Abstract
Keywords
Colorectal Cancer Apoptosis Long Noncoding RNA HOTAIR Bax Bcl2
1. Background
Colorectal cancer (CRC) has been recorded as one of the global top 10 cancers allocating rank four after skin, lung, and breast (1, 2). In 2017, 1.8 million incident cases of CRC along with 896000 deaths were reported. CRC is more prevalent in men than women (1/26 for men vs. 1/40 for women) (3, 4). Family history of the disease, inflammatory bowel disease, obesity, and alcohol and tobacco consumption are well-documented risk factors of CRC (5-7). Although this type of cancer has mostly been observed in adults, CRC has been diagnosed at any age (8). Dysregulation of genes may affect the cellular behavior and promote tumorigenesis (9, 10). Long noncoding RNAs (lncRNAs) are lengthy non-protein coding transcripts which are actively involved in modulating critical cellular signaling pathways containing cell cycle, apoptosis, and differentiation (11, 12). Abnormal expression levels of lncRNAs have been described in different human tumors (13). HOX transcript antisense RNA (HOTAIR) is one of the well-studied lncRNAs which has been dysregulated in a variety of solid tumors (13, 14). The literature demonstrated that HOTAIR is associated with apoptosis. Increased expression of HOTAIR may suppress apoptosis (15, 16)]. Gene silencing through RNA interference (RNAi) strategy is recently suggested as the therapeutic method against cancer (17, 18). This is a non-invasive approach which can effectively target the critical pathways of cell proliferation, growth, or invasion (19).
2. Objectives
The present study aimed to investigate the effect of HOTAIR silencing in triggering apoptosis through targeting Bax and Bcl2 in cellular model of CRC.
3. Methods
3.1. Cell Lines/Culture Condition
The cellular model of CRC Caco-2 was purchased from the national cell bank of the Pasteur Institute, Tehran, Iranian Biological Resource Center. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Life Technologies, USA) containing 2mM Glutamine, 10% FBS, 1% penicillin, and 1% streptomycin (Life Technologies, USA). Cells were incubated at 37°C and atmosphere of 5% CO2.
3.2. HOTAIR siRNA Transfection
The 3 × 104 cells were plated onto the 6-well plates and treated with 50 nM siRNA duplexes (siHOTAIR I, SASI Hs02 00380445) for 48h using LipofectamineTM 2000 (Invitrogen, USA) according to the user guideline. Concentration of siRNA was set based on the concentration in our pilot research, and found this concentration more suitable for knocking out.MISSION® siRNA Universal Negative Control #1, SIGMA/SIC001) was used as negative control (20). The mock test was defined as cells treated with lipofectamine and without siRNA.
3.3. RNA Extraction and Real-Time Polymerase Chain Reaction
Following 48h of treatment with siRNA, total RNA was extracted from transfected cells using Trizol reagent (Invitrogen, USA) based on manufacturer protocol. The RNAs were reverse transcribed to cDNA through Primer-Script one step real-time polymerase chain reaction (RT-PCR) kit (Biofact). The cDNA samples were amplified using the SYBR green kit (Biofact) during real-time PCR. The primers used in this study are listed in Table 1. Relative gene expression was calculated as 2−ΔΔCt.
Used Primers in This Study
| Target Gene | Sequence Primer | Amplicon Length |
|---|---|---|
| HOTAIR | F':AGGCCCTGCCTTCTGCCT | 147 bp |
| R:TGCTCTCTTACCCCCACGGA | ||
| BAX | F':TTCATCCAGGATCGAGCAGG | 103 bp |
| R:TGAGACACTCGCTCAGCTTC | ||
| BCL2 | F:GAGGATTGTGGCCTTCTTTG | 143 bp |
| R:CGTTATCCTGGATCCAGGTG | ||
| GAPDH | F:GTGAACCATGAGAAGTATGACAAC | 123bp |
| R:CATGAGTCCTTCCACGATACC |
3.4. Statistical Analyses
Data were analyzed using a one-way analysis of variance with Newman-Keuls correction for multiple comparisons using the GraphPad Prism version 6 (GraphPad Software, USA). The nonparametric tests were used if normal distribution was not met. P-value less than 0.05 was considered as a statistically significant difference.
4. Results
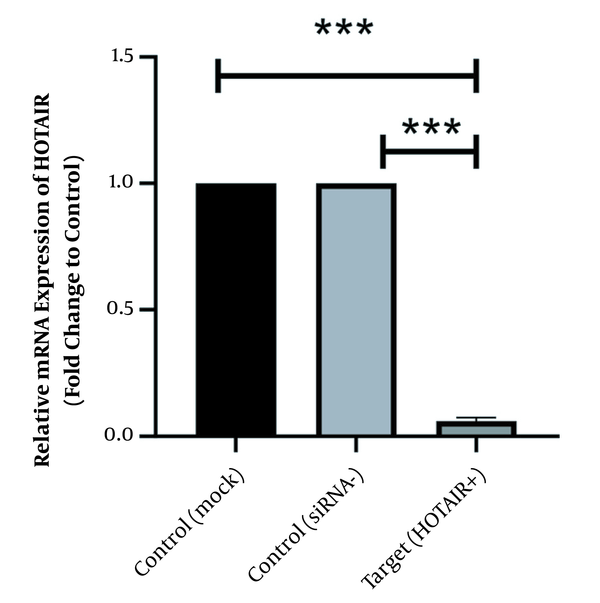
As illustrated in Figure 1 HOTAIR is strongly expressed in normal culture of Caco-2 cells before siRNA treatment. However, its expression level has been significantly downregulated since the HOTAIR-specific siRNA was added into the culture medium. The fold-change was 0.06 ± 0.011 (P-value < 0.001).
Effects of HOTAIR specific siRNA on suppressing HOTAIR transcript after treatment with 50 ng siRNA after 48 hours of treatment. Data expressed as mean ± standard deviation (the stars *** show the P-value less than 0.001 compared to the control group).
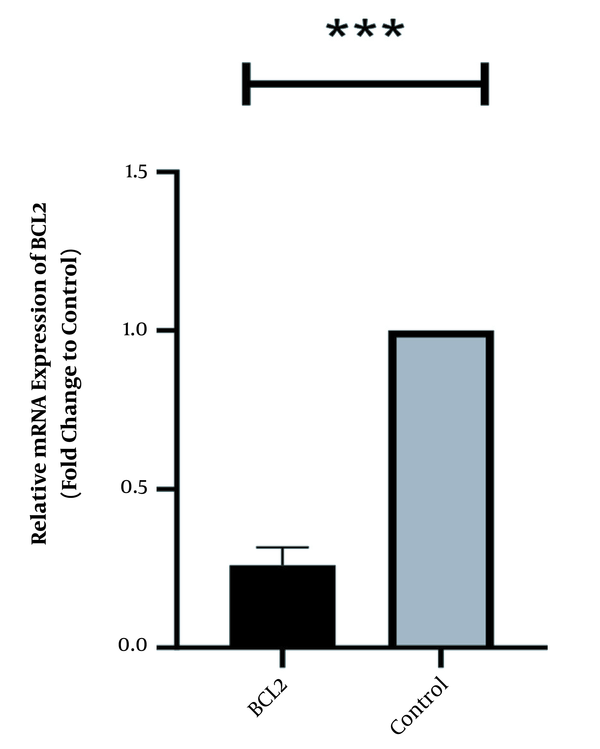
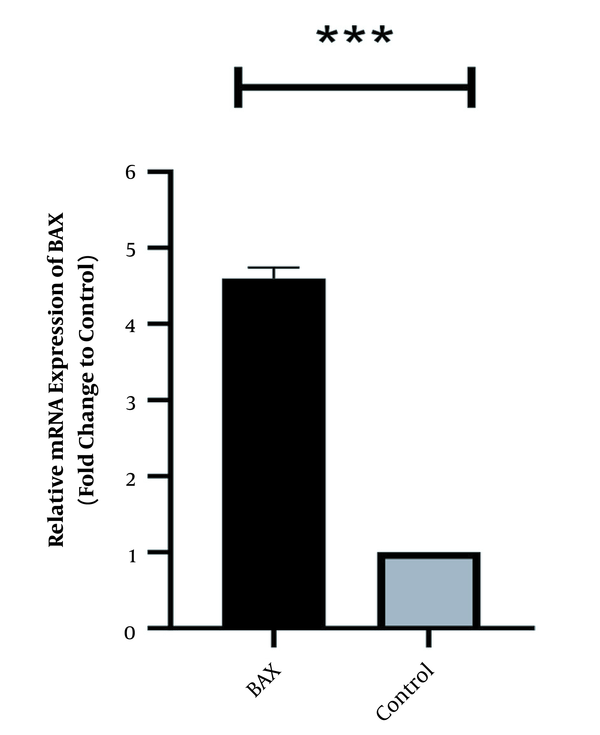
In the same way, the expression level of Bcl2 was high in normal culture of Caco-2 and downregulated after 48 h of treatment with HOTAIR-specific siRNA. The corresponding fold-change was 0.26 ± 0.042 (P-value < 0.001) (Figure 2). Conversely, the expression level of Bax was weak in Caco-2 cells and significantly increased as the cells were treated with HOTAIR-siRNA. The calculated fold-change was 4.6 ± 0.031 (P-value < 0.001) (Figure 3).
HOTAIR specific siRNA effects on suppressing Bcl2 transcript after treatment with 50 ng HOTAIR-siRNA after 48 hours of treatment. Data expressed as mean ± standard deviation (the stars *** show the P-value less than 0.001 compared to the control group).
HOTAIR specific siRNA effects on suppressing Bax transcript after treatment with 50 ng HOTAIR-siRNA after 48 hours of treatment. Data expressed as mean ± standard deviation (*** P < 0.001 compared to control; the stars *** show the P-value less than 0.001 compared to the control group).
5. Discussion
The present study showed the effects of HOTAIR silencing on the induction of apoptosis. Two well-known modulators of apoptosis, Bax, and Bcl2, were traced. Data showed Increased expression of Bax and decreased expression of Bcl2. Previously studies indicated that inhibition of HOTAIR can promotes apoptosis through different signaling pathways. However, the underlying mechanism is not well-understood in CRC. The Bax protein, which is a pro-apoptosis factor, activates apoptosis, while the Bcl2 is an anti-apoptotic protein. The Bax/Bcl2 ratio acts as a controller which decides whether cells turn on apoptosis or not. At the lower levels of ratio, the apoptosis is suppressed (21). Similarly, previous studies showed the high expression of Bcl2 in different cancers, which made them resistant to chemo- or radio-therapy (22). This is the reason that the oncoprotein Bcl2 has been proposed as therapeutic target for cancer through apoptosis induction (23). We also found that Bax had low level of expression, and its expression increased around 4-fold following siRNA treatment. The low expression of this gene was previously reported in colorectal and lung cancers (24, 25). It has been demonstrated that Bax permeabilizes the mitochondrial membrane to release cytochrome c and eventually apoptosis (26). Therefore, agents that activate Bax can be considered as promising anticancer drug. Several drugs have been confirmed or candidate to activate Bax directly or indirectly. Bortezomib, Ixabepilone, and Sorafenib are three examples of Bax activators (26). In conclusion, high level of Bcl2 protein and low level of Bax can inhibit apoptosis in cancer cells, while low amount of Bcl2 and high amount of Bax make cancer cells susceptible to the apoptosis. As we observed here, HOTAIR silencing can act as apoptosis inducer, which is partly due to the activation of Bax and inhibition of Bcl2. It would be ideal to consider the growth inhibitory effect of HOTAIR silencing on mouse models for CRC. Such a study might clarify whether HOTAIR silencing can provide a promising strategy to cure cancers. It is necessary to remind that apoptosis is only one among hundreds of molecular pathways under the control of HOTAIR. This lengthy noncoding transcript regulates variety of cellular pathways, including cell cycle, differentiation, and growth (27). Therefore, HOTAIR silencing may inhibit the cancer progression through different pathways, which can be considered in future studies.
Acknowledgements
References
-
1.
Vinson KE, George DC, Fender AW, Bertrand FE, Sigounas G. The Notch pathway in colorectal cancer. Int J Cancer. 2016;138(8):1835-42. [PubMed ID: 26264352]. https://doi.org/10.1002/ijc.29800.
-
2.
Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016;68(1):7-11. [PubMed ID: 27067591]. https://doi.org/10.1007/s13304-016-0359-y.
-
3.
Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F; Global Burden of Disease Cancer Collaboration, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5(12):1749-68. [PubMed ID: 31560378]. [PubMed Central ID: PMC6777271]. https://doi.org/10.1001/jamaoncol.2019.2996.
-
4.
GBD Colorectal Cancer Collaborators. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4(12):913-33. [PubMed ID: 31648977]. [PubMed Central ID: PMC7026697]. https://doi.org/10.1016/S2468-1253(19)30345-0.
-
5.
Salehiniya H, Pouyesh V, Tarazoj AA, Mohammadian-Hafshejani A, Aghajani M, Yousefi SM, et al. Colorectal cancer in the world: incidence, mortality and risk factors. Biomed Res Ther. 2017;4(10):1656. https://doi.org/10.15419/bmrat.v4i10.372.
-
6.
Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89-103. [PubMed ID: 31616522]. [PubMed Central ID: PMC6791134]. https://doi.org/10.5114/pg.2018.81072.
-
7.
Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24(6):1207-22. [PubMed ID: 23563998]. [PubMed Central ID: PMC4161278]. https://doi.org/10.1007/s10552-013-0201-5.
-
8.
Lee K. Measuring factors affecting colorectal cancer screening behavior and preference for colorectal cancer screening tests using the health belief model and a conjoint analysis [master's thesis]. Goyang, South Korea: National Cancer Center Graduate School of Cancer Science and Policy; 2019.
-
9.
Zoratto F, Rossi L, Verrico M, Papa A, Basso E, Zullo A, et al. Focus on genetic and epigenetic events of colorectal cancer pathogenesis: Implications for molecular diagnosis. Tumour Biol. 2014;35(7):6195-206. [PubMed ID: 25051912]. https://doi.org/10.1007/s13277-014-1845-9.
-
10.
Okugawa Y, Grady WM, Goel A. Epigenetic Alterations in Colorectal Cancer: Emerging Biomarkers. Gastroenterology. 2015;149(5):1204-1225 e12. [PubMed ID: 26216839]. [PubMed Central ID: PMC4589488]. https://doi.org/10.1053/j.gastro.2015.07.011.
-
11.
Hauptman N, Glavac D. Long non-coding RNA in cancer. Int J Mol Sci. 2013;14(3):4655-69. [PubMed ID: 23443164]. [PubMed Central ID: PMC3634483]. https://doi.org/10.3390/ijms14034655.
-
12.
Zhang H, Chen Z, Wang X, Huang Z, He Z, Chen Y. Long non-coding RNA: A new player in cancer. J Hematol Oncol. 2013;6:37. [PubMed ID: 23725405]. [PubMed Central ID: PMC3693878]. https://doi.org/10.1186/1756-8722-6-37.
-
13.
Zhai N, Xia Y, Yin R, Liu J, Gao F. A negative regulation loop of long noncoding RNA HOTAIR and p53 in non-small-cell lung cancer. Onco Targets Ther. 2016;9:5713-20. [PubMed ID: 27695348]. [PubMed Central ID: PMC5033503]. https://doi.org/10.2147/OTT.S110219.
-
14.
Angelopoulou E, Paudel YN, Piperi C. Critical role of HOX transcript antisense intergenic RNA (HOTAIR) in gliomas. J Mol Med (Berl). 2020;98(11):1525-46. [PubMed ID: 32978667]. https://doi.org/10.1007/s00109-020-01984-x.
-
15.
Qu X, Alsager S, Zhuo Y, Shan B. HOX transcript antisense RNA (HOTAIR) in cancer. Cancer Lett. 2019;454:90-7. [PubMed ID: 30981759]. https://doi.org/10.1016/j.canlet.2019.04.016.
-
16.
Wu Y, Zhang L, Zhang L, Wang Y, Li H, Ren X, et al. Long non-coding RNA HOTAIR promotes tumor cell invasion and metastasis by recruiting EZH2 and repressing E-cadherin in oral squamous cell carcinoma. Int J Oncol. 2015;46(6):2586-94. [PubMed ID: 25901533]. https://doi.org/10.3892/ijo.2015.2976.
-
17.
Zhu KY, Palli SR. Mechanisms, Applications, and Challenges of Insect RNA Interference. Annu Rev Entomol. 2020;65:293-311. [PubMed ID: 31610134]. https://doi.org/10.1146/annurev-ento-011019-025224.
-
18.
Chen X, Mangala LS, Rodriguez-Aguayo C, Kong X, Lopez-Berestein G, Sood AK. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018;37(1):107-24. [PubMed ID: 29243000]. [PubMed Central ID: PMC5898634]. https://doi.org/10.1007/s10555-017-9717-6.
-
19.
Abdelrahim M, Safe S, Baker C, Abudayyeh A. RNAi and cancer: Implications and applications. J RNAi Gene Silencing. 2006;2(1):136-45. [PubMed ID: 19771215]. [PubMed Central ID: PMC2737210].
-
20.
Tang L, Zhang W, Su B, Yu B. Long noncoding RNA HOTAIR is associated with motility, invasion, and metastatic potential of metastatic melanoma. Biomed Res Int. 2013;2013:251098. [PubMed ID: 23862139]. [PubMed Central ID: PMC3687722]. https://doi.org/10.1155/2013/251098.
-
21.
Khodapasand E, Jafarzadeh N, Farrokhi F, Kamalidehghan B, Houshmand M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran Biomed J. 2015;19(2):69-75. [PubMed ID: 25864810]. [PubMed Central ID: PMC4412916]. https://doi.org/10.6091/ibj.1366.2015.
-
22.
Ghasemi A, Khanzadeh T, Zadi Heydarabad M, Khorrami A, Jahanban Esfahlan A, Ghavipanjeh S, et al. Evaluation of BAX and BCL-2 Gene Expression and Apoptosis Induction in Acute Lymphoblastic Leukemia Cell Line CCRFCEM after High- Dose Prednisolone Treatment. Asian Pac J Cancer Prev. 2018;19(8):2319-23. [PubMed ID: 30141309]. [PubMed Central ID: PMC6171400]. https://doi.org/10.22034/APJCP.2018.19.8.2319.
-
23.
Campbell KJ, Tait SWG. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018;8(5). [PubMed ID: 29769323]. [PubMed Central ID: PMC5990650]. https://doi.org/10.1098/rsob.180002.
-
24.
Aoyagi T, Morii T, Ohtsuka K, Ohnishi H, Tajima T, Yoshiyama A, et al. Lung cancer cell line sensitivity to Zoledronic acid is BAX-dependent. Anticancer Res. 2013;33(12):5357-63. [PubMed ID: 24324070].
-
25.
Manoochehri M, Karbasi A, Bandehpour M, Kazemi B. Down-regulation of BAX gene during carcinogenesis and acquisition of resistance to 5-FU in colorectal cancer. Pathol Oncol Res. 2014;20(2):301-7. [PubMed ID: 24122668]. https://doi.org/10.1007/s12253-013-9695-0.
-
26.
Liu Z, Ding Y, Ye N, Wild C, Chen H, Zhou J. Direct Activation of Bax Protein for Cancer Therapy. Med Res Rev. 2016;36(2):313-41. [PubMed ID: 26395559]. [PubMed Central ID: PMC4752390]. https://doi.org/10.1002/med.21379.
-
27.
Hajjari M, Rahnama S. Association Between SNPs of Long Non-coding RNA HOTAIR and Risk of Different Cancers. Front Genet. 2019;10:113. [PubMed ID: 30873206]. [PubMed Central ID: PMC6403183]. https://doi.org/10.3389/fgene.2019.00113.
