Abstract
Background:
One of the common treatments for cancer is chemotherapy that is usually done by intravenous injection. Central catheters and portholes are used for the intravenous administration of chemotherapy drugs through peripheral vessels.Objectives:
This study aimed to compare the caring outcomes of the two methods of drug injection through the peripheral vessels and ports in patients undergoing chemotherapy.Methods:
In this descriptive study, 68 cancer patients admitted to the Hematology and Oncology Wards of Shahid Baghaei Hospital 2 in Ahwaz during three months were evaluated in two groups of 34 patients receiving chemotherapy (one group via ports and one group via peripheral blood vessels). Data were collected by a questionnaire consisting of two parts: demographic information (sex, age, and type of disease) and information about the patient's condition (drug injection method, serum, and catheter number), and an observational checklist including inflammation rate, drug extravasation, limitation of patient movement, medical expenses, and patient satisfaction. Data were analyzed using SPSS version 24 software.Results:
According to the results of the study, the two groups were compared in terms of inflammation (P = 0.0001, T = 4.908), drug extravasation (P = 0.0001, T = 3.872), movement limitation P = 0.000, T = 4.922) the cost of treatment (P = 0.0001, χ2 = 56.973, P = 0.000, and patient satisfaction (P = 0.0001, T = -23.66).Conclusions:
Performing chemotherapy through the port has fewer side effects and brings more comfort to the patients.Keywords
1. Background
Cancer is a disease process that results from abnormal or acquired mutations causing abnormal cell behavior. Cancer does not have a single cause (1), but rather a set of different causes are involved with different manifestations, treatments, and prognosis (2, 3). The American Cancer Society estimates the number of new cancer cases and deaths. It projected 1,762,450 new cancer cases and 606,880 cancer deaths in the United States in 2019 (4). Reports released by the National Cancer Institute show that 1,658,370 new cases of cancer were diagnosed, and 589,430 deaths occurred due to cancer in 2015 (3). According to the report, the number of cancer cases was estimated at half a million by 2013, with 90,000 new cases added each year. It is also estimated that the incidence of cancer will double in the next 5 to 15 years (5). Therefore, it is expected that the incidence rate of many cancers will increase in the future and the number of new cancers is expected to increase from 10 million in 2000 to 15 million in 2020, with an estimated 60% of these new cases happening in less developed sectors of the world (6, 7). Researchers agree that cancer treatment is medically necessary and that the treatments used for cancer patients are usually aggressive and severe, which require a large number of resources. Cancer treatment is very expensive so that it not only threatens well-being but also endangers financial security (8, 9).
Various methods such as surgery, radiation therapy, chemotherapy, Hematopoietic Stem Cell Transplantation (HSCT), temperature elevation, and target-based therapies can be used to treat cancer, depending on the stage and medical history of the patients mentioned above (8, 9). Each method can be used alone or in combination with other treatment methods. Most of these methods have specific side effects that vary according to the type of treatment, the duration, and the amount of drug use (10).
Chemotherapy as a cancer treatment has an important role in extending the lifespan of the patients. During the chemotherapy process, cytotoxic drugs are usually used. The main problem of chemotherapy is the side effects of drugs that often stop the treatment (11). Approximately two-thirds of the patients undergo chemotherapy as an important component of a cancer treatment program that can sometimes have many negative effects, such as nausea, vomiting, fatigue, depression, hair loss, and decreased capacity. Cancer patients experience physical and mental problems such as: variety of infections, oral ulcers, anemia, and sleep problems (12). This leads to increased hospitalization costs, reduced performance, and decreased quality of life (13, 14). Today, there are various methods of chemotherapy (15). Chemotherapy can be used in conjunction with surgery, radiation therapy, or both (2). The goals of chemotherapy include definitive treatment, control, and relief, which must be realistic because these goals will determine the drugs used and the severity of the treatment plan. Chemotherapy agents may be administered in hospitals, outpatient centers, or even at home. Chemotherapy drugs are classified into three categories of non-blistering, irritant, and blistering based on the possible risk of tissue damage if the drug is inadvertently withdrawn (16), It includes mild to severe damage to various parts of the body such as tendons, muscles, nerves and blood vessels. (16).
Using intravenous devices is nowadays one of the most common aggressive ways in health care aiming at prescribing intravenous fluids, medications, blood products, nutritional fluids, and hemodynamic evaluation of critically ill patients (4, 5). Chemotherapy drugs are injected through peripheral vessels, implanted intravenous access devices, or central catheters (1).
The most common method of chemotherapy is peripheral vascular injection. Often, a suitable vein is selected at the patient's hand or leg. While this method is easy and fast, it does not require many facilities and is considered to be the best treatment for many cancer patients. However, its use causes numerous complications, the most common of which is phlebitis (17, 18). The rate of infection in peripheral arteries with catheters has been reported from 2.3% to 67% (7). This complicates the patient with symptoms such as pain, warmth, redness, swelling, and stiffness, making it impossible for the patient to continue infusion through the vein (8). Researchers have found that the highest incidence of phlebitis occurs within 48 hours after catheter placement. Therefore, intravenous catheters should be replaced during the first 48 hours (19). Changing the injection site is stressful not only for the patient but also for the nurse who is primarily responsible for care. In addition, it saves a lot of money and time. (11).
One of the implanted access devices is the implant port (20). Unlike the peripheral veins, these veins (central veins) are not visible and palpable under the skin and are much larger in diameter. There are such veins throughout the body, but they are most common in the subclavian region. The port has a metal or plastic housing that is inserted into the chest. The size of the containers available in the market varies depending on the size and age of the patient. The top of the chamber is covered by a soft silicone membrane centered at the needle port (Nidel Haber). The complex eventually attaches to a polyurethane catheter, the other end of which is inserted into the central vein (21). Like any other invasive device, the port may also have problems such as catheter path obstruction, thrombosis, torsion of the reservoir, infectious problems, and the possibility of the catheter being separated (20). Numerous studies have been carried out in this regard, including the Tabari et al. study, which showed that of 34 patients with port, four patients had a transient obstruction, and one patient had catheter obstruction. Infection and inflammation of the skin of the reservoir were ported out of the port due to inadequate response to treatment and antibiotic therapy. In other words, 20.58% of the patients had trouble using the port (22). In another study, Ting Yua Wang et al. reported a port infection rate in patients with hematologic malignancy ranging from 7% to 19% (23). Research results indicate no difference in cost between the two methods of port and peripheral catheter but it is valuable in terms of survival and stress reduction (24). Despite the reported results, several studies have reported port complications, including persistent pain, pneumothorax, intravenous thrombosis, port infection, dislocation, obstruction, pinch-off syndrome, and catheter leakage (11).
Since nurses in all hospital departments, clinics, and even homes have to undergo intravenous injection and angiocut attachment, and in all hospital shifts, angiocut care is a component of the nurse's agenda, intravenous treatment is one of the most common procedures performed in hospitals around the world (9). Studies have shown contradictory results regarding the availability of the vein, costs, aggressive methods, length of stay, and patient and nurse satisfaction, which can affect patient care outcomes.22.14.18
The important of challenge is the limited use of ports compared to peripheral catheters despite 30 years of port (catheter complications), limited studies of the efficacy of venous ports, and the task of educating and guiding the patient in obtaining the best therapeutic-care approach as well as given the specific role. To provide appropriate strategies and guidance for patients.
2. Objectives
This study aimed to compare the health care outcomes of using two catheter and port catheter in patients undergoing chemotherapy in health centers undergoing treatment at Ahvaz Jundishapur University. The results of this study can be a documented guideline for suggesting the use of an appropriate method in chemotherapy.
3. Methods
The present study is a descriptive-analytical study to compare the caring outcomes of two methods of injection through ports and peripheral arteries in patients undergoing chemotherapy in Ahwaz health centers in 2017. The study population included cancer patients undergoing chemotherapy, referring to Shahid Baqaei 2 educational center in Ahvaz. After approval of the Ethics Committee (dated 15/07/96 Code of Ethics: IR.AJUMS.RES.1396.541) and obtaining permission to perform research, the researcher prepared a questionnaire, referred to the hospital, and obtained written consent from the patients. The previous time he completed the questionnaire. The sample included patients who referred to Shahid Baqaei 2 Ahwaz Medical Center for chemotherapy, had completed at least one course of chemotherapy, were currently undergoing chemotherapy, or referred for chemotherapy. Patients who had received antibiotics or intravenous feeding were excluded.
Based on statistical calculations and according to the study by Ge et al. (25), 34 people were determined in each group (Equations 1-4)
A total of 68 cancer patients who had undergone chemotherapy were divided into two equal groups of 34 patients. One group received peripheral vasculature, and the other group received chemotherapy ports. In this study, sampling was done in two ways. A convenience sampling method was used in the port group (due to limited population) and simple random sampling in the venous group.
The inclusion criteria were an age of 25 - 65 years, hospitalization for chemotherapy, and a minimum treatment course of three days.
Exclusion criteria included the use of TPN in the treatment and use of peripheral vessels in the patient with the port from the study.
In this study, patients with peripheral arteries and patients with ports were evaluated daily for three months during hospitalization for chemotherapy. Patients were informed that the results of the study would be completely confidential and used only for research purposes and that the patient identity would remain confidential for both groups under study, care was performed by the researcher., the researcher was on the site to closely monitor patients' problems. Patient satisfaction or dissatisfaction was asked with direct questioning. The location of the catheter was observed daily. The signs and symptoms of phlebitis were checked on a checklist, as follows: Grade 0 (no phlebitis): No pain at the injection site, no redness, swelling, and stiffness of the vein,
Grade1: Erythema or redness in the area of access to the vessel with or without pain
Grade2: Pain in the area of access to the vessel - erythema, edema, or both.
Grade3: Pain in the area where the vessel reaches - edema erythema or both - formation of a palpable venous cord layer (one inch or less).
grade4: Pain in the area of access to the vessel with erythema - Formation of a palpable venous cord layer (more than an inch) - Purulent discharge and drainage. The cost-effectiveness was assessed by comparing costs over a treatment period. The probability of chemotherapy leakage was also assessed in both groups according to the checklist. Also, the motor constraints caused by the presence of an angiocut or a port were questioned. Patients' satisfaction was assessed in both groups. It should be noted that this article is extracted from a student thesis.
The independent t-test was used to compare the quantitative variables between the two groups, and the Mann-Whitney test was used if the distribution was not normal, or the qualitative and rank variables would be analyzed. All analyses were performed with SPSS version 24 software, and the significance level was set at 0.05.
4. Results
The participants were 68 cancer patients with a mean and standard deviation of 41.41 and 12.65 years and a mean and standard deviation of disease history of 2.66 and 1.35 years, respectively. Of them, 36 were female (52.9%), and 32 were male (47.1%). Tables 1 to 5 show the findings of the research hypotheses.
According to the results in Table 1, the T statistical index is at the level of P = 0.0001, which is statistically significant. Also, the lower limit of the confidence interval is 1.039, and its upper limit is 2.456, which does not include zero. These results confirm the first hypothesis of the study. In addition, according to the results in Table 1, the mean inflammation rate in the peripheral vascular drug injection group (1.78) was higher than the mean inflammation rate in the port injection group (0.03).
| Group | Mean ± SD | T | P Value | Lower Limit | Upper Limit |
|---|---|---|---|---|---|
| Peripheral vascular | 1.78 ± 2.07 | 4.908 | 0.0001 | 1.039 | 2.456 |
| Port | 0.03 ± 0.17 |
According to the results of Table 2, the T statistical index is at the level of P = 0.0001, which is statistically significant. Also, the lower limit of the confidence interval is 0.552, and its upper limit is 1.649, which does not include zero. These results support the second hypothesis of the study. In addition, according to the results in Table 2, the mean of drug leakage in the peripheral vascular injection group (5.29) was higher than the mean of drug leakage in the port injection group (4.21).
| Group | Mean ± SD | T | P Value | Lower Limit | Upper Limit |
|---|---|---|---|---|---|
| Peripheral vascular | 5.29 ± 1.55 | 3.872 | 0.0001 | 0.527 | 1.649 |
| Port | 4.21 ± 0.54 |
According to the results in Table 3, the T statistical index is at P = 0.0001, which is statistically significant. Also, the lower limit of the confidence interval is -1.188 and the upper limit is -0.502, which does not include zero. These results confirm the third hypothesis of the study. In addition, according to the results in Table 3, the mean patient satisfaction during mobility and displacement in the drug injection group via ports (4.73) was higher than the mean patient satisfaction during the mobility and displacement in the peripheral vascular drug injection group. (4.21). In measuring the patients' movement restriction, their satisfaction during mobility and mobility was measured, with a high score indicating high patient satisfaction during mobility and low mobility restriction, and vice versa.
| Group | Mean ± SD | T | P Value | Lower Limit | Upper Limit |
|---|---|---|---|---|---|
| Peripheral vascular | 3.88 ± 0.15 | -4.922 | 0.0001 | -1.188 | -0.502 |
| Port | 4.73 ± 0.08 |
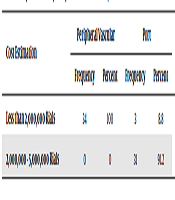
The results in Table 4 indicate that all patients in the peripheral vascular injection group estimated the cost to be less than 2000,000 Rials. In port injection patients, 8.8% of the patients reported a cost of less than 2000,000 Rials and 91.2% of the patients reported a cost between 200000 - 2000000 Tomans. The results of the Chi-square test showed a significant difference between the two groups in terms of cost estimation (P < 0.0001) and thus the two groups were not homogeneous in terms of costs.
| Cost Estimation | Peripheral Vascular | Port | Chi- Square | P Value | ||
|---|---|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | |||
| Less than 2,000,000 Rials | 34 | 100 | 3 | 8.8 | 56.973 | 0.0001 |
| 2,000,000 - 5,000,000 Rials | 0 | 0 | 31 | 91.2 | ||
According to the results in Table 5, the T statistical index is at the level of P = 0.0001, which is statistically significant. Also, the lower limit value of the confidence interval is -4.2273, and its upper limit is -1.534, which does not include zero. These results confirm the fifth hypothesis of the study. In addition, according to the results in Table 5, the mean of patient satisfaction in the port injection group (18.73) was higher than the mean of patient satisfaction in the peripheral vascular injection group (15.82).
| Group | Mean ± SD | T | P Value | Lower Limit | Upper Limit |
|---|---|---|---|---|---|
| Peripheral vascular | 15.82 ± 3.36 | -4.236 | 0.0001 | -4.273 | -1.534 |
| Port | 18.73 ± 2.08 |
5. Discussion
A comparative study was conducted to assess the complication rates of peripheral arteries and ports in patients with non-hematological malignancies. We demonstrated a lower occurrence of overall complications, particularly the late complications, in patients with port devices than in those with peripheral artery lines. The purpose of this study was to compare the health care outcomes of the two methods of injection through ports and peripheral arteries in patients undergoing chemotherapy in Ahvaz health centers in 2017.
Concerning the first aim of the present study, to compare the rate of inflammation in the two groups of peripheral vascular injection and port injection, the results confirmed the first hypothesis. These results showed that inflammation occurred more in the peripheral vascular injection group than in the port injection group. Pate et al. showed that porting is an inexpensive and rapid treatment that, despite low complications, has high therapeutic efficacy (26). It also provides more comfort for the patients and their companions, as shown in the present study, as well. Also, the intravenous length of a peripheral artery line is longer than that of a port device, further increasing the surface area for the propagation of thrombosis. The thrombosis rate in this study may be related to the predominant use of thrombogenic chemotherapy (24).
Regarding the second aim of the study, to compare the rate of drug leakage in the two groups of peripheral injection and port drug injection, the results showed that the rate of drug leakage was higher in the peripheral vascular injection group than in the drug injection group. Catheter-associated thrombosis may occur spontaneously or due to a prothrombotic state associated with underlying malignancy or treatment (27). The association between cancer and thrombosis arises as a consequence of cancer treatment and direct vessel trauma, which is because of long-term central venous catheter placement (24).
Concerning the third aim of the study, to compare patient movement restriction in two groups of peripheral vascular drug injection and port injection, the results showed that the limitation of movement and displacement was more in the peripheral vascular drug injection group than in the port injection group. Ge et al. showed that the satisfaction rate was higher in the port group, which is consistent with the results of the present study.
Regarding the fourth goal of the study, to compare the cost of peripheral vascular and percutaneous injection of drugs in the two groups, it could be concluded that the cost of treatment was more in the drug injection group than in the group injected with peripheral arteries and this can be deterrent to financially impaired patients.
Concerning the fifth goal of the study, to compare the patient satisfaction in the two groups (port and peripheral injection), the results showed that patient's satisfaction was more the port than peripheral injection. In their study, Kim et al. (2012), given the low level of problems, demonstrated the safety of port use in patients receiving chemotherapy, which is consistent with the results of the present study.
In conclusion, the results showed that the rates of inflammation, drug leakage, and movement restriction were higher in patients undergoing chemotherapy through peripheral arteries than in patients in the portal injection group. Also, patients were more satisfied with port injection. However, the costs were estimated to be higher in the port injection group than in the peripheral blood injection group. Therefore, it is suggested that patients be selected through port education.
5.1. Limitations
A major limitation of this study was that the target sample size was not achieved due to slow patient recruitment. This was in great part due to patient preference for the type of CVC device. Physician preference and concerns regarding the logistics of timely port insertion further attenuated patient recruitment. Inadequate sample size may have led to the lack of statistical significance in the time to the first major complication observed.
Acknowledgements
References
-
1.
Vescia S, Baumgartner AK, Jacobs VR, Kiechle-Bahat M, Rody A, Loibl S, et al. Management of venous port systems in oncology: a review of current evidence. Ann Oncol. 2008;19(1):9-15. doi: 10.1093/annonc/mdm272. [PubMed: 17846025].
-
2.
Smeltzer S, Bare B. Brunner & Suddarth's textbook of medical-surgical nursing. 10th ed. Lippincott Williams & Wilkins; 2004.
-
3.
Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer. 2018;124(13):2785-800. doi: 10.1002/cncr.31551. [PubMed: 29786848]. [PubMed Central: PMC6033186].
-
4.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551. [PubMed: 30620402].
-
5.
Farhood B, Geraily G, Alizadeh A. Incidence and mortality of various cancers in Iran and compare to other countries: a review article. Iran J Public Health. 2018;47(3):309.
-
6.
Kanavos P. The rising burden of cancer in the developing world. Ann Oncol. 2006;17 Suppl 8:viii15-23. doi: 10.1093/annonc/mdl983. [PubMed: 16801335].
-
7.
Longo CJ, Bereza BG. A comparative analysis of monthly out-of-pocket costs for patients with breast cancer as compared with other common cancers in Ontario, Canada. Curr Oncol. 2011;18(1):e1-8. doi: 10.3747/co.v18i1.681. [PubMed: 21331267]. [PubMed Central: PMC3031360].
-
8.
Pearson ML. Guideline for prevention of intravascular device-related infections. Part i. Intravascular device-related infections: An overview. The hospital infection control practices advisory committee. Am J Infect Control. 1996;24(4):262-77. doi: 10.1016/s0196-6553(96)90058-9. [PubMed: 8870910].
-
9.
Demiralp M, Oflaz F, Komurcu S. Effects of relaxation training on sleep quality and fatigue in patients with breast cancer undergoing adjuvant chemotherapy. J Clin Nurs. 2010;19(7-8):1073-83. doi: 10.1111/j.1365-2702.2009.03037.x. [PubMed: 20492052].
-
10.
Isikhan V, Guner P, Komurcu S, Ozet A, Arpaci F, Ozturk B. The relationship between disease features and quality of life in patients with cancer--I. Cancer Nurs. 2001;24(6):490-5. doi: 10.1097/00002820-200112000-00012. [PubMed: 11762512].
-
11.
Farrow HA, Rand JS, Burgess DM, Coradini M, Vankan DM. Jugular vascular access port implantation for frequent, long-term blood sampling in cats: methodology, assessment, and comparison with jugular catheters. Res Vet Sci. 2013;95(2):681-6. doi: 10.1016/j.rvsc.2013.03.021. [PubMed: 23706903].
-
12.
Guo Z, Tang HY, Li H, Tan SK, Feng KH, Huang YC, et al. The benefits of psychosocial interventions for cancer patients undergoing radiotherapy. Health Qual Life Outcomes. 2013;11:121. doi: 10.1186/1477-7525-11-121. [PubMed: 23866850]. [PubMed Central: PMC3721996].
-
13.
Ghavam-Nasiri M, Heshmati Nabavi F, Anvari K, Habashi Zadeh A, Moradi M, Neghabi G, et al. The effect of individual and group self-care education on quality of life in patients receiving chemotherapy: A randomized clinical trial. Iran J Med Edu. 2012;11(8):874-84.
-
14.
Safaee A, Zeighami B, Tabatabaee HR, Moghimi Dehkordi B. Quality of life and related factors in breast cancer patients under chemotherapy. Iran J Epidemiol. 2008;3(3):61-6.
-
15.
Hatam N, Bastani P, Ahmadloo N, Ahmadkia Daliri AA. Impacts of treatment management methods oncontrol of toxicity and side effects of chemotherapy regimens in breast cancer. J Ilam Uni Med Sci. 2012;20(1):42-50.
-
16.
Perez Fidalgo JA, Garcia Fabregat L, Cervantes A, Margulies A, Vidall C, Roila F, et al. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23 Suppl 7:vii167-73. doi: 10.1093/annonc/mds294. [PubMed: 22997449].
-
17.
Nekuzad N, Torab TA, Mojab F, Alavi-Majd H, Azadeh P, Ehtejab G. Effect of external use of sesame oil in the prevention of chemotherapy-induced phlebitis. Iran J Pharm Res. 2012;11(4):1065.
-
18.
Schmid MW. Risks and complications of peripherally and centrally inserted intravenous catheters. Crit Care Nurs Clin North Am. 2000;12(2):165-74. [PubMed: 11249361].
-
19.
Borzou SR, Zamanparvar AR, Farmani A, Saremi E, Zandiyeh BSM, Salavati M. Assessment of shelf life of intravenous catheters with incidence of superficial phlebitis in hospitalized patients. Avicenna J Nurs Midwifery Care. 2009;17(12):63-73.
-
20.
An H, Ryu CG, Jung EJ, Kang HJ, Paik JH, Yang JH, et al. Insertion of totally implantable central venous access devices by surgeons. Ann Coloproctol. 2015;31(2):63-7. doi: 10.3393/ac.2015.31.2.63. [PubMed: 25960974]. [PubMed Central: PMC4422989].
-
21.
Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA Cancer J Clin. 2008;58(6):323-46. doi: 10.3322/CA.2008.0015. [PubMed: 18971486].
-
22.
Tabari AK, Saeeda M, Rouzrokh M, Mirshemirani A. Applying totally implantable venous access devices (TIVAD) in children: The first iranian experience. Iran J Blood Cancer. 2010;2(3):127-30.
-
23.
Wang TY, Lee KD, Chen PT, Chen MC, Chen YY, Huang CE, et al. Incidence and risk factors for central venous access port-related infection in Chinese cancer patients. J Formos Med Assoc. 2015;114(11):1055-60. doi: 10.1016/j.jfma.2015.06.013. [PubMed: 26253646].
-
24.
Patel GS, Jain K, Kumar R, Strickland AH, Pellegrini L, Slavotinek J, et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer. 2014;22(1):121-8. doi: 10.1007/s00520-013-1941-1. [PubMed: 24005884].
-
25.
Ge F, Cang J, Xue ZG. Safety and effectiveness of central vein catheters indwelling with subcutaneous port in patients undergoing chemotherapy. Zhonghua yi xue za zhi. 2008;88(33):2331-4.
-
26.
Kim D, Ryu D, Jung H, Lee S. Evaluation of complications of totally implantable central venous port system insertion. Exp Ther Med. 2019. doi: 10.3892/etm.2019.7185.
-
27.
Bishop L, Dougherty L, Bodenham A, Mansi J, Crowe P, Kibbler C, et al. Guidelines on the insertion and management of central venous access devices in adults. Int J Lab Hematol. 2007;29(4):261-78. doi: 10.1111/j.1751-553X.2007.00931.x. [PubMed: 17617077].