Abstract
Background:
We analyzed different methods used to assess the radiological responses of patients undergoing neoadjuvant chemotherapy and metastasectomy treatment for liver metastases associated with colorectal cancer (CRC) by comparing the response evaluation criteria in solid tumors (RECIST) 1.1, the modified RECIST, and the criteria of the European Association for the Study of the Liver (EASL) methods and the histological response obtained after metastasectomy.Objectives:
We aimed to determine the optimal radiological method to assess the response of colorectal liver metastases to neoadjuvant chemotherapy.Patients and Methods:
We conducted a retrospective study of CRC patients treated for liver metastases who had received neoadjuvant chemotherapy in our hospital between January 2000 and December 2017. We analyzed the agreement between the methods for analyzing the radiological response using the quadratic weighted kappa coefficient (κ). We studied the overall survival and analyzed factors related to survival using the Kaplan-Meier method. We performed multivariate analysis to study the prognostic factors of survival. We analyzed the relationship between the radiological and histological responses using Goodman and Kruskal's gamma (γ).Results:
A significant agreement was observed between the modified RECIST and EASL methods (κ = 0.841, P < 0.001). Cox regression multivariate analysis indicated the RECIST 1.1 criteria as an independent prognostic factor (P = 0.03). The γ value showed a significant relationship between the three radiological response methods and histological response.Conclusion:
In our study, we showed that using RECIST 1.1 criteria is the ideal radiological analysis method for studying CRC liver metastases treated with neoadjuvant chemotherapy when compared to other methods that are based on functional imaging markers.Keywords
Radiological Response Liver Metastasis Colorectal Cancer Neoadjuvant Chemotherapy
1. Background
Resection of liver metastases originating from colorectal cancer (CRC) with tumor-free margins is considered the most relevant treatment to obtain a long-term survival estimated between 25 and 50% (1, 2). In patients for whom it is not feasible to perform surgery as a curative treatment, other strategies have been proposed, such as treatment with neoadjuvant chemotherapy and subsequent surgical rescue (3, 4), with 50% response rates in unresectable liver metastases and a curative surgery rate of 20%. Due to new chemotherapeutic strategies, new therapeutic possibilities have been proposed because there are no significant differences in overall survival between patients undergoing complete resection with those undergoing resection with microscopic margin involvement plus adjuvant chemotherapeutic treatment (5).
To define which patients are candidates for surgical rescue, the response evaluation criteria in solid tumors (RECIST) are used, now in its the 2009 revised form (RECIST 1.1) (6), which is based on the comparison of the size of metastases before and after neoadjuvant treatment. However, the use of RECIST in neoadjuvant treatment with monoclonal antibodies has generated controversy when comparing the radiological and histological responses, given that these treatments act by increasing the period of metastatic stability but have a very limited effect on metastasis size (7, 8), and thus, patients with prolonged survival are sometimes classified as exhibiting an absence of response. Therefore, other radiological criteria, such as the modified RECIST criteria (mRECIST) and the criteria of the European Association for the Study of the Liver (EASL), have been proposed for the analysis of radiological response to treatment with tumor monoclonal antibodies, such as hepatocarcinoma treatment (9).
2. Objectives
The objective of the present study was to establish the best imaging method for the analysis of the response of CRC liver metastases to neoadjuvant treatment by analyzing the data resulting from the application of RECIST 1.1, mRECIST, and EASL criteria and comparing these data with the survival data and the histological response of patients who made up our sample.
3. Patients and Methods
3.1. Patients and Treatment
We performed a retrospective analysis of patients treated for liver metastases originating from CRC who had received neoadjuvant chemotherapy in our hospital between January 2000 and December 2017. Patients diagnosed with CRC liver metastasis during the period of analysis who received systemic neoadjuvant treatment were administered classic chemotherapeutic agents or were given a combination of these treatments and monoclonal antibody therapy. Patients were required to undergo imaging studies obtained via computerized tomography (CT) in the portal phase before and after neoadjuvant treatment. In addition, all patients had to have undergone surgical metastasectomy after neoadjuvant treatment. Patients were rejected if it was not possible to recover all the necessary information (20 patients), if they had completed loco-regional therapy (four patients), if they had not completed their follow-up in their autonomous communities of origin (two patients), or if they did not complete neoadjuvant chemotherapy treatment due to poor tolerance (one patient). We also had to exclude 20 patients for whom the CT images were acquired too late, impeding the correct application of the mRECIST and EASL criteria. In total, the sample consisted of 77 patients.
3.2. Radiological Response
All patients underwent abdominal-pelvic CT scans in the portal phase with a 5-mm section thickness. The radiological response was assessed according to the RECIST 1.1, mRECIST, and EASL criteria (Table 1). In the RECIST 1.1 and mRECIST criteria, only two target lesions per organ were analyzed (in this case, two liver metastases). Those liver metastases whose size was at least twice the thickness between the cuts made during computed tomography were considered measurable; in our case, the sizes of the lesions were not less than 1 cm. The RECIST 1.1 criteria are based on the sum of the largest diameters of the lesions, while the mRECIST and EASL methods analyze functional radiological markers, measuring the greatest length of the enhanced area of metastasis in the case of mRECIST and the area in the case of the EASL method (10).
Radiological Response Criteria
| RECIST 1.1 | mRECIST | EASL | |
|---|---|---|---|
| Complete response | Disappearance of lesions | Disappearance of lesions | Disappearance of lesions |
| Partial response | Decrease of ≥ 30% in the sum of the largest diameter | Decrease of ≥ 30% in the one-dimensional sum of the viable remnant | Decrease of ≥ 50% in the two-dimensional sum of the viable remnant |
| Disease stable | Does not meet PR or DP criteria | Does not meet PR or DP criteria | Does not meet PR or DP criteria |
| Disease progression | Increase of ≥ 20% in the sum of the largest diameter | Increase of ≥ 20% in the one-dimensional sum of the viable remnant | Increase of ≥ 25% in the sum of the two-dimensional product of the viable remnant |
In this way, patients are classified into five categories depending on the results obtained. complete remission (CR) is considered when the target lesions have completely disappeared. If the metastasis has responded but not to the point of completely disappearing, it is considered a partial response (PR). Disease progression (DP) involves a lack of response to neoadjuvant treatment, and the disease is classified as stable (DS) when the criteria of progression and PR are not met.
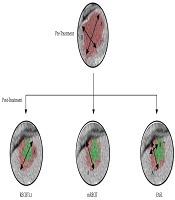
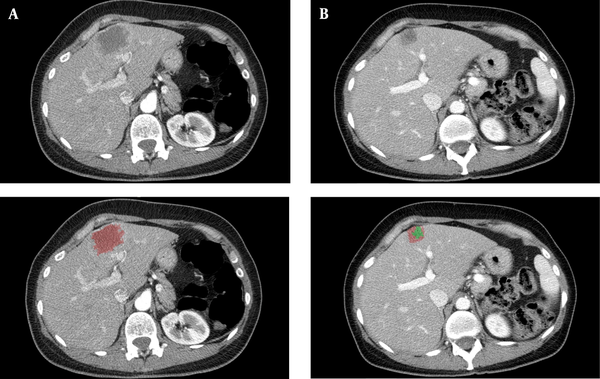
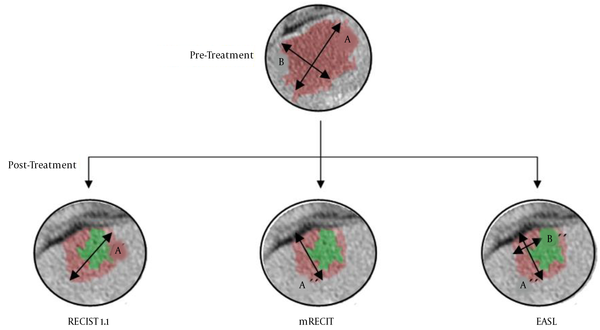
Figure 1 shows a clinical case of one of the patients included in this study, where we can observe the radiological response after neoadjuvant chemotherapy. Figure 2 illustrates the differences among the three methods for analyzing the radiological response. The measurements taken are: A = 35 mm, B = 20 mm, A’ = 20 mm, A’’ = 18 mm, B’’ = 7 mm. According to these, for the RECIST 1.1 method, the patient had a partial response (57,14%); for the mRECIST and EASL methods, there is a partial response, as well (51,43% and 18%, respectively).
A, Patient with colorectal liver metastases before neoadjuvant chemotherapeutic treatment. B, The same patient after neoadjuvant chemotherapeutic treatment.
Calculation method for RECIST 1.1: A'/A × 100; Calculation method for mRECIST: A''/A × 100; Calculation method for EASL: (A'' × B'')/(A × B) × 100
3.3. Histological Response
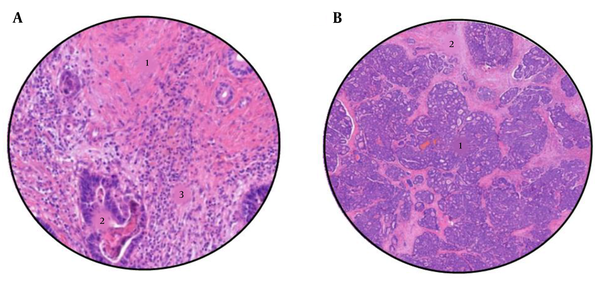
The histological response was analyzed following the criteria proposed by Rubbia-Brandt (11), for which the association with the overall survival of patients has already been demonstrated (12). The metastasis samples were analyzed on crystals stained with hematoxylin and eosin (H-E), and different histological components, such as fibrosis, mucin, necrosis, and viable tumor cells, were observed. The results were classified into five groups according to the tumor regression grade (TRG), with grade 1 indicating a complete response, in which tumor cells are absent and the metastasis has been replaced by fibrous tissue, and grade 5 indicating the absence of response, in which the presence of tumor cells and necrotic areas predominate. The samples were analyzed by a gastrointestinal pathologist and were subsequently evaluated again by another non-service specialist. Figure 3 shows two different cases of patients with different degrees of radiological response to treatment, pointing out its different characteristics.
A, Sample from a colorectal liver metastases after neoadjuvant chemotherapy, with a tumour regression grade of 2 (TRG2). 1: Abundant fibrosis, 2: Few tumour cells, and 3: Lynphocytic infiltrate. B, Sample from a colorectal liver metastases after neoadjuvant chemotherapy, with a tumour regression grade of 5 (TRG5). 1: Abundant tumour cells, and 2: Areas of necrosis.
3.4. Statistical Analysis
The data are presented as the frequency and percentage for categorical variables and the mean and standard deviation (SD) or the median and interquartile range (IQR) for quantitative variables. The concordance between the radiological response analysis methods was assessed using the quadratic weighted kappa coefficient (κ). The following parameters were used as references: κ between 0.21 and 0.40 was weak, κ between 0.41 and 0.60 was moderate, κ between 0.61 and 0.80 was good, and a value of κ greater than 0.80 was considered excellent. The relationship between the radiological and histological response was calculated using Goodman-Kruskal’s gamma (γ). Overall survival was calculated from the time of diagnosis until the date of death from any cause or until the last date of follow-up of the patient. The probability of survival as a function of time was calculated using the Kaplan-Meier (KM) method. A log-rank test was used to compare overall survival between the groups. A Cox regression model was performed to evaluate the hazard ratio (HR) and 95% confidence interval (95% CI) in each category within the radiological response analysis method (CR vs. PR vs. DS vs. DP) adjusted for sex, age, previous metastasectomy, metastases resectability, primary tumor origin, largest metastasis size, metastases number, Tumor Burden score (TBS) (13), type of chemotherapy used, and TRG. For the analysis, Stata 14/SE (Stata Co., College Station, Tx) was used.
4. Results
4.1. Patient Characteristics and Treatment
The sample included 77 patients, 47 (60.8%) men and 30 (39.2%) women, with a mean age of 60.9 years (SD = 10.1). All the patients had CRC with liver metastatic involvement and were exclusively treated with classic cytotoxic drugs (51.5%), mainly based on oxaliplatin (41.2%). A total of 41.2% of the patients received chemotherapy regimens that included classic cytotoxic drugs and monoclonal antibodies, and the majority of these patients were treated with bevacizumab (24.7%). The median time between the diagnosis of metastasis and surgery was seven months (IQR = 2 - 23). The majority of the patients had two liver metastases (IQR = 1 - 11), with a diameter of 20 mm (IQR = 2 - 160) (Table 2).
| Demographic data | Values |
|---|---|
| Total number | 77 |
| Sex | |
| Male | 47 (60.8) |
| Female | 30 (39.2) |
| Age at diagnosis, y (SD) | 60.9 (10.008) |
| Origin of the primary tumor | |
| Rectum | 32 (38.1) |
| Sigmoid | 23 (28.9) |
| Left colon | 5 (10.3) |
| Right colon | 17 (22.7) |
| Previous metastasectomy | |
| Yes | 3 (4.1) |
| No | 74 (95.9) |
| Resectability | |
| Resectable | 14 (18.6) |
| Unresectable | 63 (81.4) |
| Metastasis | |
| Synchronous | 63 (81.4) |
| Metachronous | 14 (18.55) |
| Number of metastases | 2 (2) |
| Largest diameter, mm | 20 (23) |
| TBS, tumor burden score | 3.16 (3.28) |
| Involvement of the margins | |
| Involved | 27 (35.1) |
| Tumor-free | 38 (49.5) |
| Without SI | 12 (15.5) |
| Type of chemotherapy | |
| Cytotoxic | 40 (51.5) |
| Capecitabine | 1 (2.1) |
| Oxaliplatin | 35 (41.2) |
| Irinotecan | 4 (8.2) |
| Cytostatic | 32 (41.2) |
| Cetuximab | 4 (5.2) |
| Bevacizumab | 19 (24.7) |
| Panitumumab | 9 (11.3) |
| Unknown | 5 (7.2) |
| Follow-up, mo | 35 (35) |
| Time to SI, mo | 7 (4) |
| Recurrence | |
| No | 11 (14.4) |
| Yes | 52 (68) |
| Unknown | 14 (17.6) |
| Death | |
| Yes | 52 (64.9) |
| No | 24 (34) |
| Unknown | 1 (1) |
| Death by tumor | |
| Yes | 46 (59.8) |
| No | 29 (37.1) |
| Unknown | 2 (3.1) |
4.2. Response to Neoadjuvant Chemotherapy According to Radiological and Histological Criteria
An excellent agreement was found between the mRECIST and EASL methods (κ = 0.841, P < 0.001). However, the agreement between the RECIST 1.1 criteria and the criteria of the other two methods was weak (κ = 0.218, P < 0.001 compared to mRECIST; and κ = 0.227, P < 0.001 compared to EASL).
In Table 3, the results of the histological analysis of the samples and the values resulting from the application of the radiological criteria are compared. The results obtained using the mRECIST and EASL criteria are practically superimposable. However, discrepancies existed when comparing the results obtained using mRECIST and EASL with the results derived from the RECIST 1.1 criteria. The mRECIST and EASL criteria better estimated the radiological responses. The RECIST 1.1 results were more consistent with the histological results although they did not precisely identify patients with a complete response (only four cases, compared to nine cases based on microscopic analysis).
| RECIST 1.1 | mRECIST | EASL | TRG | |
|---|---|---|---|---|
| Complete response | 4 | 12 | 12 | 9 |
| Responses | 38 | 56 | 55 | 34 |
| No response | 39 | 21 | 22 | 43 |
| Total | 77 | 77 | 77 | 77 |
4.3. Relationship Between Radiological and Histological Responses
In this study, γ showed a very significant relationship between the three radiological response methods and the pathological response, with γ = 0.4921 and P < 0.001 between RECIST 1.1 and TRG, γ = 0.3194 and P = 0.001 between mRECIST and TRG, and finally, γ = 0.3156 and P = 0.001 between EASL and TRG.
The KM survival curves show significant differences in patient survival as a function of the type of radiological response (CR, PR, DS, and DP) (Table 4 and Figure 4) using any of the three types of criteria.
Overall Survival According to Kaplan-Meier Analysis, Comparison of Probability With Log-Rank Test
| Survival, mo | RECIST 1.1 | mRECIST | EASL |
|---|---|---|---|
| CR | 132.333 | 76.724 | 76.724 |
| PR | 76.962 | 52.277 | 55.207 |
| DS | 46.675 | 40.255 | 33.839 |
| DP | 36.320 | 23.333 | 19.689 |
| p | 0.004 | 0.010 | < 0.001 |
A, Kaplan-Meier survival curve according to the RECIST 1.1 method, comparing CR vs. PR vs. DS vs. DP. It shows a significant increase in survival as radiological response improves. B, Kaplan-Meier survival curve according to the mRECIST method, comparing CR vs. PR vs. DS vs. DP. It shows a significant increase in survival as radiological response improves. C, Kaplan-Meier survival curve according to the EASL method, comparing CR vs. PR vs. DS vs. DP. It shows a significant increase in survival as radiological response improves. RECIST 1.1, response evaluation criteria in solid tumours updated in its 1.1 version; mRECIST, modified RECIST; EASL, European association for the study of the liver; CR, complete response; PR, partial response; DS, disease stable; DP, disease progressive.
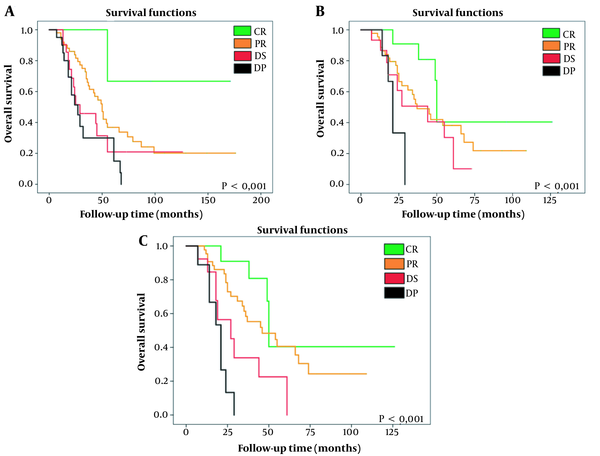
Survival was prolonged by up to 132.3 months in patients with a CR evaluated using the RECIST 1.1 criteria or by 76.7 months if they were classified as a CR by the mRECIST and EASL criteria. In contrast, in patients with DP, overall survival was reduced to 36.3, 23.3, and 19.7 months according to the RECIST 1.1, mRECIST, and EASL evaluations, respectively.
4.4. Prognostic Value of Radiological and Histological Responses
When performing the multivariate analysis, adjusting the survival according to the Cox regression, it must be considered that the variable EASL is collinear with mRECIST. In the complete response category, both variables coincide completely, which makes it impossible to analyze them within the same model, and thus, only the variable RECIST 1.1 is compared with the variable mRECIST. Only RECIST 1.1 is statistically significant (P = 0.03). The HR for each category was also calculated, resulting in an increasing HR value as the radiological response worsened (CR → PR → DS → DP). The values are listed in Table 5. Besides, RECIST 1.1 shows an HR of 0.034 (95% CI: 0.002 - 0.719) for CR and an HR up to 5.810 (95% CI: 0.522 - 11.121) for DP, and mRECIST shows the values of HR 0.716 (95% CI: 0.212 - 2.403) for CR and 0.704 (95% CI: 0.286 - 7.264) for DP.
| RECIST 1.1 | mRECIST | |||||
|---|---|---|---|---|---|---|
| Category | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
| CR | 0.034 | 0.002 - 0.719 | 0.037 | 0.716 | 0.212 - 2.403 | 0.584 |
| PR | 1 | - | 1 | - | ||
| DS | 2.786 | 0.448 - 4.297 | 0.085 | 0.557 | 0.272 - 5.567 | 0.459 |
| DP | 5.810 | 0.522 - 11.121 | 0.019 | 0.704 | 0.286 - 7.264 | 0.676 |
In the multivariate analysis, in addition to the RECIST 1.1 criterion, age is also an independent prognostic factor (P < 0.001, HR = 1.128, 95% CI: 1.063 - 1.196), as is the location of the primary tumor (P = 0.021, HR = 0.303, 95% CI: 0.110 - 0.835). The rest of the values are listed in Table 6.
| Variable | Hazard ratio | P-value | 95% CI |
|---|---|---|---|
| Sex | 0.385 | 0.057 | 0.145 - 1.030 |
| Age | 1.128 | 0.000 | 1.063 - 1.196 |
| Previous surgery | 31.491 | 0.050 | 1.001 - 990.422 |
| Resectability | 0.361 | 0.081 | 0.115 - 1.135 |
| Location of the main tumor | |||
| Sigmoid | 0.303 | 0.021 | 0.110 - 0.835 |
| Right sided | 0.416 | 0.141 | 0.130 - 1.336 |
| Rectum | 1 | - | |
| Left sided | 2.462 | 0.122 | 0.786 - 7.708 |
| Greatest diameter | 1.020 | 0.121 | 0.995 - 1.047 |
| Involvement of margins | 0.888 | 0.779 | 0.388 - 2.035 |
| RECIST 1.1 | |||
| CR | 0.034 | 0.037 | 0.002 - 0.719 |
| PR | 1 | - | |
| DS | 2.786 | 0.085 | 0.448 - 4.297 |
| DP | 5.810 | 0.019 | 0.522 - 11.121 |
| mRECIST | |||
| CR | 0.716 | 0.584 | 0.212 - 2.403 |
| PR | 1 | - | |
| DS | 0.557 | 0.459 | 0.272 - 5.567 |
| DP | 0.704 | 0.676 | 0.286 - 7.264 |
| Number of metastases | 1.086 | 0.602 | 0.797 - 1.478 |
| TBS | |||
| p0.25 | 0.978 | 0.972 | 0.270 - 3.537 |
| p0.50 | 0.660 | 0.580 | 0.152 - 2.871 |
| p0.75 | 0.267 | 0.252 | 0.028 - 2.562 |
| p1.00 | 1 | - | |
| Type of chemotherapy | 1.630 | 0.157 | 0.829 - 3.205 |
| Tumor regression grade | |||
| TRG1 | 1 | - | |
| TRG2 | 1.617 | 0.602 | 2.67 - 9.713 |
| TRG3 | 1.414 | 0.706 | 0.233 - 8.571 |
| TRG4 | 2.829 | 0.225 | 0.528 - 15.149 |
| TRG5 | 5.394 | 0.079 | 0.821 - 35.421 |
5. Discussion
Current treatment strategies for patients with borderline resectable metastasis include neoadjuvant chemotherapy (14, 15), in which classical cytotoxic drugs, such as oxaliplatin or irinotecan, are used. These cytotoxic drugs can enhance overall survival in patients with resectable metastases by up to 63.7 months (95% CI: 52.7 - 87.3) compared to 55 months with surgery alone (95% CI: 41.9 - 79.4) (16, 17). The new strategies also incorporate monoclonal antibodies, such as cetuximab or bevacizumab, which do not reduce the size of the metastases but have a stabilizing effect on them, leading to the question “which is the best method to establish the radiological response” (18, 19).
In our study, both mRECIST and EASL failed to identify patients in whom no histological response occurred (TRG 4 and TRG 5), overestimating the effect of neoadjuvant chemotherapy. In our analysis, the RECIST 1.1 criteria were an independent prognostic factor, and the HR values increased as the prognosis worsened. When analyzing the behavior of mRECIST and EASL, the HR value did not increase as the radiological response worsened, nor did these criteria reach statistical significance.
One explanation for the poor performance of the mRECIST and EASL criteria may lie in the very nature of the tumor itself. Unlike hepatocellular carcinoma, CRC metastasis is hypodense respecting the rest of the hepatic parenchyma, while hepatocellular carcinoma is a hypervascular tumor (20). Thus, CRC metastases are visible, especially in the portal phase, thus remaining less contrasted in CT images of the viable tumor portion, which makes analysis difficult (21).
In our study, patients who received monoclonal antibodies were also treated with classical cytotoxic drugs, whose effect on metastasis is homogeneous and induces size reductions (22). These factors favor the RECIST 1.1 analysis method over the mRECIST and EASL methods (19). When comparing the γ values, again, the RECIST 1.1 criteria indicated a greater association with the histological response than the mRECIST and EASL criteria. The mRECIST and EASL approaches overestimated the histological response, leading to poorer survival.
Regarding the histological response, the criteria proposed by Rubbia-Brandt did not reach statistical significance in our analysis (P = 0.051). When analyzing survival, the risk increased as the pathological response of the patients worsened; there is indeed an inversion between TRG 2 and 3, which is due to the laxity of the criteria in both categories proposed in the work of Rubbia-Brandt himself (11), since categories TRG 1, 4, and 5 have clear differentiating elements, such as the absence of tumor cells or the presence of necrosis. In contrast, TRGs 2 and 3 share the same characteristics, namely, fibrosis and few tumor cells, and are only differentiated by the proportion between the two characteristics, without determining the specific values.
Age, unlike other demographic prognostic factors, such as sex, can influence the survival of patients with CRC liver metastasis, and this was reflected in our analysis, which considered age to be an independent prognostic factor. Advanced age can be associated with a high operative risk and the presence of a greater number of postoperative complications (23). Additionally, the origin of the primary tumor was also an independent prognostic factor. As known, CRC does not constitute a single tumor type, and the disease evolution differs between tumors originating in the proximal (right) and the distal (left, rectum) portions of the colon. Depending on the location, different molecular and histological characteristics are identified (24), which should also be taken into account when developing chemotherapy treatment regimens.
If a correlation was demonstrated between the response to neoadjuvant treatment and overall survival, it would be possible to establish more efficient clinical trials to evaluate the action of new neoadjuvant therapies. A clear correlation would also allow the completion of adjuvant treatment in those cases with a poor histological response, to prolong the survival of patients. The demonstration of an association between the radiological and histological responses would allow unnecessary surgeries to be avoided in patients with a poor response.
Some oncological treatments, such as monoclonal antibodies, immunotherapy, or loco-regional treatment, are effective despite producing an atypical response. In patients with this type of treatment, the methods used to evaluate the radiological response based on size, such as the RECIST 1.1 criteria, show limitations because they underestimate the real response of the metastases (18). Akinwande et al. (21) compared the results offered by RECIST 1.1, mRECIST, and EASL in CRCliver metastases that had been treated with loco-regional therapy. These authors concluded that the determination of the mRECIST and EASL criteria presented a challenge in the case of CRC liver metastases because they are hypovascular lesions, unlike those in hepatocellular carcinoma, where both methods have demonstrated their superiority compared to the RECIST 1.1 criteria. This characteristic makes it difficult to visualize the viable tumor area. Moreover, these factors demonstrate that the RECIST 1.1 criteria rank better survival as a function of the type of radiological response and are an independent prognostic factor.
In 1996, Bismuth et al. (25) reported for the first time that neoadjuvant chemotherapy with oxaliplatin plus 5-fluorouracil and leucovorin allowed for the conversion of the initial unresectable liver metastases into resectable metastases, leading to a 40% increase in five-year survival. The addition of cetuximab to chemotherapy regimens such as FOLFIRI or FOLFOX has been shown to increase the response rate and prolong disease-free survival and overall survival as long as the metastases have the wild-type phenotype of the KRAS oncogene (26-28). In our study, we did not observe differences in the overall survival between patients undergoing neoadjuvant treatment using only classic cytotoxic drugs and patients undergoing treatment with those drugs combined with monoclonal antibodies. This result may occur due to the lack of information on whether patients have the wild-type KRAS oncogene given that mutations in this gene are associated with a poor response to cetuximab (29).
Concerning the limitations of our study, in addition to being a retrospective study, there was no homogeneity in the type of neoadjuvant therapy administered, the sample was small, and the study produced only 66 effects (66 deaths), which could lead to instability in the Cox regression analysis results. A decrease in the number of variables to be analyzed would offer, among other things, significant results about the degree of tumor regression.
In conclusion, as new chemotherapeutic regimens are developing, radiological changes differ from those seen when using classic cytotoxic drugs. Radiological biomarkers have proven to be useful to assess other tumors like hepatocarcinoma; however, they have not been tested before in CRC liver metastases treated with systemic chemotherapy.
The use of the RECIST 1.1 criteria is proposed as the ideal radiological analysis method for studying CRC liver metastases treated with neoadjuvant chemotherapy compared with the other methods that are based on functional imaging markers.
References
-
1.
Elias D, Liberale G, Vernerey D, Pocard M, Ducreux M, Boige V, et al. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol. 2005;12(11):900-9. [PubMed ID: 16184442]. https://doi.org/10.1245/ASO.2005.01.010.
-
2.
Hadden WJ, de Reuver PR, Brown K, Mittal A, Samra JS, Hugh TJ. Resection of colorectal liver metastases and extra-hepatic disease: a systematic review and proportional meta-analysis of survival outcomes. HPB (Oxford). 2016;18(3):209-20. [PubMed ID: 27017160]. [PubMed Central ID: PMC4814625]. https://doi.org/10.1016/j.hpb.2015.12.004.
-
3.
Lehmann K, Rickenbacher A, Weber A, Pestalozzi BC, Clavien PA. Chemotherapy before liver resection of colorectal metastases: friend or foe? Ann Surg. 2012;255(2):237-47. [PubMed ID: 22041509]. https://doi.org/10.1097/SLA.0b013e3182356236.
-
4.
Chua TC, Saxena A, Liauw W, Kokandi A, Morris DL. Systematic review of randomized and nonrandomized trials of the clinical response and outcomes of neoadjuvant systemic chemotherapy for resectable colorectal liver metastases. Ann Surg Oncol. 2010;17(2):492-501. [PubMed ID: 19856028]. https://doi.org/10.1245/s10434-009-0781-1.
-
5.
de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248(4):626-37. [PubMed ID: 18936576]. https://doi.org/10.1097/SLA.0b013e31818a07f1.
-
6.
Prajapati HJ, Spivey JR, Hanish SI, El-Rayes BF, Kauh JS, Chen Z, et al. mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE). Ann Oncol. 2013;24(4):965-73. [PubMed ID: 23223331]. https://doi.org/10.1093/annonc/mds605.
-
7.
Ronot M, Bouattour M, Wassermann J, Bruno O, Dreyer C, Larroque B, et al. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014;19(4):394-402. [PubMed ID: 24652387]. [PubMed Central ID: PMC3983809]. https://doi.org/10.1634/theoncologist.2013-0114.
-
8.
Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55(6):1309-16. [PubMed ID: 21703196]. https://doi.org/10.1016/j.jhep.2011.03.007.
-
9.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143-52. [PubMed ID: 28271869]. [PubMed Central ID: PMC5648544]. https://doi.org/10.1016/S1470-2045(17)30074-8.
-
10.
Liu L, Wang W, Chen H, Zhao Y, Bai W, Yin Z, et al. EASL- and mRECIST-evaluated responses to combination therapy of sorafenib with transarterial chemoembolization predict survival in patients with hepatocellular carcinoma. Clin Cancer Res. 2014;20(6):1623-31. [PubMed ID: 24493832]. https://doi.org/10.1158/1078-0432.CCR-13-1716.
-
11.
Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18(2):299-304. [PubMed ID: 17060484]. https://doi.org/10.1093/annonc/mdl386.
-
12.
Tanis E, Julie C, Emile JF, Mauer M, Nordlinger B, Aust D, et al. Prognostic impact of immune response in resectable colorectal liver metastases treated by surgery alone or surgery with perioperative FOLFOX in the randomised EORTC study 40983. Eur J Cancer. 2015;51(17):2708-17. [PubMed ID: 26342674]. https://doi.org/10.1016/j.ejca.2015.08.014.
-
13.
Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A, et al. The Tumor Burden Score: A New "Metro-ticket" Prognostic Tool For Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann Surg. 2018;267(1):132-41. [PubMed ID: 27763897]. https://doi.org/10.1097/SLA.0000000000002064.
-
14.
Devaud N, Kanji ZS, Dhani N, Grant RC, Shoushtari H, Serrano PE, et al. Liver resection after chemotherapy and tumour downsizing in patients with initially unresectable colorectal cancer liver metastases. HPB (Oxford). 2014;16(5):475-80. [PubMed ID: 23927606]. [PubMed Central ID: PMC4008166]. https://doi.org/10.1111/hpb.12159.
-
15.
Lam VW, Spiro C, Laurence JM, Johnston E, Hollands MJ, Pleass HC, et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol. 2012;19(4):1292-301. [PubMed ID: 21922338]. https://doi.org/10.1245/s10434-011-2061-0.
-
16.
Khoo E, O'Neill S, Brown E, Wigmore SJ, Harrison EM. Systematic review of systemic adjuvant, neoadjuvant and perioperative chemotherapy for resectable colorectal-liver metastases. HPB (Oxford). 2016;18(6):485-93. [PubMed ID: 27317952]. [PubMed Central ID: PMC4913134]. https://doi.org/10.1016/j.hpb.2016.03.001.
-
17.
Nigri G, Petrucciani N, Ferla F, La Torre M, Aurello P, Ramacciato G. Neoadjuvant chemotherapy for resectable colorectal liver metastases: what is the evidence? Results of a systematic review of comparative studies. Surgeon. 2015;13(2):83-90. [PubMed ID: 25257725]. https://doi.org/10.1016/j.surge.2014.07.005.
-
18.
Grimaldi S, Terroir M, Caramella C. Advances in oncological treatment: limitations of RECIST 1.1 criteria. Q J Nucl Med Mol Imaging. 2018;62(2):129-39. [PubMed ID: 29166754]. https://doi.org/10.23736/S1824-4785.17.03038-2.
-
19.
Rosen MA. Use of modified RECIST criteria to improve response assessment in targeted therapies: challenges and opportunities. Cancer Biol Ther. 2010;9(1):20-2. [PubMed ID: 20023419]. https://doi.org/10.4161/cbt.9.1.10716.
-
20.
Takada J, Hidaka H, Nakazawa T, Kondo M, Numata K, Tanaka K, et al. Modified response evaluation criteria in solid tumors is superior to response evaluation criteria in solid tumors for assessment of responses to sorafenib in patients with advanced hepatocellular carcinoma. BMC Res Notes. 2015;8:609. [PubMed ID: 26502722]. [PubMed Central ID: PMC4624170]. https://doi.org/10.1186/s13104-015-1565-2.
-
21.
Akinwande O, Philips P, Scoggins CR, Kelly L, Tatum C, Hahl M, et al. Comparison of tumor response assessment methods in patients with metastatic colorectal cancer after locoregional therapy. J Surg Oncol. 2016;113(4):443-8. [PubMed ID: 27060707]. https://doi.org/10.1002/jso.24141.
-
22.
Chung WS, Park MS, Shin SJ, Baek SE, Kim YE, Choi JY, et al. Response evaluation in patients with colorectal liver metastases: RECIST version 1.1 versus modified CT criteria. AJR Am J Roentgenol. 2012;199(4):809-15. [PubMed ID: 22997372]. https://doi.org/10.2214/AJR.11.7910.
-
23.
Donadon M, Lleo A, Di Tommaso L, Soldani C, Franceschini B, Roncalli M, et al. The Shifting Paradigm of Prognostic Factors of Colorectal Liver Metastases: From Tumor-Centered to Host Immune-Centered Factors. Front Oncol. 2018;8:181. [PubMed ID: 29892573]. [PubMed Central ID: PMC5985314]. https://doi.org/10.3389/fonc.2018.00181.
-
24.
Baran B, Mert Ozupek N, Yerli Tetik N, Acar E, Bekcioglu O, Baskin Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterology Res. 2018;11(4):264-73. [PubMed ID: 30116425]. [PubMed Central ID: PMC6089587]. https://doi.org/10.14740/gr1062w.
-
25.
Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224(4):509-20. discussion 520-2. [PubMed ID: 8857855]. [PubMed Central ID: PMC1235414]. https://doi.org/10.1097/00000658-199610000-00009.
-
26.
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663-71. [PubMed ID: 19114683]. https://doi.org/10.1200/JCO.2008.20.8397.
-
27.
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408-17. [PubMed ID: 19339720]. https://doi.org/10.1056/NEJMoa0805019.
-
28.
Somashekhar SP, Ashwin KR, Zaveri SS, Rauthan A, Patil P. Assessment of Tumor Response and Resection Rates in Unresectable Colorectal Liver Metastases Following Neoadjuvant Chemotherapy with Cetuximab. Indian J Surg Oncol. 2016;7(1):11-7. [PubMed ID: 27065676]. [PubMed Central ID: PMC4811818]. https://doi.org/10.1007/s13193-015-0442-8.
-
29.
De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19(3):508-15. [PubMed ID: 17998284]. https://doi.org/10.1093/annonc/mdm496.