Abstract
Background:
Midlife is associated with the development of various cardiovascular diseases such as coronary artery disease, hypertension, and heart failure. The present study aimed to investigate the effects of midlife on cardiac hypertrophy in sedentary rats and its relationship with body weight and oxidative stress.Methods:
In this experimental study, 10 rats aged 11 - 14 months and 10 rats aged 4 months were used. After keeping the rats under normal housing condition for 10 days, they were slaughtered, and the dimensions of the heart, the extent of heart tissue fibrosis, and levels of H2O2 were measured. Student’s t-test and linear regression were used for the statistical analysis.Results:
The findings of this study showed that midlife with low physical activity increases heart weight (P = 0.001), body weight (P = 0.001), and hydrogen peroxide (H2O2) levels (P = 0.001) resulting in a significantly increased ventricular wall thickness (P = 0.001) and ventricular diameter (P = 0.001). The increase in body weight and H2O2 by middle age was significantly associated with an increased ventricular wall thickness (P = 0.001), ventricular diameter (P = 0.001), and heart weight (P = 0.001). Also, there was a significant positive relationship between body weight gain and H2O2 level (P = 0.001).Conclusions:
Midlife with low physical activity is associated with pathological concentric cardiac hypertrophy in rats, and subsequently, with weight gain, increased H2O2, increased heart weight and left ventricular wall thickness, and to some extent, left ventricular internal diameter.Keywords
1. Background
Midlife is associated with the development of various cardiovascular diseases such as coronary artery disease, hypertension, and heart failure (1). Physical inactivity during midlife leads to weight gain in these individuals (2). The weight gain can result in the development of a variety of cardiovascular diseases, thereby increasing the risk of developing cardiovascular disease in adults (3). In this regard, it has been shown that every decade of midlife affects the integrity of the cardiovascular system, even in the absence of pathologic factors. These changes in the cardiovascular physiology due to midlife are different from the pathological changes and rises to its maximum level in old age (4, 5). The existing documents suggest that the aging process significantly affects the structure and function of the cardiovascular system, as aging is associated with molecular changes in the heart muscle. The variations in the function of cardiomyocytes are pivotal factors in the aging-dependent changes since they play an important role in hemodynamics of the cardiovascular system, and aging affects the function of cardiomyocytes at different levels. Midlife has a direct impact on calcium homeostasis, cardiac muscle contraction, paired stimulation of contractile elements, and cellular integrity of cardiomyocyte organelles (6, 7). These factors are essential in the neurohumoral regulation of cardiomyocyte function through adrenergic and renin-angiotensin systems (8-12). Additionally, midlife also causes changes in the components and quality of extracellular matrices, which affect not only the structures of the cardiomyocytes but also the cardiac function (13, 14). One of the most important changes in the heart structure during midlife is the cardiac hypertrophy caused by various underlying factors (15). Obesity, aging, high blood pressure, and oxidative pressure are among the most important (16-18). Also, the heart is capable of responding to environmental conditions, with the ability to grow or shrink. The heart size can increase, which depends on the strength and duration of stimulation, and can be categorized into pathological and physiological hypertrophy. Physiological hypertrophy is characterized by normal or incremental levels of increase in contractile function and the normal organization of the heart structure (19). Pathological hypertrophy is associated with increased cell death, fibrosis, and remodeling, and is characterized by decreased systolic and diastolic function, which often leads to heart failure. The stimuli are responsible for various cellular responses, including gene expression, protein synthesis, accumulation of sarcomeres, and cell metabolism, as well as the development of cardiac hypertrophy (20-22).
On the other hand, cardiac hypertrophy based on heart geometry can be divided into eccentric and concentric. Eccentric hypertrophy develops due to volume overload, and non-pathological eccentric hypertrophy is characterized by an increase in the ventricular volume and wall and septal thickness. Pathological eccentric hypertrophy usually develops due to heart diseases, such as myocardial infarction and dilated cardiomyopathy, leading to dilatation of the ventricles and elongation of cardiomyocytes. Concentric hypertrophy is associated with an increase in the wall and septal thickness and a decrease in the left ventricular dimensions (23-25). Generally, sedentary adults or elderly suffer from pathological hypertrophy. The important issue is the geometric type of hypertrophy that develops during adulthood and the effect of weight gain on it. In the review by Cuspidi et al. (2014), it has been pointed out that eccentric hypertrophy is more prevalent among the obese than concentric hypertrophy (26). Also, research has shown that midlife if accompanied by low mobility, leads to an increase in oxidative stress (27). There are also verified evidence of the effect of oxidative stress in pathological cardiac hypertrophy 16. However, there is still no clear view on how the heart dimensions change due to midlife and the effects of resulting weight gain and changes in oxidative pressure. Therefore, the purpose of this study was to compare the heart dimensions in 4-month-old (young) and 15-month-old (adults) rats, as well as to investigate the statistical correlation between weight gain and oxidative stress with dimensions and weight of the heart.
2. Methods
This experimental study was conducted using young and adult male rats. Twenty rats including 10 young rats (4 months of age) and 10 adult rats (11 - 14 months of age) were procured from one of the research centers in Iran. The rats were kept for 10 days under normal housing conditions with 12:12 h light-dark cycle at about 22°C in polycarbonate boxes with free access to food and water. Then, the rats were slaughtered under anesthetic conditions after measuring the body weight (The GF6100 scale, Japan). The laboratory stages of this research were conducted at the Tabriz University of Medical Sciences.
2.1. Measurement of Left Ventricular Wall Thickness and Internal Diameter
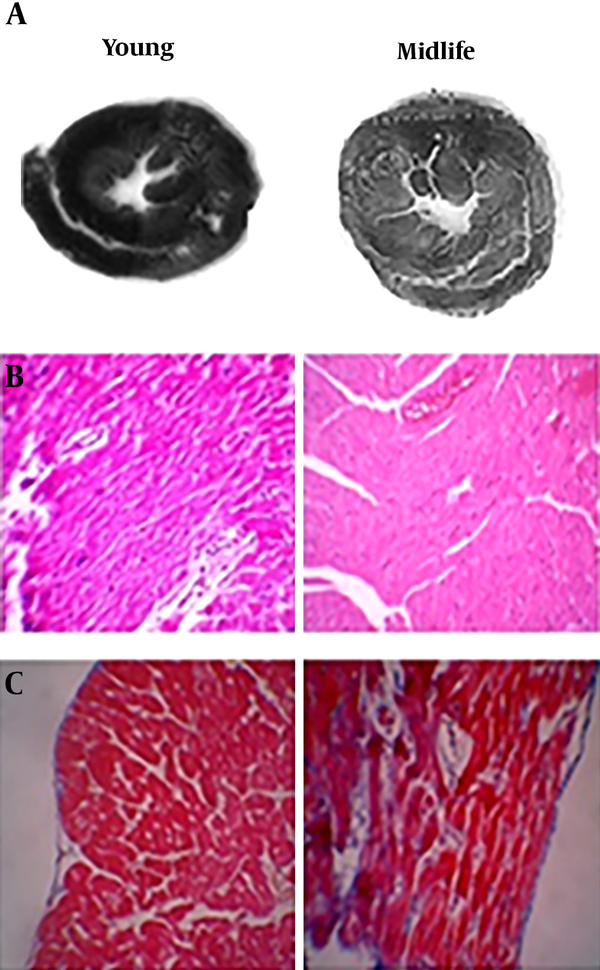
After sacrificing the rats, the heart weight was measured (The GF6100 scale, Japan), and then, the heart was dissected diagonally into two parts. One part was stored immediately at -80°C to measure immunoblotting. The other part was placed in 10% formalin, and then, embedded in paraffin and transected into 5 µm thick sections for hematoxylin and eosin staining and Masson’s trichrome staining. The heart dimensions were also measured using the ImageJ software (USA) and nanometer calibration.
2.2. Measurement of H2O2
The amount of H2O2 in the heart tissue was measured using the specific kit (Sigma-Aldrich, USA).
2.3. Statistical Analysis
The statistical tests used included Kolmogorov-Smirnov test to verify the normality of the distribution of the data, independent t-test to compare the research variables of the two groups, and linear regression to investigate the relationship between the research variables. All statistical analyses were performed using the SPSS software (Chicago, USA) version 23.
3. Results
In this study, we examined some variables in young and middle-aged rats and also looked at the relationship of the variables with each other. Accordingly, the findings of this study showed that midlife causes an increase in heart weight (P = 0.001), body weight gain (P = 0.001), and pathological cardiac hypertrophy; consequently, a significant increase was seen in the ventricular wall thickness (P = 0.001) and ventricular internal diameter (P = 0.001). There was a significant correlation between weight gain due to midlife and increase in ventricular wall thickness (r =0.959, P = 0.001), ventricular internal diameter (r = 0.771. P = 0.001) and heart weight (r = 955, P = 0.001). Also, there was a significant relationship between the amount of H2O2 and ventricular wall thickness (r = 0.886, P = 0.001), ventricular internal diameter (r = 0.761, P = 0.001) and heart weight (r = 0.891, P = 0.001). There was a significant correlation between body weight and H2O2 (r = 0.952, P = 0.001). In the young rats, there was no significant relationship between these variables (Tables 1 - 3 and Figure 1).
Comparison of the Study Variables in Young and Midlife Rats (Using T-Test)
| Variables | Midlife | Young | P Value |
|---|---|---|---|
| Internal diameter, nm | 1482.2 ± 128.47 | 464.5 ± 29.25 | 0.001 |
| Ventricular wall thickness, nm | 7978.8 ± 581.4 | 6455.5 ± 622.25 | 0.001 |
| Heart weight, g | 1.29 ± 0.14 | 0.633 ± 0.1 | 0.001 |
| Body weight, g | 646.4 ± 33.65 | 183.78 ± 14.96 | 0.001 |
| H2O2, intensity of DCF/mgP | 11.2 ± 0.85 | 7.43 ± 0.85 | 0.001 |
Relationship Between the Study Variables (Determined Using Linear Regression)
| Variables/Age | Response | |||||
|---|---|---|---|---|---|---|
| Internal Diameter | Ventricular Wall Thickness | Heart Weight | ||||
| r | P Value | r | P Value | r | P Value | |
| Body weight | ||||||
| Young | 0.609 | 0.1 | 0.02 | 0.94 | 0.309 | 0.456 |
| Midlife | 0.959 | 0.001 | 0.771 | 0.001 | 0.955 | 0.001 |
| H2O2 | ||||||
| Young | 0.275 | 0.51 | 0.156 | 0.71 | 0.368 | 0.39 |
| Midlife | 0.886 | 0.001 | 0.761 | 0.001 | 0.891 | 0.001 |
Relationship Between Body Weight and Hydrogen Peroxide
| Variables/Age | Response | |
|---|---|---|
| H2O2 | ||
| r | P Value | |
| Body weight | ||
| Young | 0.07 | 0.86 |
| Midlife | 0.952 | 0.001 |
The effect of midlife on the dimensions and tissue of the rat heart. A, comparison of heart dimensions in young and midlife; B, hematoxylin-eosin (HE) stained thin cut section of the heart ventricle; C, Mason-Trichrome coloring basis.
4. Discussion
The findings of this study showed that midlife causes weight gain and increased heart weight. The midlife was associated with the development of pathological concentric cardiac hypertrophy. Also, weight gain resulted in an increase in the left ventricular wall thickness, ventricular internal diameter, and heart weight. In line with the findings of this study, Avelar et al. (2007) demonstrated that weight gain leads to cardiac hypertrophy (28). Cuspidi et al. (2014) also showed that midlife causes cardiac hypertrophy (26). It seems that midlife, through various mechanisms, can cause weight gain and cardiac hypertrophy (26). Previous studies have revealed that weight gain is inevitable if aging is associated with physical inactivity (2) because different tissues undergo hypertrophy and fibrosis (29), as well as an increase in the body fat (2). The heart is one of the tissues that is affected significantly by aging and physical inactivity which inevitably result in high oxidative stress (30). Various studies have also shown that aging can lead to increased oxidative stress (30). Our findings also indicated that the adult rats experienced a significant increase in H2O2 levels, and a significant relationship was seen between the elevated oxidative stress and ventricular internal diameter, ventricular wall thickness, and heart weight. Other studies also found that sedentary adult men suffered from an increase in oxidative stress (12, 31). Increasing oxidative stress is due to mitochondrial impairment and damage; thus, physical inactivity can effectively cause these effects (30). Bailey-Downs et al. (2012) reported that aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice (32). Our findings also showed a significant relationship between weight gain and high levels of H2O2. The mitochondrial damage and impairment of bioenergetics caused by aging along with physical inactivity may reduce the antioxidant capacity to cope with reactive oxygen species, and this process can lead to the development of oxidative stress (30). Angiotensin-2 is one of the factors known to increase oxidative stress and cause cardiac hypertrophy, and midlife causes an increase in angiotensin-2 activity (11). The above changes can lead to fibrosis in the cardiac tissue. Imaging of heart tissue in adult rats in this study also showed fibrosis.
In this regard, our findings showed that the sedentary adult rats are confronted with pathological concentric cardiac hypertrophy. Murdolo et al. (2015) in a meta-analysis reported similar results (33). The left ventricular concentric hypertrophy is caused by diseases such as hypertension, which increases the risk of adverse cardiovascular prognosis with a high risk of death from cardiovascular disease (34). Existing evidence suggests that some degree of stress in the ventricular end-diastolic wall acts as a signal regulating hypertrophy. This can lead to more induction of left ventricular concentric hypertrophy in patients with hypertension with a small elevation of ventricular end-diastolic wall pressure, which is characterized by a significant increase in the relative wall thickness. Cardiomyocytes in myocardial concentric remodeling that have excessive thickness are indicative of increased diameters, but there is no significant increase in length. Therefore, the mean length by width (L/W) ratio is significantly reduced (35). This process is due to the orientation of the contractile sarcomere units added to cardiomyocytes (36). It seems that concentric cardiac hypertrophy appearing in adult rats may be associated with impaired blood supply to other tissues and reduce cardiac efficiency as well as cardiac and respiratory endurance.
4.1. Conclusions
To conclude, this study suggests that midlife is associated with pathological concentric cardiac hypertrophy in rats. Concomitant with weight gain, an increase is seen in the heart weight, left ventricular wall thickness, and left ventricular internal diameter. Therefore, midlife accompanied by physical inactivity causes cardiac fibrosis and impaired cardiac function.
Acknowledgements
References
-
1.
North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110(8):1097-108. [PubMed ID: 22499900]. [PubMed Central ID: PMC3366686]. https://doi.org/10.1161/CIRCRESAHA.111.246876.
-
2.
Barnes AS. Obesity and sedentary lifestyles: risk for cardiovascular disease in women. Tex Heart Inst J. 2012;39(2):224-7. [PubMed ID: 22740737]. [PubMed Central ID: PMC3384027].
-
3.
Warren TY, Barry V, Hooker SP, Sui X, Church TS, Blair SN. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc. 2010;42(5):879-85. [PubMed ID: 19996993]. [PubMed Central ID: PMC2857522]. https://doi.org/10.1249/MSS.0b013e3181c3aa7e.
-
4.
Czuriga D, Papp Z, Czuriga I, Balogh Á. Cardiac aging – a review. Eur Surg. 2011;43(2):69-77. https://doi.org/10.1007/s10353-011-0600-3.
-
5.
Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74(11):2257-62. [PubMed ID: 20962429].
-
6.
Fares E, Howlett SE. Effect of age on cardiac excitation-contraction coupling. Clin Exp Pharmacol Physiol. 2010;37(1):1-7. [PubMed ID: 19671063]. https://doi.org/10.1111/j.1440-1681.2009.05276.x.
-
7.
Rosen BD, Fernandes VR, Nasir K, Helle-Valle T, Jerosch-Herold M, Bluemke DA, et al. Age, increased left ventricular mass, and lower regional myocardial perfusion are related to greater extent of myocardial dyssynchrony in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(10):859-66. [PubMed ID: 19704101]. [PubMed Central ID: PMC2751872]. https://doi.org/10.1161/CIRCULATIONAHA.108.787408.
-
8.
Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19(7):213-20. [PubMed ID: 20382344]. [PubMed Central ID: PMC2858758]. https://doi.org/10.1016/j.tcm.2009.12.004.
-
9.
De Meyer GR, De Keulenaer GW, Martinet W. Role of autophagy in heart failure associated with aging. Heart Fail Rev. 2010;15(5):423-30. [PubMed ID: 20383579]. https://doi.org/10.1007/s10741-010-9166-6.
-
10.
Hotta H, Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr Gerontol Int. 2010;10 Suppl 1:S127-36. [PubMed ID: 20590828]. https://doi.org/10.1111/j.1447-0594.2010.00592.x.
-
11.
Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247-57. [PubMed ID: 20597104]. [PubMed Central ID: PMC3377325]. https://doi.org/10.1002/emmm.201000080.
-
12.
Tartibian B, Botelho Teixeira AM, Baghaiee B. Moderate intensity exercise is associated with decreased angiotensin-converting enzyme, increased beta2-adrenergic receptor gene expression, and lower blood pressure in middle-aged men. J Aging Phys Act. 2015;23(2):212-20. [PubMed ID: 24809305]. https://doi.org/10.1123/japa.2013-0136.
-
13.
Ma Y, Chiao YA, Zhang J, Manicone AM, Jin YF, Lindsey ML. Matrix metalloproteinase-28 deletion amplifies inflammatory and extracellular matrix responses to cardiac aging. Microsc Microanal. 2012;18(1):81-90. [PubMed ID: 22153350]. [PubMed Central ID: PMC3972008]. https://doi.org/10.1017/S1431927611012220.
-
14.
Neilan TG, Coelho-Filho OR, Shah RV, Abbasi SA, Heydari B, Watanabe E, et al. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging. 2013;6(6):672-83. [PubMed ID: 23643283]. [PubMed Central ID: PMC3683385]. https://doi.org/10.1016/j.jcmg.2012.09.020.
-
15.
Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8(1):143-64. [PubMed ID: 22108734]. [PubMed Central ID: PMC3223374]. https://doi.org/10.1016/j.hfc.2011.08.011.
-
16.
Maulik SK, Kumar S. Oxidative stress and cardiac hypertrophy: a review. Toxicol Mech Methods. 2012;22(5):359-66. [PubMed ID: 22394344]. https://doi.org/10.3109/15376516.2012.666650.
-
17.
Kavazis AN. Pathological vs. physiological cardiac hypertrophy. J Physiol. 2015;593(17):3767. [PubMed ID: 26331830]. [PubMed Central ID: PMC4575564]. https://doi.org/10.1113/JP271161.
-
18.
Chen WK, Yeh YL, Lin YM, Lin JY, Tzang BS, Lin JA, et al. Cardiac hypertrophy-related pathways in obesity. Chin J Physiol. 2014;57(3):111-20. [PubMed ID: 24826779]. https://doi.org/10.4077/CJP.2014.BAB146.
-
19.
Weeks KL, McMullen JR. The athlete's heart vs. the failing heart: can signaling explain the two distinct outcomes? Physiology (Bethesda). 2011;26(2):97-105. [PubMed ID: 21487028]. https://doi.org/10.1152/physiol.00043.2010.
-
20.
Lyon RC, Zanella F, Omens JH, Sheikh F. Mechanotransduction in cardiac hypertrophy and failure. Circ Res. 2015;116(8):1462-76. [PubMed ID: 25858069]. [PubMed Central ID: PMC4394185]. https://doi.org/10.1161/CIRCRESAHA.116.304937.
-
21.
Francis GS, McDonald KM, Cohn JN. Neurohumoral activation in preclinical heart failure. Remodeling and the potential for intervention. Circulation. 1993;87(5 Suppl):IV90-6. [PubMed ID: 8097970].
-
22.
Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14(1):38-48. [PubMed ID: 23258295]. [PubMed Central ID: PMC4416212]. https://doi.org/10.1038/nrm3495.
-
23.
Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589-600. [PubMed ID: 16936699]. https://doi.org/10.1038/nrm1983.
-
24.
Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128(1):191-227. [PubMed ID: 20438756]. https://doi.org/10.1016/j.pharmthera.2010.04.005.
-
25.
Selby DE, Palmer BM, LeWinter MM, Meyer M. Tachycardia-induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. J Am Coll Cardiol. 2011;58(2):147-54. [PubMed ID: 21718911]. [PubMed Central ID: PMC3147146]. https://doi.org/10.1016/j.jacc.2010.10.069.
-
26.
Cuspidi C, Rescaldani M, Sala C, Grassi G. Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. J Hypertens. 2014;32(1):16-25. [PubMed ID: 24309485]. https://doi.org/10.1097/HJH.0b013e328364fb58.
-
27.
Dorjgochoo T, Gao YT, Chow WH, Shu XO, Yang G, Cai Q, et al. Obesity, age, and oxidative stress in middle-aged and older women. Antioxid Redox Signal. 2011;14(12):2453-60. [PubMed ID: 21043829]. [PubMed Central ID: PMC3096497]. https://doi.org/10.1089/ars.2010.3337.
-
28.
Avelar E, Cloward TV, Walker JM, Farney RJ, Strong M, Pendleton RC, et al. Left ventricular hypertrophy in severe obesity: interactions among blood pressure, nocturnal hypoxemia, and body mass. Hypertension. 2007;49(1):34-9. [PubMed ID: 17130310]. https://doi.org/10.1161/01.HYP.0000251711.92482.14.
-
29.
Biernacka A, Frangogiannis NG. Aging and Cardiac Fibrosis. Aging Dis. 2011;2(2):158-73. [PubMed ID: 21837283]. [PubMed Central ID: PMC3153299].
-
30.
Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012;2012:646354. [PubMed ID: 21977319]. [PubMed Central ID: PMC3184498]. https://doi.org/10.1155/2012/646354.
-
31.
Baghaiee B, Botelho Teixeira AM, Tartibian B. Moderate aerobic exercise increases SOD-2 gene expression and decreases leptin and malondialdehyde in middle-aged men. Sci Sports. 2016;31(3):e55-63. https://doi.org/10.1016/j.scispo.2015.12.003.
-
32.
Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, et al. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68(7):780-92. [PubMed ID: 23213032]. [PubMed Central ID: PMC3674713]. https://doi.org/10.1093/gerona/gls238.
-
33.
Murdolo G, Angeli F, Reboldi G, Di Giacomo L, Aita A, Bartolini C, et al. Left ventricular hypertrophy and obesity: only a matter of fat? High Blood Press Cardiovasc Prev. 2015;22(1):29-41. [PubMed ID: 25117210]. https://doi.org/10.1007/s40292-014-0068-x.
-
34.
Matsui Y, Eguchi K, Shibasaki S, Ishikawa J, Shimada K, Kario K. Morning hypertension assessed by home monitoring is a strong predictor of concentric left ventricular hypertrophy in patients with untreated hypertension. J Clin Hypertens (Greenwich). 2010;12(10):776-83. [PubMed ID: 21029340]. https://doi.org/10.1111/j.1751-7176.2010.00350.x.
-
35.
Sawada K, Kawamura K. Architecture of myocardial cells in human cardiac ventricles with concentric and eccentric hypertrophy as demonstrated by quantitative scanning electron microscopy. Heart Vessels. 1991;6(3):129-42. [PubMed ID: 1833369].
-
36.
Linzbach AJ. Hypertrophy, hyperplasia and structural dilatation of the human heart. Adv Cardiol. 1976;18(0):1-14. [PubMed ID: 136171]. https://doi.org/10.1159/000399507.