rat models of CKD induced by 5/6 nephrectomy (SNX) combined with L-NNA and a 6% NaCl diet (118).
According to a study of the paracrine effects of bone marrow-derived MSCs and MSC conditioned medium on DN regeneration, exosomes improved DN by suppressing abnormal infiltration of bone marrow dendritic cells, renal fibrosis, and epithelial-mesenchymal transition (EMT) and protecting tubular endothelial cells (119). In another study, bone marrow-derived MSC-exosomes improved renal function and morphology in DN rats by upregulating autophagy markers LC3II and Beclin-1 while downregulating mTOR and fibrotic marker expression (120). In a DN rat model, exosomes derived from urine mesenchymal-like stem cells inhibited the apoptosis of podocytes and tubular endothelial cells. They also reduced urine volume and albumin excretion while increasing glomerular endothelial cell proliferation. These exosomes contain factors for promoting vascular regeneration and cell survival, such as transforming growth factor-β1, angiogenin, and bone morphogenetic protein-7 (121).
3. Conclusions
In conclusion, the protective structure of the exosome saves its cargo from degradation; thus, the molecular cargo of exosomes can play essential roles in the early diagnosis and treatment of kidney disease. However, there are not enough studies and clinical trials in this field to ensure the efficacy of MSC-exosomes in kidney treatment. There are also difficulties in selecting faster and more effective methods for isolating and purifying exosomes. Limitation of exosomal biomarkers for kidney diagnosis in the laboratory and insufficient information about the reference value of biomarkers imply that their diagnostic standard is still in its infancy. As a result, accurate detection and precise conformation of biomarker indices are required to improve exosome diagnosis application. Furthermore, our future goals are advancements in the use of MSC-exosomes in cell-free-based kidney therapy to alleviate disease pain and reduce treatment side effects in patients.
References
-
1.
Bao YW, Yuan Y, Chen JH, Lin WQ. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool Res. 2018;39(2):72-86. [PubMed ID: 29515089]. [PubMed Central ID: PMC5885387]. https://doi.org/10.24272/j.issn.2095-8137.2017.055.
-
2.
Makris K, Spanou L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin Biochem Rev. 2016;37(2):85-98. [PubMed ID: 28303073]. [PubMed Central ID: PMC5198510].
-
3.
Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241-57. [PubMed ID: 28239173]. https://doi.org/10.1038/nrneph.2017.2.
-
4.
Gaitonde DY, Cook DL, Rivera IM. Chronic Kidney Disease: Detection and Evaluation. Am Fam Physician. 2017;96(12):776-83. [PubMed ID: 29431364].
-
5.
Zoccali C, Kramer A, Jager KJ. Chronic kidney disease and end-stage renal disease-a review produced to contribute to the report 'the status of health in the European union: towards a healthier Europe'. NDT Plus. 2010;3(3):213-24. [PubMed ID: 28657040]. [PubMed Central ID: PMC5477935]. https://doi.org/10.1093/ndtplus/sfp127.
-
6.
Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-80. [PubMed ID: 21840587]. https://doi.org/10.1016/S0140-6736(11)60178-5.
-
7.
Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573-85. [PubMed ID: 32733095]. [PubMed Central ID: PMC7391926]. https://doi.org/10.1038/s41581-020-0315-4.
-
8.
Heldal K, Midtvedt K, Lonning K, Iversen T, Hernaes KH, Tsarpali V, et al. Kidney transplantation: an attractive and cost-effective alternative for older patients? A cost-utility study. Clin Kidney J. 2019;12(6):888-94. [PubMed ID: 31807304]. [PubMed Central ID: PMC6885668]. https://doi.org/10.1093/ckj/sfz018.
-
9.
Liu D, Cheng F, Pan S, Liu Z. Stem cells: a potential treatment option for kidney diseases. Stem Cell Res Ther. 2020;11(1):249. [PubMed ID: 32586408]. [PubMed Central ID: PMC7318741]. https://doi.org/10.1186/s13287-020-01751-2.
-
10.
Kaballo MA, Canney M, O'Kelly P, Williams Y, O'Seaghdha CM, Conlon PJ. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin Kidney J. 2018;11(3):389-93. [PubMed ID: 29942504]. [PubMed Central ID: PMC6007575]. https://doi.org/10.1093/ckj/sfx117.
-
11.
Wong CY. Current advances of stem cell-based therapy for kidney diseases. World J Stem Cells. 2021;13(7):914-33. [PubMed ID: 34367484]. [PubMed Central ID: PMC8316868]. https://doi.org/10.4252/wjsc.v13.i7.914.
-
12.
Kim HJ, Park JS. Usage of Human Mesenchymal Stem Cells in Cell-based Therapy: Advantages and Disadvantages. Dev Reprod. 2017;21(1):1-10. [PubMed ID: 28484739]. [PubMed Central ID: PMC5409204]. https://doi.org/10.12717/DR.2017.21.1.001.
-
13.
Rota C, Morigi M, Imberti B. Stem Cell Therapies in Kidney Diseases: Progress and Challenges. Int J Mol Sci. 2019;20(11). [PubMed ID: 31181604]. [PubMed Central ID: PMC6600599]. https://doi.org/10.3390/ijms20112790.
-
14.
Musial-Wysocka A, Kot M, Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28(7):801-12. [PubMed ID: 31018669]. [PubMed Central ID: PMC6719501]. https://doi.org/10.1177/0963689719837897.
-
15.
Ghayour MB, Abdolmaleki A, Fereidoni M. Use of Stem Cells in the Regeneration of Peripheral Nerve Injuries: an Overview. Neurosci J Shefaye Khatam. 2015;3(1):84-98. https://doi.org/10.18869/acadpub.shefa.3.1.84.
-
16.
Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8(7). [PubMed ID: 31311206]. [PubMed Central ID: PMC6678302]. https://doi.org/10.3390/cells8070727.
-
17.
Nasrollahi Nia F, Asadi A, Zahri S, Abdolmaleki A. Biosynthesis, characterization and evaluation of the supportive properties and biocompatibility of DBM nanoparticles on a tissue-engineered nerve conduit from decellularized sciatic nerve. Regen Ther. 2020;14:315-21. [PubMed ID: 32467828]. [PubMed Central ID: PMC7243182]. https://doi.org/10.1016/j.reth.2020.03.004.
-
18.
Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18(1):449. [PubMed ID: 33246476]. [PubMed Central ID: PMC7691969]. https://doi.org/10.1186/s12967-020-02622-3.
-
19.
Toh WS, Lai RC, Zhang B, Lim SK. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans. 2018;46(4):843-53. [PubMed ID: 29986939]. [PubMed Central ID: PMC6103455]. https://doi.org/10.1042/BST20180079.
-
20.
Jin C, Wu P, Li L, Xu W, Qian H. Exosomes: Emerging Therapy Delivery Tools and Biomarkers for Kidney Diseases. Stem Cells Int. 2021;2021:7844455. [PubMed ID: 34471412]. [PubMed Central ID: PMC8405320]. https://doi.org/10.1155/2021/7844455.
-
21.
Sonoda H, Lee BR, Park KH, Nihalani D, Yoon JH, Ikeda M, et al. miRNA profiling of urinary exosomes to assess the progression of acute kidney injury. Sci Rep. 2019;9(1):4692. [PubMed ID: 30886169]. [PubMed Central ID: PMC6423131]. https://doi.org/10.1038/s41598-019-40747-8.
-
22.
Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52. [PubMed ID: 34267223]. https://doi.org/10.1038/s41572-021-00284-z.
-
23.
Thomas ME, Blaine C, Dawnay A, Devonald MA, Ftouh S, Laing C, et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87(1):62-73. [PubMed ID: 25317932]. https://doi.org/10.1038/ki.2014.328.
-
24.
Machado MN, Nakazone MA, Maia LN. Acute kidney injury based on KDIGO (Kidney Disease Improving Global Outcomes) criteria in patients with elevated baseline serum creatinine undergoing cardiac surgery. Rev Bras Cir Cardiovasc. 2014;29(3):299-307. [PubMed ID: 25372901]. [PubMed Central ID: PMC4412317]. https://doi.org/10.5935/1678-9741.20140049.
-
25.
Li PK, Burdmann EA, Mehta RL; World Kidney Day Steering Committee. Acute kidney injury: global health alert. Kidney Int. 2013;83(3):372-6. [PubMed ID: 23302721]. https://doi.org/10.1038/ki.2012.427.
-
26.
Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3(4):948-54. [PubMed ID: 18417742]. [PubMed Central ID: PMC2440279]. https://doi.org/10.2215/CJN.05431207.
-
27.
Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382(9887):170-9. [PubMed ID: 23727171]. https://doi.org/10.1016/S0140-6736(13)60647-9.
-
28.
Case J, Khan S, Khalid R, Khan A. Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract. 2013;2013:479730. [PubMed ID: 23573420]. [PubMed Central ID: PMC3618922]. https://doi.org/10.1155/2013/479730.
-
29.
Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2(7):364-77. [PubMed ID: 16932465]. https://doi.org/10.1038/ncpneph0218.
-
30.
Priyamvada PS, Jayasurya R, Shankar V, Parameswaran S. Epidemiology and Outcomes of Acute Kidney Injury in Critically Ill: Experience from a Tertiary Care Center. Indian J Nephrol. 2018;28(6):413-20. [PubMed ID: 30647494]. [PubMed Central ID: PMC6309393]. https://doi.org/10.4103/ijn.IJN_191_17.
-
31.
Prakash J, Singh TB, Ghosh B, Malhotra V, Rathore SS, Vohra R, et al. Changing epidemiology of community-acquired acute kidney injury in developing countries: analysis of 2405 cases in 26 years from eastern India. Clin Kidney J. 2013;6(2):150-5. [PubMed ID: 26019843]. [PubMed Central ID: PMC4432435]. https://doi.org/10.1093/ckj/sfs178.
-
32.
Cerdá J, Bagga A, Kher V, Chakravarthi RM. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008;4(3):138-53. [PubMed ID: 18212780]. https://doi.org/10.1038/ncpneph0722.
-
33.
Lewington AJ, Cerda J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84(3):457-67. [PubMed ID: 23636171]. [PubMed Central ID: PMC3758780]. https://doi.org/10.1038/ki.2013.153.
-
34.
Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482-93. [PubMed ID: 23744003]. [PubMed Central ID: PMC3805065]. https://doi.org/10.2215/CJN.00710113.
-
35.
Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365(9457):417-30. [PubMed ID: 15680458]. https://doi.org/10.1016/S0140-6736(05)17831-3.
-
36.
Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-9. [PubMed ID: 23062091].
-
37.
Prieto-Garcia L, Pericacho M, Sancho-Martinez SM, Sanchez A, Martinez-Salgado C, Lopez-Novoa JM, et al. Mechanisms of triple whammy acute kidney injury. Pharmacol Ther. 2016;167:132-45. [PubMed ID: 27490717]. https://doi.org/10.1016/j.pharmthera.2016.07.011.
-
38.
Tariq R, Singal AK. Management of Hepatorenal Syndrome: A Review. J Clin Transl Hepatol. 2020;8(2):192-9. [PubMed ID: 32832400]. [PubMed Central ID: PMC7438356]. https://doi.org/10.14218/JCTH.2020.00011.
-
39.
Blantz RC. Pathophysiology of pre-renal azotemia. Kidney Int. 1998;53(2):512-23. [PubMed ID: 9461116]. https://doi.org/10.1046/j.1523-1755.2003_t01-1-00784.x.
-
40.
Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2(2):1303-53. [PubMed ID: 23798302]. [PubMed Central ID: PMC3919808]. https://doi.org/10.1002/cphy.c110041.
-
41.
Hegarty NJ, Young LS, Kirwan CN, O'Neill AJ, Bouchier-Hayes DM, Sweeney P, et al. Nitric oxide in unilateral ureteral obstruction: effect on regional renal blood flow. Kidney Int. 2001;59(3):1059-65. [PubMed ID: 11231361]. https://doi.org/10.1046/j.1523-1755.2001.0590031059.x.
-
42.
Prakash J, Sen D, Kumar NS, Kumar H, Tripathi LK, Saxena RK. Acute renal failure due to intrinsic renal diseases: review of 1122 cases. Ren Fail. 2003;25(2):225-33. [PubMed ID: 12739829]. https://doi.org/10.1081/jdi-120018723.
-
43.
Lv JC, Zhang LX. Prevalence and Disease Burden of Chronic Kidney Disease. Adv Exp Med Biol. 2019;1165:3-15. [PubMed ID: 31399958]. https://doi.org/10.1007/978-981-13-8871-2_1.
-
44.
Prowle JR, Forni LG, Bell M, Chew MS, Edwards M, Grams ME, et al. Postoperative acute kidney injury in adult non-cardiac surgery: joint consensus report of the Acute Disease Quality Initiative and PeriOperative Quality Initiative. Nat Rev Nephrol. 2021;17(9):605-18. [PubMed ID: 33976395]. [PubMed Central ID: PMC8367817]. https://doi.org/10.1038/s41581-021-00418-2.
-
45.
Ferenbach DA, Bonventre JV. Acute kidney injury and chronic kidney disease: From the laboratory to the clinic. Nephrol Ther. 2016;12 Suppl 1:S41-8. [PubMed ID: 26972097]. [PubMed Central ID: PMC5475438]. https://doi.org/10.1016/j.nephro.2016.02.005.
-
46.
Zafrani L, Ince C. Microcirculation in Acute and Chronic Kidney Diseases. Am J Kidney Dis. 2015;66(6):1083-94. [PubMed ID: 26231789]. https://doi.org/10.1053/j.ajkd.2015.06.019.
-
47.
Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20(3):297-305. [PubMed ID: 21422927]. https://doi.org/10.1097/MNH.0b013e328344c3d4.
-
48.
Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, et al. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol. 2012;23(5):868-83. [PubMed ID: 22383695]. [PubMed Central ID: PMC3338296]. https://doi.org/10.1681/ASN.2011080851.
-
49.
Benjamin O, Lappin SL. End-Stage Renal Disease. Treasure Island (FL): StatPearls; 2021.
-
50.
Aghadavod E, Khodadadi S, Baradaran A, Nasri P, Bahmani M, Rafieian-Kopaei M. Role of Oxidative Stress and Inflammatory Factors in Diabetic Kidney Disease. Iran J Kidney Dis. 2016;10(6):337-43. [PubMed ID: 27903991].
-
51.
Onuigbo MA, Agbasi N. Diabetic Nephropathy and CKD-Analysis of Individual Patient Serum Creatinine Trajectories: A Forgotten Diagnostic Methodology for Diabetic CKD Prognostication and Prediction. J Clin Med. 2015;4(7):1348-68. [PubMed ID: 26239680]. [PubMed Central ID: PMC4519794]. https://doi.org/10.3390/jcm4071348.
-
52.
Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967-78. [PubMed ID: 6307529]. https://doi.org/10.1016/0092-8674(83)90040-5.
-
53.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412-20. [PubMed ID: 3597417].
-
54.
Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. [PubMed ID: 30815248]. [PubMed Central ID: PMC6377728]. https://doi.org/10.1186/s13578-019-0282-2.
-
55.
Ma ZJ, Yang JJ, Lu YB, Liu ZY, Wang XX. Mesenchymal stem cell-derived exosomes: Toward cell-free therapeutic strategies in regenerative medicine. World J Stem Cells. 2020;12(8):814-40. [PubMed ID: 32952861]. [PubMed Central ID: PMC7477653]. https://doi.org/10.4252/wjsc.v12.i8.814.
-
56.
Tschuschke M, Kocherova I, Bryja A, Mozdziak P, Angelova Volponi A, Janowicz K, et al. Inclusion Biogenesis, Methods of Isolation and Clinical Application of Human Cellular Exosomes. J Clin Med. 2020;9(2). [PubMed ID: 32041096]. [PubMed Central ID: PMC7074492]. https://doi.org/10.3390/jcm9020436.
-
57.
Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337-62. [PubMed ID: 22831642]. https://doi.org/10.1146/annurev-cellbio-092910-154152.
-
58.
Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288(15):10849-59. [PubMed ID: 23439645]. [PubMed Central ID: PMC3624465]. https://doi.org/10.1074/jbc.M112.446831.
-
59.
Borges FT, Reis LA, Schor N. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res. 2013;46(10):824-30. [PubMed ID: 24141609]. [PubMed Central ID: PMC3854311]. https://doi.org/10.1590/1414-431X20132964.
-
60.
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241-4. [PubMed ID: 21989406]. [PubMed Central ID: PMC3245025]. https://doi.org/10.1093/nar/gkr828.
-
61.
Kim DK, Kang B, Kim OY, Choi DS, Lee J, Kim SR, et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles. 2013;2. [PubMed ID: 24009897]. [PubMed Central ID: PMC3760654]. https://doi.org/10.3402/jev.v2i0.20384.
-
62.
Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116-25. [PubMed ID: 24959705]. https://doi.org/10.1016/j.ceb.2014.05.004.
-
63.
Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684-707. [PubMed ID: 32206116]. [PubMed Central ID: PMC7069071]. https://doi.org/10.7150/thno.41580.
-
64.
Sidhom K, Obi PO, Saleem A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int J Mol Sci. 2020;21(18). [PubMed ID: 32899828]. [PubMed Central ID: PMC7556044]. https://doi.org/10.3390/ijms21186466.
-
65.
Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270(2):211-26. [PubMed ID: 12379326]. https://doi.org/10.1016/s0022-1759(02)00330-7.
-
66.
Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940-8. [PubMed ID: 22503788]. https://doi.org/10.1016/j.bbagen.2012.03.017.
-
67.
Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles. 2013;2. [PubMed ID: 24009896]. [PubMed Central ID: PMC3760653]. https://doi.org/10.3402/jev.v2i0.19861.
-
68.
Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396-405. [PubMed ID: 26241750]. [PubMed Central ID: PMC4656109]. https://doi.org/10.1016/j.jconrel.2015.07.030.
-
69.
Yu LL, Zhu J, Liu JX, Jiang F, Ni WK, Qu LS, et al. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. Biomed Res Int. 2018;2018:3634563. [PubMed ID: 30148165]. [PubMed Central ID: PMC6083592]. https://doi.org/10.1155/2018/3634563.
-
70.
Lai RC, Arslan F, Tan SS, Tan B, Choo A, Lee MM, et al. Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles. J Mol Cell Cardiol. 2010;48(6):1215-24. [PubMed ID: 20064522]. https://doi.org/10.1016/j.yjmcc.2009.12.021.
-
71.
Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo AB, et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9:47. [PubMed ID: 21513579]. [PubMed Central ID: PMC3100248]. https://doi.org/10.1186/1479-5876-9-47.
-
72.
Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56(2):293-304. [PubMed ID: 22285593]. https://doi.org/10.1016/j.ymeth.2012.01.002.
-
73.
Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Korvala J, Salo T, Sormunen R, et al. Human saliva-derived exosomes: comparing methods of isolation. J Histochem Cytochem. 2015;63(3):181-9. [PubMed ID: 25473095]. [PubMed Central ID: PMC4340734]. https://doi.org/10.1369/0022155414564219.
-
74.
Liangsupree T, Multia E, Riekkola ML. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. 2021;1636:461773. [PubMed ID: 33316564]. https://doi.org/10.1016/j.chroma.2020.461773.
-
75.
Wu Y, Deng W, Klinke D2. Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst. 2015;140(19):6631-42. [PubMed ID: 26332016]. [PubMed Central ID: PMC4986832]. https://doi.org/10.1039/c5an00688k.
-
76.
Wang YT, Shi T, Srivastava S, Kagan J, Liu T, Rodland KD. Proteomic Analysis of Exosomes for Discovery of Protein Biomarkers for Prostate and Bladder Cancer. Cancers (Basel). 2020;12(9). [PubMed ID: 32825017]. [PubMed Central ID: PMC7564640]. https://doi.org/10.3390/cancers12092335.
-
77.
Akers JC, Ramakrishnan V, Nolan JP, Duggan E, Fu CC, Hochberg FH, et al. Comparative Analysis of Technologies for Quantifying Extracellular Vesicles (EVs) in Clinical Cerebrospinal Fluids (CSF). PLoS One. 2016;11(2). e0149866. [PubMed ID: 26901428]. [PubMed Central ID: PMC4763994]. https://doi.org/10.1371/journal.pone.0149866.
-
78.
Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine. 2011;7(6):780-8. [PubMed ID: 21601655]. [PubMed Central ID: PMC3280380]. https://doi.org/10.1016/j.nano.2011.04.003.
-
79.
Gardiner C, Ferreira YJ, Dragovic RA, Redman CW, Sargent IL. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J Extracell Vesicles. 2013;2. [PubMed ID: 24009893]. [PubMed Central ID: PMC3760643]. https://doi.org/10.3402/jev.v2i0.19671.
-
80.
Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. [PubMed ID: 31394127]. https://doi.org/10.1016/j.lfs.2019.116733.
-
81.
Merdalimova A, Chernyshev V, Nozdriukhin D, Rudakovskaya P, Gorin D, Yashchenok A. Identification and Analysis of Exosomes by Surface-Enhanced Raman Spectroscopy. Applied Sciences. 2019;9(6). https://doi.org/10.3390/app9061135.
-
82.
Gurunathan S, Kang MH, Kim JH. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int J Nanomedicine. 2021;16:1281-312. [PubMed ID: 33628021]. [PubMed Central ID: PMC7898217]. https://doi.org/10.2147/IJN.S291956.
-
83.
Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells. 2019;8(4). [PubMed ID: 30987213]. [PubMed Central ID: PMC6523673]. https://doi.org/10.3390/cells8040307.
-
84.
Choi JY, Kim S, Kwak HB, Park DH, Park JH, Ryu JS, et al. Extracellular Vesicles as a Source of Urological Biomarkers: Lessons Learned From Advances and Challenges in Clinical Applications to Major Diseases. Int Neurourol J. 2017;21(2):83-96. [PubMed ID: 28673066]. [PubMed Central ID: PMC5497201]. https://doi.org/10.5213/inj.1734961.458.
-
85.
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214-22. [PubMed ID: 20138817]. https://doi.org/10.1016/j.scr.2009.12.003.
-
86.
Harrell CR, Jovicic N, Djonov V, Volarevic V. Therapeutic Use of Mesenchymal Stem Cell-Derived Exosomes: From Basic Science to Clinics. Pharmaceutics. 2020;12(5). [PubMed ID: 32456070]. [PubMed Central ID: PMC7313713]. https://doi.org/10.3390/pharmaceutics12050474.
-
87.
Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells. 2020;9(5). [PubMed ID: 32392899]. [PubMed Central ID: PMC7290908]. https://doi.org/10.3390/cells9051157.
-
88.
Deng H, Sun C, Sun Y, Li H, Yang L, Wu D, et al. Lipid, Protein, and MicroRNA Composition Within Mesenchymal Stem Cell-Derived Exosomes. Cell Reprogram. 2018;20(3):178-86. [PubMed ID: 29782191]. https://doi.org/10.1089/cell.2017.0047.
-
89.
Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM. Current Knowledge and Future Perspectives on Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Agent. Int J Mol Sci. 2020;21(3). [PubMed ID: 31979113]. [PubMed Central ID: PMC7036914]. https://doi.org/10.3390/ijms21030727.
-
90.
Janockova J, Slovinska L, Harvanova D, Spakova T, Rosocha J. New therapeutic approaches of mesenchymal stem cells-derived exosomes. J Biomed Sci. 2021;28(1):39. [PubMed ID: 34030679]. [PubMed Central ID: PMC8143902]. https://doi.org/10.1186/s12929-021-00736-4.
-
91.
Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3(8). [PubMed ID: 29669940]. [PubMed Central ID: PMC5931131]. https://doi.org/10.1172/jci.insight.99263.
-
92.
Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. Int J Nanomedicine. 2019;14:2847-59. [PubMed ID: 31114198]. [PubMed Central ID: PMC6488158]. https://doi.org/10.2147/IJN.S200036.
-
93.
Han C, Sun X, Liu L, Jiang H, Shen Y, Xu X, et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016;2016:7653489. [PubMed ID: 26770213]. [PubMed Central ID: PMC4684885]. https://doi.org/10.1155/2016/7653489.
-
94.
Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15(3):4142-57. [PubMed ID: 24608926]. [PubMed Central ID: PMC3975389]. https://doi.org/10.3390/ijms15034142.
-
95.
Sonoda H, Oshikawa-Hori S, Ikeda M. An Early Decrease in Release of Aquaporin-2 in Urinary Extracellular Vesicles After Cisplatin Treatment in Rats. Cells. 2019;8(2). [PubMed ID: 30744167]. [PubMed Central ID: PMC6407024]. https://doi.org/10.3390/cells8020139.
-
96.
Panich T, Chancharoenthana W, Somparn P, Issara-Amphorn J, Hirankarn N, Leelahavanichkul A. Urinary exosomal activating transcriptional factor 3 as the early diagnostic biomarker for sepsis-induced acute kidney injury. BMC Nephrol. 2017;18(1):10. [PubMed ID: 28061889]. [PubMed Central ID: PMC5219663]. https://doi.org/10.1186/s12882-016-0415-3.
-
97.
Zou YF, Wen D, Zhao Q, Shen PY, Shi H, Zhao Q, et al. Urinary MicroRNA-30c-5p and MicroRNA-192-5p as potential biomarkers of ischemia-reperfusion-induced kidney injury. Exp Biol Med (Maywood). 2017;242(6):657-67. [PubMed ID: 28056546]. [PubMed Central ID: PMC5685255]. https://doi.org/10.1177/1535370216685005.
-
98.
Lv CY, Ding WJ, Wang YL, Zhao ZY, Li JH, Chen Y, et al. A PEG-based method for the isolation of urinary exosomes and its application in renal fibrosis diagnostics using cargo miR-29c and miR-21 analysis. Int Urol Nephrol. 2018;50(5):973-82. [PubMed ID: 29330775]. https://doi.org/10.1007/s11255-017-1779-4.
-
99.
Li W, Yang S, Qiao R, Zhang J. Potential Value of Urinary Exosome-Derived let-7c-5p in the Diagnosis and Progression of Type II Diabetic Nephropathy. Clin Lab. 2018;64(5):709-18. [PubMed ID: 29739042]. https://doi.org/10.7754/Clin.Lab.2018.171031.
-
100.
Wang LP, Gao YZ, Song B, Yu G, Chen H, Zhang ZW, et al. MicroRNAs in the Progress of Diabetic Nephropathy: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2019;2019:3513179. [PubMed ID: 30984273]. [PubMed Central ID: PMC6431481]. https://doi.org/10.1155/2019/3513179.
-
101.
Lange T, Artelt N, Kindt F, Stracke S, Rettig R, Lendeckel U, et al. MiR-21 is up-regulated in urinary exosomes of chronic kidney disease patients and after glomerular injury. J Cell Mol Med. 2019;23(7):4839-43. [PubMed ID: 31066165]. [PubMed Central ID: PMC6584549]. https://doi.org/10.1111/jcmm.14317.
-
102.
Yu Y, Bai F, Qin N, Liu W, Sun Q, Zhou Y, et al. Non-Proximal Renal Tubule-Derived Urinary Exosomal miR-200b as a Biomarker of Renal Fibrosis. Nephron. 2018;139(3):269-82. [PubMed ID: 29539618]. https://doi.org/10.1159/000487104.
-
103.
Kumari M, Mohan A, Ecelbarger CM, Gupta A, Prasad N, Tiwari S. miR-451 Loaded Exosomes Are Released by the Renal Cells in Response to Injury and Associated With Reduced Kidney Function in Human. Front Physiol. 2020;11:234. [PubMed ID: 32322216]. [PubMed Central ID: PMC7158952]. https://doi.org/10.3389/fphys.2020.00234.
-
104.
Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22(5):772-80. [PubMed ID: 23082760]. [PubMed Central ID: PMC3578372]. https://doi.org/10.1089/scd.2012.0266.
-
105.
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4(2):34. [PubMed ID: 23618405]. [PubMed Central ID: PMC3707035]. https://doi.org/10.1186/scrt194.
-
106.
Zhu G, Pei L, Lin F, Yin H, Li X, He W, et al. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J Cell Physiol. 2019;234(12):23736-49. [PubMed ID: 31180587]. https://doi.org/10.1002/jcp.28941.
-
107.
Wang B, Jia H, Zhang B, Wang J, Ji C, Zhu X, et al. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res Ther. 2017;8(1):75. [PubMed ID: 28388958]. [PubMed Central ID: PMC5385032]. https://doi.org/10.1186/s13287-016-0463-4.
-
108.
Lindoso RS, Collino F, Bruno S, Araujo DS, Sant'Anna JF, Tetta C, et al. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev. 2014;23(15):1809-19. [PubMed ID: 24669934]. [PubMed Central ID: PMC4103261]. https://doi.org/10.1089/scd.2013.0618.
-
109.
Zhang G, Zou X, Huang Y, Wang F, Miao S, Liu G, et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect Against Acute Kidney Injury Through Anti-Oxidation by Enhancing Nrf2/ARE Activation in Rats. Kidney Blood Press Res. 2016;41(2):119-28. [PubMed ID: 26894749]. https://doi.org/10.1159/000443413.
-
110.
Jia H, Liu W, Zhang B, Wang J, Wu P, Tandra N, et al. HucMSC exosomes-delivered 14-3-3zeta enhanced autophagy via modulation of ATG16L in preventing cisplatin-induced acute kidney injury. Am J Transl Res. 2018;10(1):101-13. [PubMed ID: 29422997]. [PubMed Central ID: PMC5801350].
-
111.
Shen B, Liu J, Zhang F, Wang Y, Qin Y, Zhou Z, et al. CCR2 Positive Exosome Released by Mesenchymal Stem Cells Suppresses Macrophage Functions and Alleviates Ischemia/Reperfusion-Induced Renal Injury. Stem Cells Int. 2016;2016:1240301. [PubMed ID: 27843457]. [PubMed Central ID: PMC5098097]. https://doi.org/10.1155/2016/1240301.
-
112.
Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173-85. [PubMed ID: 27156061]. https://doi.org/10.1016/j.ijcard.2016.04.061.
-
113.
Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92(1):114-24. [PubMed ID: 28242034]. [PubMed Central ID: PMC5483390]. https://doi.org/10.1016/j.kint.2016.12.023.
-
114.
Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, et al. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol Ther. 2016;24(7):1290-301. [PubMed ID: 27203438]. [PubMed Central ID: PMC5088767]. https://doi.org/10.1038/mt.2016.90.
-
115.
Choi HY, Lee HG, Kim BS, Ahn SH, Jung A, Lee M, et al. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem Cell Res Ther. 2015;6:18. [PubMed ID: 25889661]. [PubMed Central ID: PMC4393614]. https://doi.org/10.1186/s13287-015-0012-6.
-
116.
Eirin A, Zhu XY, Jonnada S, Lerman A, van Wijnen AJ, Lerman LO. Mesenchymal Stem Cell-Derived Extracellular Vesicles Improve the Renal Microvasculature in Metabolic Renovascular Disease in Swine. Cell Transplant. 2018;27(7):1080-95. [PubMed ID: 29954220]. [PubMed Central ID: PMC6158551]. https://doi.org/10.1177/0963689718780942.
-
117.
Zhu F, Chong Lee Shin OLS, Pei G, Hu Z, Yang J, Zhu H, et al. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget. 2017;8(41):70707-26. [PubMed ID: 29050313]. [PubMed Central ID: PMC5642588]. https://doi.org/10.18632/oncotarget.19979.
-
118.
van Koppen A, Joles JA, van Balkom BW, Lim SK, de Kleijn D, Giles RH, et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One. 2012;7(6). e38746. [PubMed ID: 22723882]. [PubMed Central ID: PMC3378606]. https://doi.org/10.1371/journal.pone.0038746.
-
119.
Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N, et al. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep. 2016;6:34842. [PubMed ID: 27721418]. [PubMed Central ID: PMC5056395]. https://doi.org/10.1038/srep34842.
-
120.
Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, et al. Mesenchymal Stem Cell-Derived Exosomes Ameliorated Diabetic Nephropathy by Autophagy Induction through the mTOR Signaling Pathway. Cells. 2018;7(12). [PubMed ID: 30467302]. [PubMed Central ID: PMC6315695]. https://doi.org/10.3390/cells7120226.
-
121.
Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC, et al. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:24. [PubMed ID: 26852014]. [PubMed Central ID: PMC4744390]. https://doi.org/10.1186/s13287-016-0287-2.