Abstract
Keywords
1. Context
Aging is associated with the prevalence of a different cardiovascular disease such as coronary artery disease, hypertension, and heart failure (1). Every decade of increase in age affects the totality of the cardiovascular system even in the absence of pathological factors (2-5). Aging influences the function of cardiomyocytes, and has a direct effect on the calcium homeostasis, contraction of the heart muscle, contraction excitation pair, and cellular integrity of the organelles of cardiomyocytes (6, 7). These factors have a considerable effect on neurohumoral regulation of the function of cardiomyocytes from the adrenergic system as well as the renin-angiotensin system (8, 9). In addition, middle age and old age cause alterations in the components and quality of extracellular matrices, which affect both the structure of cardiac cells and cardiac function (10-12). On the other hand, increasing age is associated with variations in many proteins, which affect the cellular structure and function of the heart. One of these proteins is Klotho.
2. Evidence Acquisition
A total of 66 papers published in PubMed and SID between 1997 and 2018 were selected. The search was performed using the keywords of exercise training, Klotho, oxidative stress, and aging. The exclusion criteria included duplicate titles and articles unrelated to Klotho and aging. At first, 150 articles were found and eventually, 66 articles were selected.
3. Klotho
Klotho (α-Klotho) has found a great deal of attention because of being involved in different biological processes, many of which are associated with human longevity (13). Although the molecular mechanism of action of Klotho is not well known, recent studies suggest high pleiotropy (when a gene affects two or several irrelevant phenotypical traits) of this protein with different biological functions and downstream targets (13). The function of Klotho involves regulating energy metabolism, anti-inflammation, anti-oxidative stress, ion transfer regulation, and mineral metabolism regulation (14). Many of these effects are associated with maintaining the health of blood vessels, and the changes in Klotho levels are also associated with cardiovascular diseases (13, 15).
In the body of living creatures, Klotho protein is present as a form of membrane transporter, involved in transferring the signals of fibroblast growth phosphatonin 23 (FGF23) factor (16). It has also been detected as a water-soluble endocrine factor in the blood and cerebrospinal fluid (16). Its soluble form (∼130 kDa) is dominant in the human body, which declines with aging (17) and can develop in different ways including splicing of substitute RNA (18). In this process, the cleavage of α-Klotho occurs in a process called α-cut by α-secretases A disintegrin, metalloproteinase domain-containing proteins 10 and 17 (ADAM10 and ADAM17, respectively) and β-secretase β-APP cleaving enzyme 1 (BACE1) (19). The cleaved product is a soluble α-Klotho protein (∼130 kDa) that lacks both transmembrane and intracellular domains. The remaining fragment, which remains embedded in the cell membrane, is cleaved by γ-secretase (19, 20). Another mechanism called β-cut, promoted by insulin stimulation, cleaves α-Klotho between the KL1 and KL2 domains and generates two fragments (∼65 kDa each) (19).
Nevertheless, in other studies, the chromosomal position of Klotho in mice has been detected as 13q12, which includes a weight of 50 kDa and 5 exons (21). Similarly, 12q12 position (22) in rats and 13a12 in humans have been detected for its gene (23). According to available findings, the membrane type of Klotho has a double internal replication K1 and K2, which can be released and changed into the soluble form in response to cleavage by α/β-secretases (23). Meanwhile, there is also another type of Klotho called secretory Klotho, whose function has still remained unknown (23). Notably, in some references, soluble Klotho is also called secretory Klotho (22).
Deficiency of Klotho protein was first known to be associated with cardiovascular diseases, based on research on mice with low levels of Klotho (24). It is associated with human symptoms including increased atherosclerosis, extensive internal calcification of the aorta, and both internal calcification and thickening of the vessel walls (24, 25). The majority of research on Klotho has focused on its role as a renal cofactor for FGF23 binding (26). In the kidney, Klotho causes phosphaturic effect of FGF23 and inhibition of the active form of vitamin D (26, 27). Nevertheless, the presence of soluble Klotho and its effects are significant on different tissues of the body including the heart (14, 28).
3.1. The Effect of Aging on Klotho
Phosphatopathies can generally be observed in patients with kidney diseases (29). Kidney diseases are associated with a progressive reduction in kidney function in older adults (30). Over 26 American people (13% of the entire population of the US) have kidney diseases (31). Patients with kidney diseases face diminished Klotho levels (32). Thus, one of the main reasons for reduced Klotho in adults can be the kidney dysfunction because a major part of Klotho is produced in the kidneys (29, 33). Nevertheless, recent studies have shown that blood vessels can also produce Klotho (34). It appears that tunica media produces Klotho, as shown in immunochemical studies of the tunica media in healthy individuals and aorta of rats, but there are some disagreements in this regard. Scialla et al. reported absolutely no expression of any type of Klotho in the vessels of humans or rats (34). On the other hand, Lindberg et al. indicated low levels of this protein (35). The discrepancies can be attributed to the region where sample are taken as well as the technical and experimental methods. Overall, what can be inferred from the research is that low levels of Klotho are expressed in the vessels. It has also been found that aging or kidney diseases reduce production of Klotho in the vessels (15). Evidence suggests that there is an inverse relationship between oxidative stress, nutritional status and inactivity in older adults (36). Inadequate intake of antioxidant-containing foods and sedentary life style leads to increased oxidative in older people stress (36, 37). In this regard Mitobe and et al reported oxidative stress decreases Klotho expression in a mouse kidney cell line (38).
3.2. Effect of Gender Difference on Klotho Levels
The effect of gender on Klotho is not clear. Some studies have shown that soluble Klotho levels were higher in women than in men with acromegaly, but estrogen status cannot account for this difference (39). However, it is clear that Klotho is reduced due to aging.
4. The Relationship Between Klotho and Aging-Associated Diseases
4.1. Klotho and Oxidative Stress
Some studies have examined the relationship between oxidative stress and Klotho. These studies have found that when Klotho protein binds to cellular receptors on its surface, it inhibits FOXO phosphorylation and promotes its nuclear transfer (40). Then, FOXO is directly bound to the generators of the SOD2 enzyme, causing its overregulation. In this way, it facilitates removal of reactive oxygen species and helps resistance against oxidative stress (40). Nevertheless, it is not clear how Klotho causes resistance against oxidative stress through inducing SOD2 expression (40). This mechanism appears to be one of the antiaging pathways by which Klotho can contribute to increased longevity, as Klotho is able to increase other antioxidant enzymes including catalase (41). According to some researchers, Klotho can have an anti-aging function in different ways including inhibiting the insulin-IGF1 signaling and increasing resistance against oxidative stress (40, 42). It appears that the ability of Klotho in activating FOXO firstly depends on its ability in inhibiting the insulin/IGF-1/PI3K/Akt signaling although Klotho might activate FOXO through other pathways (40). For example, FOXO can be activated by inhibiting serum- and glucocorticoid-inducible kinase (SGK) and activation of c-Jun NH2-terminal kinase (43, 44).
4.2. Klotho and MAPK
Oxidative stress is able to activate MAPKs through apoptosis signal-regulating kinase 1 (ASK1), thus enhancing the activity of MAPKs (37) ASK1 or kinase-kinase MAPK (MAP3K5) is able to activate MAPKs including p38 in response to factors such as oxidative stress (37). Furthermore, Klotho is able to deactivate ASK1. Possibly, Klotho can reduce ASK1 either directly or through reducing oxidative stress, though this mechanism is hypothetical (45). On the other hand, H2O2 is able to activate ERK1/2 through Raf-Ras pathway as well as the kinase tyrosine pathway including platelet-derived growth factor subunit B and Src as well as protein kinase C delta (PKC-delta) (46). Klotho appears to be effective in ERK1/2 reduction through reducing oxidative stress and inhibiting Raf-Ras pathway. Although Klotho affects PDGF-beta and PKD-delta along with Src, no research is available on this effect.
4.3. Klotho and TRPC6
Recent studies have shown that soluble Klotho protects the heart against driving forces of cardiac hypertrophy (47). A study found that Klotho can protect the heart by inhibiting Transient Receptor Potential Cation Channel Subfamily C Member 6 (TRPC6) (48). The response of the heart to the damage and stress factors often causes the development of heart failure and death through pathological growth and remodeling (49). One of the key steps that regulate development of pathological growth of heart and remodeling is the activity of Calmodulin-dependent serine threonine calcineurin phosphatase protein, which is activated through abnormal calcium signaling (50). The family of TRPC channels are calcium-permeable cation channels which can be present in the membrane of medications (51). TRPC family includes seven members, categorized into two groups based on their function and structure (51). Current evidence suggests that the calcium cascade through the cardiac TRPC signal is crucial in signaling the pathway of calcineurin and hypertrophic growth of the heart (51). Expression of TRPC channels in hypertrophic hearts increases in response to stimulation of different factors, where inhibiting these factors can have a protective role for the heart. Likewise, soluble Klotho has been found to inhibit TRPC6 channels and protect the heart against pathological growth and remodeling (52).
Although the relationship between Klotho and MAPK can also be notable, its mechanism is still unknown. According to previous studies, oxidative stress is one of the factors that stimulates ERK1/2 activity through Ras activation. On the other hand, Klotho is also considered a factor that reduces oxidative stress (53, 54). Thus, Klotho may result in diminished ERK1/2 activity through reducing oxidative stress. Another research has discovered that Klotho is able to deactivate P38 (55). P38 has been defined as a factor that enhances TRPC6 activity (56). Accordingly, Klotho may lead to inhibition of TRPC6 channels through reducing p38 activity.
Other studies have introduced other mechanisms for the effect of Klotho on cardiac hypertrophy through TRPC6. According to these researchers, IGF-1 receptors can cause activation of PI3k and enhanced activity of TRPC6. Nevertheless, Klotho is able to inhibit the activity of IGF-1 receptors of its downstream signals (48).
4.4. Others Mechanisms
Studies suggest that reduced Klotho can stimulate Wnt signaling pathway and cause aging either directly or through inducing reduction of stem cells (57). Klotho reduction inhibits autophagy, where this reduction results in direct acceleration of aging as well as reduction in and aging of stem cells (58). The reduction in Klotho increases IGF-1 signaling and oxidative stress, antidiuretic hormone, and aldosterone (58). All these processes can accelerate the course of aging and its associated diseases in some way (58).
Recent studies have emphasized the protective role of Klotho in the vascular system, for example, maintaining endothelial homeostasis and vascular function (59, 60). Clinical studies have indicated that low Klotho levels are associated with arterial stiffness in patients with kidney disease (61). Furthermore, Klotho can stimulate nitric oxide synthetase and NO production, such that in animal samples with low levels of Klotho, NO level has been associated with a reduction in its production (62). Nevertheless, it should be emphasized that various factors are involved in NO production and one cannot consider Klotho as the only factor or the major factor.
4.5. The Effect of Exercise on Klotho
Studies on the effect of exercise training on Klotho are limited. Nevertheless, the investigations by Matsubara et al. (2014) are notable in this area. They found that 12 weeks of aerobic exercise results in elevation of Klotho and improved vasodilation in postmenopausal women of 50-76 years old (63). Mostafidi et al. (2016) also reported that athletes enjoy higher levels of Klotho compared to non-athlete individuals (64). It has also been discovered that PPAR-γ is one of the main pathways of Klotho production, and its elevation results in the production of Klotho in rats’ kidneys (65). Exercise might be effective in producing Klotho by activating PPAR-γ, though this mechanism is hypothetical. The reason is that despite increased expression of PPAR-γ in skeletal muscles, no clear results have been found regarding its expression in kidneys induced by exercise. Some studies have suggested that elevation of angiotensin-3 results in diminished production of Klotho in the kidneys (65). Therefore, a decline of angiotensin-2 resulting from exercise can be another possible mechanism for Klotho production, which should be taken into account in case of exercise-induced Klotho elevation (65, 66). In this regard, our research indicated that moderate-intensity aerobic exercise for eight weeks leads to enhanced serum Klotho (37). In this research, we indicated that elevation of Klotho in response to exercise results in diminished oxidative stress. This, in turn, led to a significant reduction in P38 and ERK1/2 levels. All of these results led to diminished pathological cardiac hypertrophy and development of physiological hypertrophy in middle-aged Wistar rats (37).
5. Conclusions
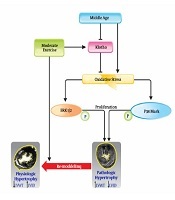
Aging has different effects on the cardiovascular system as well as proteins such as Klotho. Since the role of Klotho on the cardiovascular system has been confirmed, its reduced values in response to aging can lead to increased oxidative stress, enhanced activity of MAPK and TRPC6 signals. These signals lead to cardiac fibrosis and development of pathological hypertrophy. However, doing exercise can prevent it or at least control it. Indeed, exercise can reduce the activity of MAPK and possibly TRPC6 pathways through enhancing Klotho and reducing oxidative stress. This mechanism has been shown in Figure 1.
The mechanism of effect of exercise and middle-age on cardiac hypertrophy (37).

References
-
1.
Tartibian B, Botelho Teixeira AM, Baghaiee B. Moderate intensity exercise is associated with decreased angiotensin-converting enzyme, increased beta2-adrenergic receptor gene expression, and lower blood pressure in middle-aged men. J Aging Phys Act. 2015;23(2):212-20. [PubMed ID: 24809305]. https://doi.org/10.1123/japa.2013-0136.
-
2.
Baghaiee B, Botelho Teixeira AM, Tartibian B. Moderate aerobic exercise increases SOD-2 gene expression and decreases leptin and malondialdehyde in middle-aged men. Sci Sport. 2016;31(3):e55-63. https://doi.org/10.1016/j.scispo.2015.12.003.
-
3.
Czuriga D, Papp Z, Czuriga I, Balogh Á. Cardiac aging – a review. Euro Surg. 2011;43(2):69-77. https://doi.org/10.1007/s10353-011-0600-3.
-
4.
Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74(11):2257-62. [PubMed ID: 20962429]. https://doi.org/10.1253/circj.cj-10-0910.
-
5.
Baghaiee B, Siahkouhian M, Karimi P, Botelho Teixeira AM, Dabagh Nikoo Kheslat S. Weight gain and oxidative stress in midlife lead to pathological concentric cardiac hypertrophy in sedentary rats. J Clin Res Paramed Sci. 2018;7(1). https://doi.org/10.5812/jcrps.79957.
-
6.
Fares E, Howlett SE. Effect of age on cardiac excitation-contraction coupling. Clin Exp Pharmacol Physiol. 2010;37(1):1-7. [PubMed ID: 19671063]. https://doi.org/10.1111/j.1440-1681.2009.05276.x.
-
7.
Rosen BD, Fernandes VR, Nasir K, Helle-Valle T, Jerosch-Herold M, Bluemke DA, et al. Age, increased left ventricular mass, and lower regional myocardial perfusion are related to greater extent of myocardial dyssynchrony in asymptomatic individuals: The multi-ethnic study of atherosclerosis. Circulation. 2009;120(10):859-66. [PubMed ID: 19704101]. [PubMed Central ID: PMC2751872]. https://doi.org/10.1161/CIRCULATIONAHA.108.787408.
-
8.
Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: The role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19(7):213-20. [PubMed ID: 20382344]. [PubMed Central ID: PMC2858758]. https://doi.org/10.1016/j.tcm.2009.12.004.
-
9.
De Meyer GR, De Keulenaer GW, Martinet W. Role of autophagy in heart failure associated with aging. Heart Fail Rev. 2010;15(5):423-30. [PubMed ID: 20383579]. https://doi.org/10.1007/s10741-010-9166-6.
-
10.
Ma Y, Chiao YA, Zhang J, Manicone AM, Jin YF, Lindsey ML. Matrix metalloproteinase-28 deletion amplifies inflammatory and extracellular matrix responses to cardiac aging. Microsc Microanal. 2012;18(1):81-90. [PubMed ID: 22153350]. [PubMed Central ID: PMC3972008]. https://doi.org/10.1017/S1431927611012220.
-
11.
Neilan TG, Coelho-Filho OR, Shah RV, Abbasi SA, Heydari B, Watanabe E, et al. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: Relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging. 2013;6(6):672-83. [PubMed ID: 23643283]. [PubMed Central ID: PMC3683385]. https://doi.org/10.1016/j.jcmg.2012.09.020.
-
12.
Baghaiee B, Siahkouhian M, Karimi P, Botelho Teixeira AM, Dabagh Nikoo Kheslat S, Ebrahimi K. Effect of exercise training and middle-age on pathological and physiological cardiac hypertrophy. J Clin Res Paramed Sci. 2018;7(1). https://doi.org/10.5812/jcrps.79968.
-
13.
Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, et al. Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24(9):3438-50. [PubMed ID: 20466874]. [PubMed Central ID: PMC2923354]. https://doi.org/10.1096/fj.10-154765.
-
14.
Donate-Correa J, Mora-Fernandez C, Martinez-Sanz R, Muros-de-Fuentes M, Perez H, Meneses-Perez B, et al. Expression of FGF23/KLOTHO system in human vascular tissue. Int J Cardiol. 2013;165(1):179-83. [PubMed ID: 21945708]. https://doi.org/10.1016/j.ijcard.2011.08.850.
-
15.
Donate-Correa J, Martin-Nunez E, Mora-Fernandez C, Muros-de-Fuentes M, Perez-Delgado N, Navarro-Gonzalez JF. Klotho in cardiovascular disease: Current and future perspectives. World J Biol Chem. 2015;6(4):351-7. [PubMed ID: 26629318]. [PubMed Central ID: PMC4656911]. https://doi.org/10.4331/wjbc.v6.i4.351.
-
16.
Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242(3):626-30. [PubMed ID: 9464267]. https://doi.org/10.1006/bbrc.1997.8019.
-
17.
Xiao NM, Zhang YM, Zheng Q, Gu J. Klotho is a serum factor related to human aging. Chin Med J (Engl). 2004;117(5):742-7. [PubMed ID: 15161545].
-
18.
Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424(1-2):6-10. [PubMed ID: 9537505]. https://doi.org/10.1016/s0014-5793(98)00127-6.
-
19.
Xu Y, Sun Z. Molecular basis of Klotho: From gene to function in aging. Endocr Rev. 2015;36(2):174-93. [PubMed ID: 25695404]. [PubMed Central ID: PMC4399270]. https://doi.org/10.1210/er.2013-1079.
-
20.
Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, et al. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583(19):3221-4. [PubMed ID: 19737556]. [PubMed Central ID: PMC2757472]. https://doi.org/10.1016/j.febslet.2009.09.009.
-
21.
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45-51. [PubMed ID: 9363890]. https://doi.org/10.1038/36285.
-
22.
Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104(50):19796-801. [PubMed ID: 18056631]. [PubMed Central ID: PMC2148378]. https://doi.org/10.1073/pnas.0709805104.
-
23.
Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, et al. Molecular cloning of rat klotho cDNA: Markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun. 1998;251(3):920-5. [PubMed ID: 9791011]. https://doi.org/10.1006/bbrc.1998.9576.
-
24.
Fukino K, Suzuki T, Saito Y, Shindo T, Amaki T, Kurabayashi M, et al. Regulation of angiogenesis by the aging suppressor gene klotho. Biochem Biophys Res Commun. 2002;293(1):332-7. [PubMed ID: 12054604]. https://doi.org/10.1016/S0006-291X(02)00216-4.
-
25.
Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, et al. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276(2):767-72. [PubMed ID: 11027545]. https://doi.org/10.1006/bbrc.2000.3470.
-
26.
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281(10):6120-3. [PubMed ID: 16436388]. [PubMed Central ID: PMC2637204]. https://doi.org/10.1074/jbc.C500457200.
-
27.
Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, et al. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146(11):4647-56. [PubMed ID: 16081635]. [PubMed Central ID: PMC4140631]. https://doi.org/10.1210/en.2005-0670.
-
28.
Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125(18):2243-55. [PubMed ID: 22492635]. https://doi.org/10.1161/CIRCULATIONAHA.111.053405.
-
29.
Kuro-o M. Klotho and the aging process. Korean J Intern Med. 2011;26(2):113-22. [PubMed ID: 21716585]. [PubMed Central ID: PMC3110841]. https://doi.org/10.3904/kjim.2011.26.2.113.
-
30.
So S, Stevenson J, Lee V. Kidney diseases in the elderly. Advanced age geriatric care. Springer; 2019. p. 131-44. https://doi.org/10.1007/978-3-319-96998-5_16.
-
31.
Zoccali C, Kramer A, Jager KJ. Epidemiology of CKD in Europe: An uncertain scenario. Nephrol Dial Transplant. 2010;25(6):1731-3. [PubMed ID: 20501467]. https://doi.org/10.1093/ndt/gfq250.
-
32.
Lu X, Hu MC. Klotho/FGF23 axis in chronic kidney disease and cardiovascular disease. Kidney Dis (Basel). 2017;3(1):15-23. [PubMed ID: 28785560]. [PubMed Central ID: PMC5527179]. https://doi.org/10.1159/000452880.
-
33.
Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112(17):2627-33. [PubMed ID: 16246962]. https://doi.org/10.1161/CIRCULATIONAHA.105.553198.
-
34.
Scialla JJ, Lau WL, Reilly MP, Isakova T, Yang HY, Crouthamel MH, et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83(6):1159-68. [PubMed ID: 23389416]. [PubMed Central ID: PMC3672330]. https://doi.org/10.1038/ki.2013.3.
-
35.
Lindberg K, Olauson H, Amin R, Ponnusamy A, Goetz R, Taylor RF, et al. Arterial klotho expression and FGF23 effects on vascular calcification and function. PLoS One. 2013;8(4). e60658. [PubMed ID: 23577141]. [PubMed Central ID: PMC3618102]. https://doi.org/10.1371/journal.pone.0060658.
-
36.
Moreira PL, Villas Boas PJ, Ferreira AL. Association between oxidative stress and nutritional status in the elderly. Rev Assoc Med Bras (1992). 2014;60(1):75-83. [PubMed ID: 24918857]. https://doi.org/10.1590/1806-9282.60.01.016.
-
37.
Baghaiee B, Karimi P, Siahkouhian M, Pescatello LS. Moderate aerobic exercise training decreases middle-aged induced pathologic cardiac hypertrophy by improving Klotho expression, MAPK signaling pathway, and oxidative stress status in Wistar rats. Iran J Basic Med Sci. 2018;21(9):911-9. [PubMed ID: 30524691]. [PubMed Central ID: PMC6272071].
-
38.
Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol. 2005;101(2):e67-74. [PubMed ID: 15976510]. https://doi.org/10.1159/000086500.
-
39.
Sze L, Schmid C. Effects of age, sex, and estrogen on serum phosphorus: Role for growth hormone and klotho? Am J Kidney Dis. 2014;64(1):157-8. [PubMed ID: 24954457]. https://doi.org/10.1053/j.ajkd.2014.03.021.
-
40.
Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280(45):38029-34. [PubMed ID: 16186101]. [PubMed Central ID: PMC2515369]. https://doi.org/10.1074/jbc.M509039200.
-
41.
Kimura T, Shiizaki K, Akimoto T, Shinzato T, Shimizu T, Kurosawa A, et al. The impact of preserved Klotho gene expression on antioxidative stress activity in healthy kidney. Am J Physiol Renal Physiol. 2018;315(2):F345-52. [PubMed ID: 29693450]. https://doi.org/10.1152/ajprenal.00486.2017.
-
42.
Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308(5730):1909-11. [PubMed ID: 15879174]. https://doi.org/10.1126/science.1106653.
-
43.
Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol. 2001;21(3):952-65. [PubMed ID: 11154281]. [PubMed Central ID: PMC86685]. https://doi.org/10.1128/MCB.21.3.952-965.2001.
-
44.
Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23(24):4802-12. [PubMed ID: 15538382]. [PubMed Central ID: PMC535088]. https://doi.org/10.1038/sj.emboj.7600476.
-
45.
Hsieh CC, Kuro-o M, Rosenblatt KP, Brobey R, Papaconstantinou J. The ASK1-Signalosome regulates p38 MAPK activity in response to levels of endogenous oxidative stress in the Klotho mouse models of aging. Aging (Albany NY). 2010;2(9):597-611. [PubMed ID: 20844314]. [PubMed Central ID: PMC2984608]. https://doi.org/10.18632/aging.100194.
-
46.
Lee YJ, Cho HN, Soh JW, Jhon GJ, Cho CK, Chung HY, et al. Oxidative stress-induced apoptosis is mediated by ERK1/2 phosphorylation. Exp Cell Res. 2003;291(1):251-66. [PubMed ID: 14597424]. https://doi.org/10.1016/s0014-4827(03)00391-4.
-
47.
Ding HY, Ma HX. Significant roles of anti-aging protein klotho and fibroblast growth factor23 in cardiovascular disease. J Geriatr Cardiol. 2015;12(4):439-47. [PubMed ID: 26347327]. [PubMed Central ID: PMC4554784]. https://doi.org/10.11909/j.issn.1671-5411.2015.04.017.
-
48.
Xie J, Cha SK, An SW, Kuro OM, Birnbaumer L, Huang CL. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun. 2012;3:1238. [PubMed ID: 23212367]. [PubMed Central ID: PMC3526952]. https://doi.org/10.1038/ncomms2240.
-
49.
van Berlo JH, Elrod JW, Aronow BJ, Pu WT, Molkentin JD. Serine 105 phosphorylation of transcription factor GATA4 is necessary for stress-induced cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2011;108(30):12331-6. [PubMed ID: 21746915]. [PubMed Central ID: PMC3145698]. https://doi.org/10.1073/pnas.1104499108.
-
50.
Wang Y, Tandan S, Hill JA. Calcineurin-dependent ion channel regulation in heart. Trends Cardiovasc Med. 2014;24(1):14-22. [PubMed ID: 23809405]. [PubMed Central ID: PMC3830706]. https://doi.org/10.1016/j.tcm.2013.05.004.
-
51.
Eder P, Molkentin JD. TRPC channels as effectors of cardiac hypertrophy. Circ Res. 2011;108(2):265-72. [PubMed ID: 21252153]. https://doi.org/10.1161/CIRCRESAHA.110.225888.
-
52.
Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: A new therapeutic target? Circulation. 2004;109(13):1580-9. [PubMed ID: 15066961]. https://doi.org/10.1161/01.CIR.0000120390.68287.BB.
-
53.
Arany I, Megyesi JK, Nelkin BD, Safirstein RL. STAT3 attenuates EGFR-mediated ERK activation and cell survival during oxidant stress in mouse proximal tubular cells. Kidney Int. 2006;70(4):669-74. [PubMed ID: 16788692]. https://doi.org/10.1038/sj.ki.5001604.
-
54.
Arany I, Faisal A, Nagamine Y, Safirstein RL. p66shc inhibits pro-survival epidermal growth factor receptor/ERK signaling during severe oxidative stress in mouse renal proximal tubule cells. J Biol Chem. 2008;283(10):6110-7. [PubMed ID: 18174162]. https://doi.org/10.1074/jbc.M708799200.
-
55.
Song S, Gao P, Xiao H, Xu Y, Si LY. Klotho suppresses cardiomyocyte apoptosis in mice with stress-induced cardiac injury via downregulation of endoplasmic reticulum stress. PLoS One. 2013;8(12). e82968. [PubMed ID: 24340070]. [PubMed Central ID: PMC3858310]. https://doi.org/10.1371/journal.pone.0082968.
-
56.
Thodeti CK, Paruchuri S, Meszaros JG. A TRP to cardiac fibroblast differentiation. Channels (Austin). 2013;7(3):211-4. [PubMed ID: 23511028]. [PubMed Central ID: PMC3710348]. https://doi.org/10.4161/chan.24328.
-
57.
Ahrens HE, Huettemeister J, Schmidt M, Kaether C, von Maltzahn J. Klotho expression is a prerequisite for proper muscle stem cell function and regeneration of skeletal muscle. Skelet Muscle. 2018;8(1):20. [PubMed ID: 29973273]. [PubMed Central ID: PMC6030782]. https://doi.org/10.1186/s13395-018-0166-x.
-
58.
Bian A, Neyra JA, Zhan M, Hu MC. Klotho, stem cells, and aging. Clin Interv Aging. 2015;10:1233-43. [PubMed ID: 26346243]. [PubMed Central ID: PMC4531025]. https://doi.org/10.2147/CIA.S84978.
-
59.
Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, et al. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci. 2000;57(5):738-46. [PubMed ID: 10892340]. https://doi.org/10.1007/s000180050038.
-
60.
Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248(2):324-9. [PubMed ID: 9675134]. https://doi.org/10.1006/bbrc.1998.8943.
-
61.
Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8(2). e56695. [PubMed ID: 23431388]. [PubMed Central ID: PMC3576368]. https://doi.org/10.1371/journal.pone.0056695.
-
62.
Rakugi H, Matsukawa N, Ishikawa K, Yang J, Imai M, Ikushima M, et al. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31(1):82-7. [PubMed ID: 17709902]. https://doi.org/10.1007/s12020-007-0016-9.
-
63.
Matsubara T, Miyaki A, Akazawa N, Choi Y, Ra SG, Tanahashi K, et al. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am J Physiol Heart Circ Physiol. 2014;306(3):H348-55. [PubMed ID: 24322608]. https://doi.org/10.1152/ajpheart.00429.2013.
-
64.
Mostafidi E, Moeen A, Nasri H, Ghorbani Hagjo A, Ardalan M. Serum klotho levels in trained athletes. Nephrourol Mon. 2016;8(1). e30245. [PubMed ID: 26981496]. [PubMed Central ID: PMC4780197]. https://doi.org/10.5812/numonthly.30245.
-
65.
Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, et al. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111(6):833-41. [PubMed ID: 12639989]. [PubMed Central ID: PMC153766]. https://doi.org/10.1172/JCI16290.
-
66.
Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension. 2002;39(4):838-43. [PubMed ID: 11967236]. https://doi.org/10.1161/01.hyp.0000013734.33441.ea.