Abstract
Objectives:
This study investigated the effects of an eight-week aerobic exercise program and use of garlic extract on serum atrial natriuretic peptide (ANP) and NT-pro brain natriuretic peptide (BNP) levels in obese hypertensive patients.Methods:
A total of 50 male obese hypertensive patients with a mean age of 53 ± 7.6 years were recruited in this study. They were randomly assigned to five groups, including aerobic exercise, garlic extract, aerobic exercise + garlic extract, placebo, and control. The experimental groups were subjected to eight weeks of aerobic exercise (three sessions per week, each session for 35 - 60 min with a maximum heart rate of 50% - 70%) and/or garlic extract supplement (6 g daily). Blood samples were collected before interventions and 48 h after the last exercise session. Statistical analysis of data was performed by dependent t-test and Analysis of Covariance (ANCOVA).Results:
Body weight, body mass index (BMI), systolic blood pressure, diastolic blood pressure, and NT-pro BNP in aerobic exercise, garlic extract, and garlic extract + aerobic exercise reduced significantly compared to control group (P ≤ 0.05). Body weight, BMI, and ANP decreased significantly in aerobic and aerobic + garlic extract groups compared to garlic group (P ≤ 0.001). Moreover, ANP reduced more significantly in aerobic exercise + garlic extract group than in aerobic exercise group (P ≤ 0.05). In addition, NT-pro BNP decreased more significantly in aerobic exercise + garlic extract group than in garlic extract group (P ≤ 0.01).Conclusions:
It seems that both aerobic exercise and garlic extract are able to decrease obesity and hypertension. However, their simultaneous use has no additional effects on hypertension control although it influences the body composition and natriuretic peptides.Keywords
Aerobic Exercise Garlic Extract Hypertension, ANP NT-Pro BNP
1. Background
The prevalence of overweight and obesity has doubled worldwide since 1980 so that approximately one-third of the global population is ranked as obese or overweight (1). Obesity negatively affects almost all physiologic functions of the body and is a significant threat for public health. Obesity increases the risk of numerous diseases such as diabetes (2), cardiovascular diseases (1, 2), different cancers (3), and musculoskeletal disorders (4), all of which have negative effects on the quality of life, efficiency, and healthcare costs. The risk of cardiovascular diseases is higher in overweight people, and physical activity is considered a supplement to obesity treatment regimen and cardiovascular system health (5). Maintaining systolic blood pressure at about 140 mmHg reduces stroke by 28% - 44% and ischemic heart disease by 20% - 35% depending on the patient’s age. Lifestyle adjustment, including exercise, weight loss, and reduced sodium intake play a key role in controlling systolic blood pressure (6). In addition to the blood circulation, the heart acts as an endocrine gland and secrets natriuretic peptides. Natriuretic peptides include atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), the important factors for the diagnosis of cardiac disorders and diseases associated with them (7). ANP is seen as storage granules in mammalian atrial myocardial cells in a 28-amino acid sequence synthesized from a polypeptide. BNP with a 32-amino acid sequence is secreted from the Purkinje cells and heart ventricles (8). Increased ANP secretion is considered an important neutralizing mechanism that excretes sodium and increases vascular expansion by inhibiting the secretion of renin-angiotensin-aldosterone and sympathetic nerve. BNP is released as a result of pressure on the walls as well as ventricular dilation and is disintegrated into active (BNP) and passive (NT-pro BNP) hormones. The plasma half-life of BNP is only 20 min, while that of the NT-pro BNP is about 60 - 120 min. BNP and NT-pro BNP reduce blood pressure by increasing glomerular filtration and sodium reabsorption (9). Studies have shown an association between ANP and BNP and diseases such as congestive heart failure, stage 1 hypertension, myocardial infarction, nephrotic syndrome, ventricular fibrillation, and coronary artery occlusion (10). ANP and BNP are secreted from the heart continually, but the amount of secretion varies in response to different stimuli, and various factors are involved in their synthesis control and secretion. It has been shown that atrial wall stretch is the most important stimulus for the secretion of cardiac hormones (11). Further, endothelin stimulates ANP secretion, and inhibition of endothelin receptor reduces its response to hypoxic conditions (12). Cardiac hormones play a pivotal role in the regulation of blood pressure and volume (13). Yet, their concentration is variable in different physiologic conditions. Sport activity, a non-pharmaceutic intervention for prevention and even improvement of cardiovascular diseases such as blood pressure (5), is able to change the levels of natriuretic peptides. Pirooz et al. (14) reported ANP reduction and lack of NT-pro BNP change following eight weeks of endurance exercise in the healthy young participants, while endurance and combined endurance and resistance activities did not change the levels of these peptides. Moreover, Bordbar et al. (15) indicated NT-pro BNP level was unchanged after eight weeks of endurance exercise in the healthy adults, but it was followed by a significant increase after endurance exercise. In addition, Beltran Valls et al. (16) reported an unchanged NT-pro BNP level after a 12-week endurance exercise program in the elderly. Furthermore, Berent et al. (17) found NT-pro BNP level reduced after four weeks of aerobic exercise in the cardiac patients. Although a number of studies have investigated the chronic effects of sports activities on natriuretic peptides, the changes of these peptides have not been explored owing to adaptability to aerobic exercise in blood pressure conditions.
Garlic is a plant that has been widely used for the treatment of illnesses associated with aging. It has potent antioxidant properties (18), reduces blood glucose, blood lipid, blood pressure, and blood concentration, and prevents atherosclerosis and cardiovascular diseases (19). Garlic affects the vessels and increases blood flow to arteries and capillaries (20). The beneficial effects of sports activities and consumption of garlic supplement on cardiovascular health seem to be associated with cardiac hormone changes.
2. Objectives
Therefore, the present study was aimed to investigate the effect of an eight-week aerobic exercise program and use of garlic extract on serum ANP and BNP levels in obese hypertensive patients.
3. Methods
In this quasi-experimental study, 50 male obese hypertensive patients, aged 53 ± 7.6 years, were recruited voluntarily from region three of Tehran, Iran. The participants had no history of tobacco and alcohol consumption as well as other diseases. Obesity was diagnosed by body mass index (BMI) formula and BMI ≥ 30 was considered obesity.
3.1. Sample and Sampling Method
In this quasi-experimental study, 50 male obese hypertensive patients, aged 53 ± 7.6 years, were recruited. The participants had no history of tobacco and alcohol consumption as well as other diseases. Obesity was diagnosed by body mass index (BMI) formula and BMI ≥ 30 was considered obesity. The blood pressure of the participants was measured by OMRON M2 digital barometer with accuracy of 0.1 mmHg. Systolic blood pressure > 140 mmHg and diastolic blood pressure ≥ 90 mmHg in at least two separate measurements in two different days were considered hypertension (21). Then, the participants were randomly divided into five groups, including aerobic exercise, garlic extract, aerobic exercise + garlic extract, placebo, and control. The experimental groups were subjected to eight weeks of exercise and/or garlic extract supplement.
3.2. Intervention
The garlic supplement group received 1 garlic capsule (Nature’s Bounty, U.S.) daily (each capsule containing 1000 mg garlic extract). The placebo group received 1 dextrose capsule (1000 mg) (22). The aerobic exercise protocol included three sessions of exercise per week, each session for 35 - 60 min. Each session included 10 min warm-up (jogging and stretching), 20 - 45 min treadmill running, and 5 min cool-down at the end of the exercise. In the first week, exercise started with a maximum heart rate of 50% - 55%, including 20 min treadmill running. In the second week, exercise started with a maximum heart rate of 55% - 60% for 30 min. In the third week, exercise started with a maximum heart rate of 60% - 70% for 40 min and continued to week six. From week six on, exercise initiated with a maximum heart rate of 70% - 75% for 45 min and continued to week eight. The maximum heart rate was computed by the 220-age equation for each participant. The intensity of exercise was controlled via heart rate at specific intervals (every five seconds) by a heart rate monitor (Polar, Finland) (14). At the beginning of the study, 5 cc blood was taken from the patients’ brachial vein following 12 fasting hours and kept in the test tubes containing EDTA. The blood samples were immediately transferred to the laboratory for the following measurements. In the laboratory, all samples were centrifuged by a centrifuge machine (MSE, U.K) at 3500 rpm for 15 min at 4°C. Serum was poured in 0.5 mL Eppendorf microtubes by a sampler and kept at -80°C. Then, 48 hours after the last exercise session, the posttest blood samples were taken in the same conditions as those of the pretest samples. ANP concentration was measured by an ELISA kit (ABCAM) with catalog number ab245705 and 26/2 pg/mL sensitivity and NT-pro BNP concentration was measured by an ELISA kit (ABSAM) with catalog number ab193712 and 0.14 ng/mL sensitivity according to the manufacturer’s instructions.
3.3. Data Analysis
SPSS (version 16) software was used for the analysis of data. Normal distribution of data was examined by Kolmogorov-Smirnov test and homogeneity of variances was studied by Levin’s test. Intergroup comparison was performed by dependent t-test and between-group comparison was done by one-way ANOVA and ANCOVA. In the case of significance, the Bonferroni follow-up test was applied. P ≤ 0.05 was considered significant.
3.4. Ethical Considerations
This study is taken from a research project approved by Kermanshah University of Medical Sciences (ethical code: IR.KUMS.REC.1398.656) and registered in the Iranian Registry of Clinical Trials (no.: IRCT20190918044813N1). All participants were informed of the study procedures and included in the study after completing informed consent forms. It is noteworthy that the required medical care was taken into account during blood sampling and exercise.
4. Results
Data were presented as mean ± SEM (standard error of the mean). The results of the pretest showed no significant difference in body weight, blood pressure, ANP, and BNP among the study groups (P > 0.05). However, to eliminate the effect of the covariate variable (pretest scores), ANCOVA test was used for between-group comparisons in the posttest.
The results of the posttest indicated a significant reduction in the mean body weight in the aerobic exercise group (t = 12.329, P ≤ 0.001), garlic extract group (t = 2.792, P ≤ 0.05), and aerobic exercise + garlic extract group (t = 10.350, P ≤ 0.001). The findings of ANCOVA test showed a significant difference among the study groups in the weight variable (F = 48.120, P ≤ 0.001) so that a significant decrease was observed in the body weight between the control group and aerobic exercise (P ≤ 0.001), garlic extract (P ≤ 0.05), and aerobic exercise + garlic extract (P ≤ 0.001) groups. Moreover, the aerobic exercise and aerobic exercise + garlic extract groups showed a significantly higher weight loss than the garlic extract group (P ≤ 0.001) (Table 1).
| Groups | Weight | BMI | Systolic Blood Pressure | Diastolic Blood Pressure |
|---|---|---|---|---|
| Aerobic exercise | ||||
| Pre test | 5.5 ± 106.9 | 0.9 ± 33.4 | 1.6 ± 145.3 | 1.9 ± 93.1 |
| Post test | 5.1 ± 98.0 | 0.8 ± 30.5 | 1.8 ± 134.9 | 1.2 ± 88.6 |
| Garlic extract | ||||
| Pre test | 5.3 ± 105.3 | 1.1 ± 33.1 | 1.5 ± 143.5 | 5.5 ± 93.7 |
| Post test | 5.5 ± 103.2 | 1.2 ± 32.3 | 1.7 ± 135.8 | 1.4 ± 88.9 |
| Aerobic exercise + garlic extract | ||||
| Pre test | 5.9 ± 105.7 | 1.2 ± 32.7 | 1.4 ± 147.1 | 1.7 ± 94.6 |
| Post test | 5.1 ± 95.1 | 1 ± 29.6 | 1.8 ± 133.5 | 1.0 ± 86.2 |
| Placebo | ||||
| Pre test | 5.7 ± 111.2 | 1.2 ± 33.9 | 1.7 ± 146.6 | 1.4. ± 91.5 |
| Post test | 5.8 ± 110.9 | 1.3 ± 33.8 | 1.8 ± 147.9 | 1.4 ± 92.3 |
| Control | ||||
| Pre test | 6.5 ± 106.1 | 1.2 ± 33.4 | 1.5 ± 147.6 | 1.8 ± 94.3 |
| Post test | 6.4 ± 107.6 | 1.3 ± 33.8 | 1.7 ± 148.2 | 1.5 ± 93.0 |
The results of the posttest showed mean BMI significantly reduced in the aerobic exercise (t = 12.429, P ≤ 0.001), garlic extract (t = 3.207, P ≤ 0.05), and aerobic exercise + garlic extract (t = 9.858, P ≤ 0.0001) groups. Further, the results of ANCOVA showed a significant difference in BMI among study groups (F = 34.632, P ≤ 0.001) so that a significant reduction in was found BMI between the control group and aerobic exercise (P ≤ 0.001), garlic extract (P ≤ 0.05), and aerobic exercise + garlic extract (P ≤ 0.001) groups. Moreover, the aerobic exercise and aerobic exercise + garlic extract groups indicated a significantly higher BMI decrease than garlic extract group (P ≤ 0.001) (Table 1).
Furthermore, the posttest showed mean systolic and diastolic blood pressure reduced significantly in the aerobic exercise (t = 7.992, P ≤ 0.001 and t = 7.997, P ≤ 0.001, respectively), garlic extract (t = 5.164, P ≤ 0.001 and t = 10.286, P ≤ 0.001, respectively), and aerobic exercise + garlic extract (t = 6.013, P ≤ 0.001 and t = 5.273, P ≤ 0.001, respectively) groups. The results of the ANCOVA test showed a significant difference among study groups in systolic blood pressure and diastolic blood pressure (F = 22.393, P ≤ 0.001 and F = 14.329, P ≤ 0.001). The systolic and diastolic blood pressure significantly reduced in the aerobic exercise (P ≤ 0.001 and P ≤ 0.05, respectively), garlic extract (P ≤ 0.001 and P ≤ 0.05, respectively), and aerobic exercise + garlic extract (P ≤ 0.001 and P ≤ 0.001, respectively) groups compared to control group (Table 1).
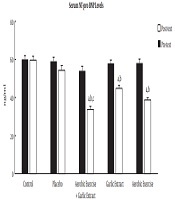
The posttest showed serum ANP and BNP levels significantly reduced in the aerobic exercise (t = 11.979, P ≤ 0.001 and t = 7.389, P ≤ 0.001, respectively), aerobic exercise + garlic extract (t = 13.728, P ≤ 0.001 and t = 7.069, P ≤ 0.001, respectively) groups, but only BNP level decreased significantly in the garlic extract group (t = 7.287, P ≤ 0.001). The findings of the ANCOVA test indicated a significant difference among study groups in ANP and BNP levels (F = 31.247, P ≤ 0.001 and F = 73.153, P ≤ 0.001). A significant decrease was found in ANP and BNP levels between the control group and aerobic exercise (P ≤ 0.001) and aerobic exercise + garlic extract (P ≤ 0.001) groups, but this significant decrease was observed only in BNP in the garlic extract group (P ≤ 0.05). In addition, there was a significantly higher ANP reduction in the aerobic exercise and aerobic exercise + garlic extract groups than in garlic extract group (P ≤ 0.001). Furthermore, the aerobic exercise + garlic extract group underwent a significantly higher ANP reduction than aerobic exercise group (P ≤ 0.05). As for BNP, the aerobic exercise + garlic extract group experienced a significantly higher BNP decrease than garlic extract group (P ≤ 0.01) (Figures 1 and 2).
Serum ANP levels in study groups. A, significant difference with pretest (P ≤ 0.001); B, significant difference with the control group (P ≤ 0.001); C, significant difference with garlic extract (P ≤ 0.01); D, significant difference with aerobic exercise (P ≤ 0.05).
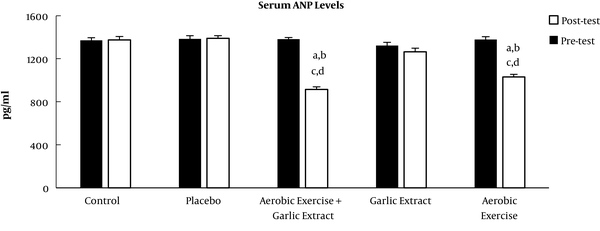
Serum NT-pro BNP levels in study groups, A, significant difference with pretest (P ≤ 0.001); B, significant difference with control group (P ≤ 0.05); C, significant difference with garlic extract (P ≤ 0.01).
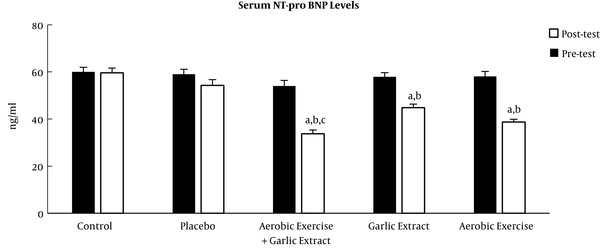
The posttest showed serum ANP and BNP levels significantly reduced in the aerobic exercise (t = 11.979, P ≤ 0.001 and t = 7.389, P ≤ 0.001, respectively), aerobic exercise + garlic extract (t = 13.728, P ≤ 0.001 and t = 7.069, P ≤ 0.001, respectively) groups, but only BNP level decreased significantly in the garlic extract group (t = 7.287, P ≤ 0.001). The findings of the ANCOVA test indicated a significant difference among study groups in ANP and BNP levels (F = 31.247, P ≤ 0.001 and F = 73.153, P ≤ 0.001). A significant decrease was found in ANP and BNP levels between the control group and aerobic exercise (P ≤ 0.001) and aerobic exercise + garlic extract (P ≤ 0.001) groups, but this significant decrease was observed only in BNP in the garlic extract group (P ≤ 0.05). In addition, there was a significantly higher ANP reduction in the aerobic exercise and aerobic exercise + garlic extract groups than in garlic extract group (P ≤ 0.001). Furthermore, the aerobic exercise + garlic extract group underwent a significantly higher ANP reduction than aerobic exercise group (P ≤ 0.05). As for BNP, the aerobic exercise + garlic extract group experienced a significantly higher BNP decrease than garlic extract group (P ≤ 0.01) (Figures 1 and 2).
5. Discussion
This study was aimed to investigate the effect of eight-week aerobic exercise program and garlic extract consumption on serum ANP and NT-pro BNP levels in obese hypertensive patients. Both aerobic exercise and garlic extract could significantly reduce systolic and diastolic blood pressure in the patients. However, there was no significant difference between a combination of these two interventions and each intervention alone in controlling blood pressure. Numerous studies have reported decreased blood pressure following regular exercise (23, 24) or garlic supplement consumption (25).
It has been shown that exercise makes a balance between vasodilation and vasoconstriction cytokines such as nitric oxide (26), prostacyclin, and thromboxane (27). The possible mechanisms of the antihypertensive function of garlic can be associated with its semi-prostaglandin effects, which reduces the resistance of peripheral vessels (28). Garlic decreases the E2 prostaglandin and B2 thromboxane (29). Further, gamma-glutamyl cysteine is a compound in garlic that inhibits the angiotensin-converting enzyme (30). Garlic also inhibits endothelin-1-induced contraction in a dose-dependent manner (31). This study evaluated the weight loss mechanisms and changing levels of natriuretic peptides, which can potentially influence blood pressure.
As expected, regular aerobic exercise used in the present study reduced the weight and BMI of the obese hypertensive participants. Interestingly, regular use of garlic extract also caused a significant reduction in body weight and BMI in the participants. Yet, this is in contrast with the results of Mahdavi-Roshan et al. (25), indicating no change in BMI following three months of garlic extract consumption in patients with cardiac failure. This difference can be related to the primary BMI of the participants. The BMI of the participants of the above study was 25.5 kg/m2, while the participants of the present study had a BMI above 30. It has been reported that systolic blood pressure is directly associated with BMI in patients with hypertension (25).
Although the changes of serum ANP and NT-pro BNP levels due to severe exercise were not taken into account, its high increase following exercise has been reported previously, which can be considered a mechanism for the decreased weight and BMI in this study. The levels of natriuretic peptides are inversely associated with visceral fat (24) and BMI (32). These observations show that obesity may reduce with the lipolytic effects of these peptides (33). It has also been shown that ANP acts as a lipolytic hormone and can increase the free fatty acids and heighten oxidation in skeletal muscles, liver, and adipose tissue (34). Aerobic exercise reduced serum ANP and Nt-pro BNP levels in obese hypertensive patients, while garlic extract only reduced serum NT-pro BNP level.
Liu et al. (35) reported a drop in Nt-pro-BNP level following the use of black garlic supplement in patients with coronary artery disease. Yet, there are contradictory reports about the effect of exercise on the resting levels of natriuretic peptides. Pirooz et al. (14) reported the reduction of ANP and no change in NT-pro BNP level after eight weeks of resistance exercise in the healthy young participants, while resistance exercise and combined resistance and endurance exercises did not change these peptides. Further, Beltran Valls et al. (16) found no change in NT-pro BNP level following twelve weeks of resistance exercise in the healthy elderly. Berent et al. (17) also reported the decrease of NT-pro BNP after four weeks of aerobic exercise in the cardiac patients.
It seems that the participants recruited in various studies are one of the factors involved in this contradiction. A review of randomized controlled trials found aerobic and resistance exercises decreased natriuretic peptides, especially NT-pro BNP in patients with cardiac failure (36). Plasma NT-pro BNP is known as a useful biomarker for the diagnosis and prognosis of cardiac failure in clinical trials (37). In this regard, a review by Hamasaki (38) showed that exercise increased the natriuretic peptides; however, these peptides decreased 72 h after exercise.
Interestingly, the reduced levels of natriuretic peptides in the healthy participants return to the resting state after a while, while they remain reduced in the participants suffering from cardiac failure (38). The decreased level of serum natriuretic peptides in the obese hypertensive patients observed in the present study also confirms the previous reports and indicates that exercise is able to significantly reduce the increased natriuretic peptides caused by myocardial impairment. The association between exercise and natriuretic peptides has also been reported from other aspects. Both chronic hypertension and exercise cause cardiac hypertrophy. A feature of exercise-induced hypertrophy is the lower expression of natriuretic peptides compared to blood pressure-induced hypertrophy (39).
This report can explain the reduction of increased natriuretic peptides following aerobic exercise in obese hypertensive patients. Although the natriuretic peptides increase in obese hypertensive patients, sensitivity to them seems to decrease. Exercise is probably able to moderate the abnormally increased levels of these peptides in hypertensive patients by increasing their sensitivity, including their receptors, and control the blood pressure simultaneously, a mechanism that needs further studies to confirm.
5.1. Conclusions
The use of garlic extract reduced the systolic and diastolic blood pressure in obese hypertensive patients, which is followed by reduced weight, BMI, and serum NT-pro BNP levels. On the other hand, regular aerobic exercise reduced natriuretic peptides (ANP and NT-pro BNP), body weight, BMI, and blood pressure. However, concurrent use of aerobic exercise and garlic extract had no additional effect on blood pressure control although it mostly influenced the body composition and levels of natriuretic peptides.
References
-
1.
Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6-10. [PubMed ID: 30253139]. https://doi.org/10.1016/j.metabol.2018.09.005.
-
2.
Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS One. 2013;8(7). e65174. [PubMed ID: 23935815]. [PubMed Central ID: PMC3728292]. https://doi.org/10.1371/journal.pone.0065174.
-
3.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer--viewpoint of the iarc working group. N Engl J Med. 2016;375(8):794-8. [PubMed ID: 27557308]. [PubMed Central ID: PMC6754861]. https://doi.org/10.1056/NEJMsr1606602.
-
4.
Anandacoomarasamy A, Caterson I, Sambrook P, Fransen M, March L. The impact of obesity on the musculoskeletal system. Int J Obes (Lond). 2008;32(2):211-22. [PubMed ID: 17848940]. https://doi.org/10.1038/sj.ijo.0803715.
-
5.
Sarvottam K, Yadav RK. Obesity-related inflammation & cardiovascular disease: Efficacy of a yoga-based lifestyle intervention. Indian J Med Res. 2014;139(6):822-34. [PubMed ID: 25109716]. [PubMed Central ID: PMC4164994].
-
6.
Choudhury A, Lip GY. Exercise and hypertension. J Hum Hypertens. 2005;19(8):585-7. [PubMed ID: 15905895]. https://doi.org/10.1038/sj.jhh.1001851.
-
7.
Bishop D, Edge J, Thomas C, Mercier J. Effects of high-intensity training on muscle lactate transporters and postexercise recovery of muscle lactate and hydrogen ions in women. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1991-8. [PubMed ID: 18832090]. https://doi.org/10.1152/ajpregu.00863.2007.
-
8.
Saribulbul O, Alat I, Coskun S, Apaydin AZ, Yagdi T, Kiliccioglu M, et al. The role of brain natriuretic peptide in the prediction of cardiac performance in coronary artery bypass grafting. Tex Heart Inst J. 2003;30(4):298-304. [PubMed ID: 14677740]. [PubMed Central ID: PMC307715].
-
9.
Felker GM, Whellan D, Kraus WE, Clare R, Zannad F, Donahue M, et al. N-terminal pro-brain natriuretic peptide and exercise capacity in chronic heart failure: Data from the Heart Failure and a Controlled trial investigating outcomes of exercise training (HF-ACTION) study. Am Heart J. 2009;158(4 Suppl):S37-44. [PubMed ID: 19782787]. [PubMed Central ID: PMC3748954]. https://doi.org/10.1016/j.ahj.2009.07.011.
-
10.
Ruskoaho H. Cardiac hormones as diagnostic tools in heart failure. Endocr Rev. 2003;24(3):341-56. [PubMed ID: 12788803]. https://doi.org/10.1210/er.2003-0006.
-
11.
Sarzani R, Spannella F, Giulietti F, Balietti P, Cocci G, Bordicchia M. Cardiac natriuretic peptides, hypertension and cardiovascular risk. High Blood Press Cardiovasc Prev. 2017;24(2):115-26. [PubMed ID: 28378069]. [PubMed Central ID: PMC5440492]. https://doi.org/10.1007/s40292-017-0196-1.
-
12.
Skvorak JP, Nazian SJ, Dietz JR. Endothelin acts as a paracrine regulator of stretch-induced atrial natriuretic peptide release. Am J Physiol. 1995;269(5 Pt 2):R1093-8. [PubMed ID: 7503296]. https://doi.org/10.1152/ajpregu.1995.269.5.R1093.
-
13.
Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27(1):47-72. [PubMed ID: 16291870]. https://doi.org/10.1210/er.2005-0014.
-
14.
Pirooz M, Khademi Y, HOSEINI SA. Effect of eight weeks endurance, resistance and combined training on concentration of atrial and brain peptides hormones in male students. Sport Psychol Manage Investigate. 2019;10(4):9-20.
-
15.
Bordbar S, Bigi MA, Aslani A, Rahimi E, Ahmadi N. Effect of endurance and strength exercise on release of brain natriuretic peptide. J Cardiovasc Dis Res. 2012;3(1):22-5. [PubMed ID: 22346141]. [PubMed Central ID: PMC3271676]. https://doi.org/10.4103/0975-3583.91599.
-
16.
Beltran Valls MR, Dimauro I, Brunelli A, Tranchita E, Ciminelli E, Caserotti P, et al. Explosive type of moderate-resistance training induces functional, cardiovascular, and molecular adaptations in the elderly. Age (Dordr). 2014;36(2):759-72. [PubMed ID: 24136652]. [PubMed Central ID: PMC4039278]. https://doi.org/10.1007/s11357-013-9584-1.
-
17.
Berent R, von Duvillard SP, Crouse SF, Auer J, Green JS, Sinzinger H, et al. Short-term residential cardiac rehabilitation reduces B-type natriuretic peptide. Eur J Cardiovasc Prev Rehabil. 2009;16(5):603-8. [PubMed ID: 19494782]. https://doi.org/10.1097/HJR.0b013e32832d7ca8.
-
18.
Seo DY, Lee S, Figueroa A, Kwak YS, Kim N, Rhee BD, et al. Aged garlic extract enhances exercise-mediated improvement of metabolic parameters in high fat diet-induced obese rats. Nutr Res Pract. 2012;6(6):513-9. [PubMed ID: 23346301]. [PubMed Central ID: PMC3542441]. https://doi.org/10.4162/nrp.2012.6.6.513.
-
19.
Colin-Gonzalez AL, Santana RA, Silva-Islas CA, Chanez-Cardenas ME, Santamaria A, Maldonado PD. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid Med Cell Longev. 2012;2012:907162. [PubMed ID: 22685624]. [PubMed Central ID: PMC3363007]. https://doi.org/10.1155/2012/907162.
-
20.
Al-Qattan KK, Thomson M, Al-Mutawa'a S, Al-Hajeri D, Drobiova H, Ali M. Nitric oxide mediates the blood-pressure lowering effect of garlic in the rat two-kidney, one-clip model of hypertension. J Nutr. 2006;136(3 Suppl):774S-6S. [PubMed ID: 16484561]. https://doi.org/10.1093/jn/136.3.774S.
-
21.
Brunner L, Suddarth D. Text book of medical surgical nursing. Philadelpia: Lippincott; 2008.
-
22.
Koseoglu M, Isleten F, Atay A, Kaplan YC. Effects of acute and subacute garlic supplement administration on serum total antioxidant capacity and lipid parameters in healthy volunteers. Phytother Res. 2010;24(3):374-8. [PubMed ID: 19653315]. https://doi.org/10.1002/ptr.2953.
-
23.
Punia S, Kulandaivelan S, Singh V, Punia V. Effect of aerobic exercise training on blood pressure in Indians: Systematic review. Int J Chronic Dis. 2016;2016:1370148. [PubMed ID: 27493989]. [PubMed Central ID: PMC4967448]. https://doi.org/10.1155/2016/1370148.
-
24.
Wen H, Wang L. Reducing effect of aerobic exercise on blood pressure of essential hypertensive patients: A meta-analysis. Medicine (Baltimore). 2017;96(11). e6150. [PubMed ID: 28296729]. [PubMed Central ID: PMC5369884]. https://doi.org/10.1097/MD.0000000000006150.
-
25.
Mahdavi-Roshan M, Nasrollahzadeh J, Mohammad Zadeh A, Zahedmehr A. Does garlic supplementation control blood pressure in patients with severe coronary artery disease? A clinical trial study. Iran Red Crescent Med J. 2016;18(11). e23871. [PubMed ID: 28191330]. [PubMed Central ID: PMC5292129]. https://doi.org/10.5812/ircmj.23871.
-
26.
Nyberg M, Jensen LG, Thaning P, Hellsten Y, Mortensen SP. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: Effect of high-intensity aerobic training. J Physiol. 2012;590(6):1481-94. [PubMed ID: 22271868]. [PubMed Central ID: PMC3382335]. https://doi.org/10.1113/jphysiol.2011.225136.
-
27.
Hansen AH, Nyberg M, Bangsbo J, Saltin B, Hellsten Y. Exercise training alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. Hypertension. 2011;58(5):943-9. [PubMed ID: 21896936]. https://doi.org/10.1161/HYPERTENSIONAHA.111.176529.
-
28.
Fugh-Berman A. Herbs and dietary supplements in the prevention and treatment of cardiovascular disease. Prev Cardiol. 2000;3(1):24-32. [PubMed ID: 11834913]. https://doi.org/10.1111/j.1520-037x.2000.80355.x.
-
29.
Al-Qattan KK, Khan I, Alnaqeeb MA, Ali M. Thromboxane-B2, prostaglandin-E2 and hypertension in the rat 2-kidney 1-clip model: A possible mechanism of the garlic induced hypotension. Prostaglandins Leukot Essent Fatty Acids. 2001;64(1):5-10. [PubMed ID: 11161580]. https://doi.org/10.1054/plef.2000.0232.
-
30.
Shouk R, Abdou A, Shetty K, Sarkar D, Eid AH. Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutr Res. 2014;34(2):106-15. [PubMed ID: 24461311]. https://doi.org/10.1016/j.nutres.2013.12.005.
-
31.
Ide N, Lau BH. Aged garlic extract attenuates intracellular oxidative stress. Phytomedicine. 1999;6(2):125-31. [PubMed ID: 10374252]. https://doi.org/10.1016/S0944-7113(99)80047-6.
-
32.
Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, et al. N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension. 2005;46(4):660-6. [PubMed ID: 16129819]. https://doi.org/10.1161/01.HYP.0000179575.13739.72.
-
33.
Polak J, Kotrc M, Wedellova Z, Jabor A, Malek I, Kautzner J, et al. Lipolytic effects of B-type natriuretic peptide 1-32 in adipose tissue of heart failure patients compared with healthy controls. J Am Coll Cardiol. 2011;58(11):1119-25. [PubMed ID: 21884948]. https://doi.org/10.1016/j.jacc.2011.05.042.
-
34.
Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122(12):4675-9. [PubMed ID: 23114600]. [PubMed Central ID: PMC3533552]. https://doi.org/10.1172/JCI64526.
-
35.
Liu J, Zhang G, Cong X, Wen C. Black garlic improves heart function in patients with coronary heart disease by improving circulating antioxidant levels. Front Physiol. 2018;9:1435. [PubMed ID: 30443217]. [PubMed Central ID: PMC6221913]. https://doi.org/10.3389/fphys.2018.01435.
-
36.
Smart NA, Steele M. Systematic review of the effect of aerobic and resistance exercise training on systemic brain natriuretic peptide (BNP) and N-terminal BNP expression in heart failure patients. Int J Cardiol. 2010;140(3):260-5. [PubMed ID: 19664831]. https://doi.org/10.1016/j.ijcard.2009.07.004.
-
37.
Kroon MH, van den Hurk K, Alssema M, Kamp O, Stehouwer CD, Henry RM, et al. Prospective associations of B-type natriuretic peptide with markers of left ventricular function in individuals with and without type 2 diabetes: an 8-year follow-up of the Hoorn Study. Diabetes Care. 2012;35(12):2510-4. [PubMed ID: 23033248]. [PubMed Central ID: PMC3507581]. https://doi.org/10.2337/dc11-1959.
-
38.
Hamasaki H. The effects of exercise on natriuretic peptides in individuals without heart failure. Sports (Basel). 2016;4(2). [PubMed ID: 29910280]. [PubMed Central ID: PMC5968914]. https://doi.org/10.3390/sports4020032.
-
39.
Iemitsu M, Miyauchi T, Maeda S, Sakai S, Kobayashi T, Fujii N, et al. Physiological and pathological cardiac hypertrophy induce different molecular phenotypes in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281(6):R2029-36. [PubMed ID: 11705790]. https://doi.org/10.1152/ajpregu.2001.281.6.R2029.