Abstract
Background:
The association between chronic nephrolithiasis and several systemic conditions has been established in previous studies. Patients with recurrent urinary stones generally experience more urinary calcium loss, resulting in a lower bone mineral density (BMD). Dual-energy X-ray absorptiometry (DEXA) is the standard imaging method for diagnosing a low BMD. However, imaging imposes significant cost and radiation burden on patients.Objectives:
We aimed to assess the relationship between 24-hour urinalysis biometrics and bone mineral content, retrieved from non-contrast computed tomography (CT) imaging, which is routinely preformed for urinary stone patients as a primary evaluation.Patients and Methods:
The sample population for this retrospective study included urinary stone patients, undergoing percutaneous nephrolithotomy (PCNL) during 2015-2019, with available 24-hour urinalysis and CT imaging records. Stone size was defined as the maximum stone diameter on the CT image. BMD for each subject was also calculated at the vertebral L1 level, with CT attenuation measured in Hounsfield units (HU). According to the literature, a cutoff value of 160 HU was selected to distinguish normal BMD from low BMD.Results:
The present results showed a significant association between the stone size and BMD (P < 0.05). Moreover, patients with a low BMD had a higher urinary calcium excretion in the 24-hour urinalysis (P < 0.05). Evaluation of urine chemical composition and stone size demonstrated a significant association between hypercalciuria and urinary stone volume (P < 0.05).Conclusion:
A low BMD detected by CT imaging in patients with urinary stones is associated with abnormal 24-hour urinalysis biometrics and larger stones; therefore, it should be properly assessed.Keywords
Bone Mineral Density Bone Mineral Content Computed Tomography Scan Nephrolithiasis Urinary Stones X-Ray
1. Background
Nephrolithiasis is a globally common disorder, affecting all populations regardless of geographic and socioeconomic factors (1). According to reports, 10% - 15% of people in Western countries experience nephrolithiasis during their lifetime, with an even higher risk in the Middle East (20% - 25%) (1, 2). Also, previous studies suggest a prevalence of 1.9% in the general population of Iran, especially in central and southern provinces (3, 4). There is a general consensus that a more extensive metabolic evaluation is needed for recurrent or high-risk stone formers. Patients with large stones are a subgroup of patients who need further metabolic evaluation via 24-hour urine collection (5).
Chronic nephrolithiasis is often considered the hallmark of a systemic disorder (6). The bone mineral density (BMD) is lower in renal stone formers, stemming from several proposed pathogeneses, including genetic factors, metabolic disorders (such as renal leak hypercalciuria), immunological processes, and dietary, environmental, and iatrogenic effects (7-10). A decrease in BMD has been shown to occur regardless of urinary calcium excretion, as observed in both normocalciuric and hypercalciuric patients (7, 11). This reduction in BMD is of great importance, since the overall risk of bone fracture is higher among nephrolithiasis patients as compared to the normal population (7, 12, 13).
Non-contrast computed tomography (CT) is universally recognized as the standard modality for detecting urinary stones. It has been applied in up to 71% of emergency department visits, substituting other imaging methods, such as X-ray and ultrasound (14). Guidelines provided by the American Urological Association (AUA) for the surgical management of urinary stones strongly emphasize the role of non-contrast CT scan as a preoperative assessment (15). Besides, data collection through CT imaging for urolithiasis can minimize the need for additional costs and radiation burden on patients (5). Studies conducted by Alacreu et al. (16) and Pickhardt et al. (17) established abdominal CT scan, compared to the universally standard DEXA scan, as a viable method for assessing BMD by measuring the Hounsfield units (HU) at different vertebral levels, specifically L1.
2. Objectives
We aimed to evaluate the relationship between the BMD of patients with urinary stones and the ancillary data, including stone diameter retrieved from CT images and urine biometrics in the 24-hour urinalysis. We also aimed to assess the role of non-contrast CT scan as a promising screening tool in these patients.
3. Patients and Methods
3.1. Patients and Setting
The sample population of this retrospective study included patients with kidney stones, undergoing percutaneous nephrolithotomy (PCNL) for stone removal, admitted to the urology ward of Shohada-e Tajrish Hospital, Tehran, Iran, from February 2015 to July 2019. The subjects were enrolled based on the inclusion criteria: (1) Having a predominant calcium component in the urinary calculi analysis; (2) having a non-contrast abdominopelvic CT scan a day before surgery; and (3) a complete 24-hour urine study collected at least one month after the PCNL procedure.
Of 390 PCNL patients referred to the ward in the target period, 148 patients met the inclusion criteria and were enrolled in the study. Each patient’s preoperative medical record was retrieved from the medical record database of Shohada-e Tajrish Hospital. The data included each subject’s basic information, 24-hour urine biomarkers, serum biochemical analysis, and preoperative abdominopelvic CT scan. Patients with no previous record of 24-hour urinalysis were excluded.
Urinalysis before the stone removal procedure was also considered as an exclusion criterion, since the urine biochemistry may be affected by larger stone burdens. Besides, patients receiving medical treatments for nephrolithiasis before surgery were excluded from the study to reduce outliers in the data. Patients receiving calcium or vitamin D3 supplements, as well as those with impaired parathyroid hormone (PTH) secretion, were also excluded from the study. According to the standard protocol (18), CT is routinely performed before all stone removal surgeries in our center; therefore, we did not include it in the criteria.
3.2. Radiological Analysis
CT imaging was performed using a 16-slice Somatom Sensation scanner (Siemens, Germany), calibrated daily to ensure the accuracy of attenuation measurements. A distinguished radiologist retrieved each subject’s CT data from the hospital database and analyzed the images, using an INFINITT PACS viewer, assessing bone mineral attenuation and calculating the urinary stone size. Stone size was defined as the maximum diameter of stones in either axial or coronal windows, measured in millimeters.
According to established standards for measuring bone mineral attenuation in CT images, vertebral attenuation was calculated on both coronal and axial CT cross-sections by designating an oval region of interest (ROI) with an approximate area of 2 cm2 at the L1 vertebra over the centermost area of the trabecular bone. Proper anatomical selection of the ROI was ensured by inspecting sagittal and lateral windows to avoid attenuation measurement distortions. Bone mineral attenuation was measured in Hounsfield units (HU). As established in previous studies, a diagnostic threshold of 160 HU was selected to discriminate between patients with low and normal BMD (mean sensitivity: 73.9%, mean specificity: 70.6%) (16, 17).
3.3. Statistical Analysis
Independent-samples t-test and chi-square test were performed to assess the association between the 24-hour urine biometrics, BMD, and stone size. A linear regression analysis and correlation coefficient test were used to evaluate 24-hour urine biometrics and BMD. Moreover, the normal distribution of data was evaluated using Kolmogorov-Smirnov test. Skewness and kurtosis of data were also assessed. The significance of ANOVA results was determined using Scheffe’s post-hoc test. Besides, a linear regression analysis was used to evaluate the possible cofounding effects of age and gender on the main variables of the study. A significant statistical difference was defined as a two-sided P-value less than 0.05. All statistical analyses were performed in IBM SPSS version 22.
4. Results
This study was conducted on 148 patients with urinary stones, including 85 males (57.4%) and 63 females (42.6%). Eighty-three patients were assigned to the normal BMD group (> 160 HU) and 65 patients to the low BMD group (< 160 HU). The mean age of the participants was 48.80 ± 14.11 years. Table 1 presents the basic biometric information of the patients (in two groups of low and normal BMD), including the mean stone size, mean BMI, and serum biochemical composition. Our analysis confirmed the normal distribution of data (P < 0.05). The skewness and kurtosis of all quantitative variables fell in the normal range of -2 to +2. While a direct association was observed between BMI and bone mineral attenuation (P < 0.05), no significant relationship was found between BMD and the serum biochemical composition.
Distribution of the Patients’ Basic Characteristics in the Low and Normal BMD Subgroups
| Characteristics | Low BMD (HU < 160)a (n = 65) | Normal BMD (HU > 160)a (n = 83) | P-value |
|---|---|---|---|
| Mean stone size | 37.62 ± 15.68 | 24.65 ± 9.2 | 0.03 |
| Mean age | 56.54 ± 9.89 | 43.6 ± 14.21 | 0.04 |
| Gender (M/F) | 32/33 | 53/30 | 0.07 |
| Mean BMI | 23.85 ± 4.59 | 27.97 ± 4.6 | 0.03 |
| Serum calcium | 8.34 ± 1.31 | 8.22 ± 1.07 | 0.14 |
| Serum phosphorous | 3.14 ± 1.09 | 3.26 ± 0.89 | 0.21 |
| Serum PTH level | 35.92 ± 13.62 | 33.76 ± 14.28 | 0.19 |
| Serum vitamin D level | 11.03 ± 6.91 | 28.58 ± 12.47 | 0.06 |
Table 2 presents the association between 24-hour urinalysis biometrics and BMD, with a diagnostic cut-off value of 160 HU as the discriminatory threshold. The three main urinary biomarkers (calcium, oxalate, and citrate) were compared between the two groups regarding BMD (19). A significant inverse correlation was observed when comparing urinary calcium and urinary oxalate excretions between subjects with low and normal BMD. Conversely, urinary citrate was directly correlated with bone mineral attenuation (P < 0.05 for all correlations).
Association Between 24-Hour Urinalysis Biometrics and BMD Values
| 24-Hour urinalysis | Low BMD (HU < 160) (n = 65) | Normal BMD (HU > 160) (n = 83) | P-value |
|---|---|---|---|
| Calcium | 307.95 ± 134.26 | 203.65 ± 99.01 | 0.02 |
| Oxalate | 53.22 ± 21.32 | 36.16 ± 11.77 | 0.05 |
| Citrate | 264.27 ± 187.02 | 441.24 ± 195.45 | 0.01 |
Moreover, the urinary stone size data were extracted from the hospital database and defined as previously stated. Stone size was divided into three subdomains of < 20 mm, 20 - 30 mm, and > 30 mm, as established in the literature (20-22). Also, 24-hour urine biometric features were distributed and statistically evaluated across the three stone size subdomains (Table 3). Comparison of urinary biometrics among different groups based on stone size by ANOVA test showed a significant direct association between 24-hour urinary calcium and oxalate excretions and stone size, while the amount of urinary citrate excretion was inversely correlated with the urinary stone diameter (P < 0.05). The significance of the results was investigated using Scheffe’s post-hoc test (in the calcium subdomain, < 20 mm and 20-30 mm: post-hoc P-value = 0.01, < 20 mm and > 30 mm: P = 0.01, and 20 - 30 mm and > 30 mm: P = 0.02; in the oxalate subdomain, < 20 mm and 20 - 30 mm: P = 0.03, < 20 mm and > 30 mm: P = 0.03, and 20 - 30 mm and > 30 mm: P = 0.04; and in the citrate subdomain, < 20 mm and 20 - 30 mm: P = 0.01, < 20 mm and > 30 mm: P = 0.02, and 20 - 30 mm and > 30 mm: P = 0.02).
Association Between 24-Hour Urinalysis Biometrics and Urinary Stone Size
| 24-Hour urinalysis | Mean stone size groups, mm | P-value | ||
|---|---|---|---|---|
| < 20 (n = 39) | 20 - 30 (n = 52) | > 30 (n = 57) | ||
| Calcium | 138.95 ± 43.0 | 201.06 ± 94.92 | 339.56 ± 109.54 | 0.01 |
| Oxalate | 26.9 ± 4.40 | 34.91 ± 5.07 | 58.36 ± 18.04 | 0.03 |
| Citrate | 633.95 ± 108.197 | 448.44 ± 103.95 | 163.67 ± 57.45 | 0.01 |
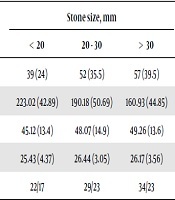
Table 4 demonstrates the relationship between urinary stone size and basic variables, including BMI, age, gender, and bone mineral attenuation for each stone size subdomain. The results of ANOVA test showed that BMD was inversely correlated with the mean urinary stone size. Patients with larger stones had a lower BMD (P < 0.05). In contrast, no significant association was found between the stone size and other parameters. The significance of data was confirmed by Scheffe’s post-hoc test (HU subdomains; < 20 mm and 20 - 30 mm: P = 0.02; < 20 mm and > 30 mm: P = 0.03; and 20 - 30 mm and > 30 mm: P = 0.03). The linear regression analysis showed that gender and age had no confounding effects on the data (P > 0.05). Also, a linear regression analysis and correlation coefficient test were used to examine the predictive role of urinary biometrics in BMD. However, none of these variables could predict BMD (P > 0.05).
Association Between Urinary Stone Size and Basic Variables in Each Stone Size Subdomain
| Stone size, mm | P-value | |||
|---|---|---|---|---|
| < 20 | 20 - 30 | > 30 | ||
| N = 148 | 39 (24) | 52 (35.5) | 57 (39.5) | |
| Mean bone mineral attenuation (HU) | 223.02 (42.89) | 190.18 (50.69) | 160.93 (44.85) | 0.03 |
| Mean age, y | 45.12 (13.4) | 48.07 (14.9) | 49.26 (13.6) | 0.07 |
| Mean BMI | 25.43 (4.37) | 26.44 (3.05) | 26.17 (3.56) | 0.18 |
| Gender (M/F) | 22/17 | 29/23 | 34/23 | 0.11 |
5. Discussion
The results of this study indicated a possible correlation between 24-hour urinalysis and BMD per abdominopelvic CT attenuation in urinary calcium stone formers. Higher urinary calcium (307.95 ± 134.26 vs. 203.65 ± 99.01) and oxalate (53.22 ± 21.32 vs. 36.16 ± 11.77) excretions were observed in the low BMD group, while hypercitraturia was more common in the normal BMD group (264.27 ± 187.02 vs. 441.24 ± 195.45). Therefore, the bone mineral net loss was directly correlated with the amount of urinary calcium and oxalate elimination, while it was inversely correlated with urinary citrate. As mentioned before, the participants in this study had calcium-predominant stones, including both calcium oxalate and calcium phosphate, with the majority having calcium oxalate stones. The basic pathogenesis of these subtypes remains similar, making deduction based on these findings feasible (5, 13).
The correlations between stone size, BMD, and urinary biometrics were also evaluated in this study. The present findings suggested a significant inverse association between BMD and the urinary stone size. Larger stones were observed in low-BMD patients, and expectedly, the urinary chemical composition in patients with larger stones was similar to that of low-BMD patients; there was a significant correlation between urinary oxalate, citrate, and calcium excretions and the mean urinary stone size (P < 0.05).
Patients with urinary stones are known to have a lower BMD than the general population; therefore, they may have a higher risk of fractures, as shown in previous studies (7, 12, 13, 23). While the etiology of this phenomenon is not clearly defined (7, 9, 24, 25), the loss of BMD may be attributed to hypercalciuria, as the most common metabolic finding in patients with urinary stones (26). Almost 80% of urinary stones contain calcium as their major component (5, 13, 27, 28). In these patients, a negative ion balance is expected to directly affect the BMD, since bones are the main calcium storage sites in the human body (29, 30).
Previous studies have demonstrated an association between chronic nephrolithiasis and reduced BMD. In this regard, Asplin et al. (31) and Pietschmann et al. (7) proposed an inverse association between urinary calcium excretion and reduced lumbar and femoral BMD, based on the gold standard DEXA imaging (7, 31). Nevertheless, the direct effects of urinary calcium excretion on BMD in urinary stone patients are subject to controversy. A study by Sakhaee et al. (9) questioned the relationship between hypercalciuria and BMD in patients with urinary stones, while Tugcu et al. (11) found decreased BMD in patients with normocalcemic stones. These discrepancies may be related to the multifactorial nature of hypercalciuria and BMD disease in nephrolithiasis or differences in sample selection, as several etiologies other than urinary calcium excretion have been proposed to contribute to bone mineral loss in case of recurrent urinary stone formation.
Previous studies have documented an inverse association between urinary sodium and spinal bone content loss (7), while further research by Sakhaee et al. (9) demonstrated a relationship between higher urinary calcium levels and lower BMD in postmenopausal women without hormone replacement therapy. While previous studies, such as the cohort conducted by Framingham et al., have established the protective effect of BMI on BMD (32), the effect of serum biochemical composition on the bone mineral content is controversial and beyond the scope of this study.
Regardless of the serological and urine chemistry results, screening for reduced BMD and the subsequent increased risk of fracture is of great importance. This study proposed non-contrast CT imaging as a standard diagnostic modality for urinary stones and a promising screening tool for these patients. While our study did not have access to the gold standard DEXA imaging for measuring BMD, the 160-HU threshold provided acceptable sensitivity and specificity in distinguishing normal from osteoporotic patients, based on previous studies (5, 16, 17)
Different cutoff values, such as 180 HU and 190 HU, have been proposed in previous studies, offering higher sensitivity for differentiating osteoporotic from osteopenic patients; however, use of such thresholds was beyond the scope of the current study (16, 17, 33). The 24-hour urinalysis has been recommended in the AUA guidelines for high-risk and chronic stone formers (18). Also, abnormalities in the urine chemical composition, such as hypercalciuria, hypocitraturia, and hyperoxaluria, are common findings in these patients, which have been recognized as risk factors for recurrent stone formation and bone mineral reduction.
Appropriate treatment regimens may reduce both kidney stone recurrence and fracture risk in patients (34, 35). However, up to 35% of patients with urinary stones have normal urine biometrics in the 24-hour analysis (19). The relationship between urine biometrics and bone mineral attenuation on CT images, as established in our study, can be useful for the mentioned cases, since the additional data provided by non-contrast CT may help clinicians in risk-assessment to decide if the patient can benefit from subsequent diagnostic or curative interventions.
Our findings suggested non-contrast abdominopelvic CT imaging as a promising and valid screening tool for examining the bone mineral content in patients with urinary stones. Categorization of patients into normal and low BMD groups based on the trabecular bone attenuation on CT images (discriminatory cut-off value of 160 HU) can help clinicians differentiate high-risk patients. Also, the association between BMD and urine chemical composition is useful in selecting patients who can benefit from further work-up, either through 24-hour urinalysis or medical management.
Moreover, high-risk patients with urinary stones have been shown to have larger stone burdens. Our findings demonstrated a similar relationship between the urinary stone diameter and 24-hour urinalysis biometrics and also between BMD and urine chemical composition. Besides, stone size was shown to be inversely correlated with BMD as an independent factor. Such an association has been partially discussed in the literature (5). In this regard, Patel et al. reported an inverse association between the stone volume and BMD in a subset analysis. Overall, it seems that stone size can help physicians identify kidney stone formers with a higher risk of fracture.
To the best of our knowledge, this is the first study evaluating the association between 24-hour urinalysis and low BMD, retrieved from the CT images of an Iranian sample population. However, some limitations must be addressed. First, although previous studies have confirmed a low BMD diagnostic threshold of 160 HU in CT images to be valid compared to DEXA, our study was only conducted based on CT results, and we did not have access to the gold standard DEXA. Also, 160 HU is the defined cut-off threshold for osteopenia; therefore, there may be a sample of patients with normal BMD, falsely included in the low BMD subgroup.
Second, lack of comprehensive patient profiles in the hospital database may partly affect the results of our analysis, as several etiologies have been proposed for BMD loss in urinary stone formers. Also, history of urinary stone symptoms and the medications used by the patients might have also affected the results (1). While we retrieved the urinary and serum biochemical data of patients from the database, their history could not be fully retrieved. Therefore, further analysis of prospectively selected groups is recommended to evaluate the risk factors for fracture risk and low BMD in patients with urinary stones.
Third, an inclusion criterion of this study was undergoing PCNL. Since patients with stones smaller than 10 - 15 mm in diameter are not candidates for this type of surgery, they were not included in our analysis. Therefore, further research may be necessary to clarify the effects of smaller urinary stones on the BMD and urinary biometrics (15).
In conclusion, our findings related to reduced BMD and urinary stone size, retrieved from non-contrast abdominal/pelvic CT images, were significantly associated with abnormal 24-hour urine biochemistry. Patients with hypercalciuria, hypocitraturia, and hyperoxaluria had a lower BMD, as well as larger urinary stones. These findings can be used as a valuable screening tool to identify urinary stone patients with a higher risk of osteoporosis and fracture and to help physicians deal with such patients properly using laboratory and medical tools.
Acknowledgements
References
-
1.
Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367(9507):333-44. [PubMed ID: 16443041]. https://doi.org/10.1016/S0140-6736(06)68071-9.
-
2.
Pak CY, Resnick MI, Preminger GM. Ethnic and geographic diversity of stone disease. Urology. 1997;50(4):504-7. [PubMed ID: 9338722]. https://doi.org/10.1016/s0090-4295(97)00307-5.
-
3.
Ketabchi AA, Aziziolahi GA. Prevalence of symptomatic urinary calculi in Kerman, Iran. Urol J. 2008;5(3):156-60. [PubMed ID: 18825621].
-
4.
Hosseini MM, Eshraghian A, Dehghanian I, Irani D, Amini M. Metabolic abnormalities in patients with nephrolithiasis: comparison of first-episode with recurrent cases in Southern Iran. Int Urol Nephrol. 2010;42(1):127-31. [PubMed ID: 19548107]. https://doi.org/10.1007/s11255-009-9599-9.
-
5.
Patel ND, Ward RD, Calle J, Remer EM, Monga M. CT-Based Diagnosis of Low Vertebral Bone Mineral Density Is Associated with Hypercalciuria and Hypocitraturia on Opportunistic Imaging. J Endourol. 2018;32(9):878-83. [PubMed ID: 29954225]. https://doi.org/10.1089/end.2018.0296.
-
6.
Heilberg IP, Weisinger JR. Bone disease in idiopathic hypercalciuria. Curr Opin Nephrol Hypertens. 2006;15(4):394-402. [PubMed ID: 16775454]. https://doi.org/10.1097/01.mnh.0000232880.58340.0c.
-
7.
Pietschmann F, Breslau NA, Pak CY. Reduced vertebral bone density in hypercalciuric nephrolithiasis. J Bone Miner Res. 1992;7(12):1383-8. [PubMed ID: 1481724]. https://doi.org/10.1002/jbmr.5650071205.
-
8.
Trinchieri A. Bone mineral content in calcium renal stone formers. Urol Res. 2005;33(4):247-53. [PubMed ID: 16078084]. https://doi.org/10.1007/s00240-005-0498-y.
-
9.
Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int. 2011;79(4):393-403. [PubMed ID: 21124301]. [PubMed Central ID: PMC3088506]. https://doi.org/10.1038/ki.2010.473.
-
10.
Sakhaee K. Nephrolithiasis as a systemic disorder. Curr Opin Nephrol Hypertens. 2008;17(3):304-9. [PubMed ID: 18408483]. https://doi.org/10.1097/MNH.0b013e3282f8b34d.
-
11.
Tugcu V, Ozbek E, Aras B, Ozbay B, Islim F, Tasci AI. Bone mineral density measurement in patients with recurrent normocalciuric calcium stone disease. Urol Res. 2007;35(1):29-34. [PubMed ID: 17160655]. https://doi.org/10.1007/s00240-006-0074-0.
-
12.
Melton L3, Crowson CS, Khosla S, Wilson DM, O'Fallon WM. Fracture risk among patients with urolithiasis: a population-based cohort study. Kidney Int. 1998;53(2):459-64. [PubMed ID: 9461107]. https://doi.org/10.1046/j.1523-1755.1998.00779.x.
-
13.
Lauderdale DS, Thisted RA, Wen M, Favus MJ. Bone mineral density and fracture among prevalent kidney stone cases in the Third National Health and Nutrition Examination Survey. J Bone Miner Res. 2001;16(10):1893-8. [PubMed ID: 11585355]. https://doi.org/10.1359/jbmr.2001.16.10.1893.
-
14.
Fwu CW, Eggers PW, Kimmel PL, Kusek JW, Kirkali Z. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;83(3):479-86. [PubMed ID: 23283137]. [PubMed Central ID: PMC3587650]. https://doi.org/10.1038/ki.2012.419.
-
15.
Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, et al. Surgical Management of Stones: American Urological Association/Endourological Society Guideline, PART I. J Urol. 2016;196(4):1153-60. [PubMed ID: 27238616]. https://doi.org/10.1016/j.juro.2016.05.090.
-
16.
Alacreu E, Moratal D, Arana E. Opportunistic screening for osteoporosis by routine CT in Southern Europe. Osteoporos Int. 2017;28(3):983-90. [PubMed ID: 28108802]. https://doi.org/10.1007/s00198-016-3804-3.
-
17.
Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158(8):588-95. [PubMed ID: 23588747]. [PubMed Central ID: PMC3736840]. https://doi.org/10.7326/0003-4819-158-8-201304160-00003.
-
18.
Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, et al. Medical management of kidney stones: AUA guideline. J Urol. 2014;192(2):316-24. [PubMed ID: 24857648]. https://doi.org/10.1016/j.juro.2014.05.006.
-
19.
Hsi RS, Sanford T, Goldfarb DS, Stoller ML. The Role of the 24-Hour Urine Collection in the Prevention of Kidney Stone Recurrence. J Urol. 2017;197(4):1084-9. [PubMed ID: 27746283]. https://doi.org/10.1016/j.juro.2016.10.052.
-
20.
ElSheemy MS, Elmarakbi AA, Hytham M, Ibrahim H, Khadgi S, Al-Kandari AM. Mini vs standard percutaneous nephrolithotomy for renal stones: a comparative study. Urolithiasis. 2019;47(2):207-14. [PubMed ID: 29549382]. https://doi.org/10.1007/s00240-018-1055-9.
-
21.
Kokov D, Manka L, Beck A, Winter A, Gerullis H, Karakiewicz PI, et al. Only Size Matters in Stone Patients: Computed Tomography Controlled Stone-Free Rates after Mini-Percutaneous Nephrolithotomy. Urol Int. 2019;103(2):166-71. [PubMed ID: 30844789]. https://doi.org/10.1159/000497442.
-
22.
Zewu Z, Cui Y, Feng Z, Yang L, Chen H. Comparison of retrograde flexible ureteroscopy and percutaneous nephrolithotomy in treating intermediatesize renal stones (2-3cm): a meta-analysis and systematic review. Int Braz J Urol. 2019;45(1):10-22. [PubMed ID: 30620157]. [PubMed Central ID: PMC6442149]. https://doi.org/10.1590/S1677-5538.IBJU.2018.0510.
-
23.
Ou SM, Chen YT, Shih CJ, Tarng DC. Increased risk of bone fracture among patients with urinary calculi: a nationwide longitudinal population-based study. Osteoporos Int. 2015;26(4):1261-9. [PubMed ID: 25524022]. https://doi.org/10.1007/s00198-014-2998-5.
-
24.
Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet. 2009;41(8):926-30. [PubMed ID: 19561606]. https://doi.org/10.1038/ng.404.
-
25.
Weisinger JR, Alonzo E, Bellorin-Font E, Blasini AM, Rodriguez MA, Paz-Martinez V, et al. Possible role of cytokines on the bone mineral loss in idiopathic hypercalciuria. Kidney Int. 1996;49(1):244-50. [PubMed ID: 8770975]. https://doi.org/10.1038/ki.1996.34.
-
26.
Coe FL, Bushinsky DA. Pathophysiology of hypercalciuria. Am J Physiol. 1984;247(1 Pt 2):F1-13. [PubMed ID: 6377922]. https://doi.org/10.1152/ajprenal.1984.247.1.F1.
-
27.
Caudarella R, Vescini F, Buffa A, La Manna G, Stefoni S. Osteoporosis and urolithiasis. Urol Int. 2004;72 Suppl 1:17-9. [PubMed ID: 15133327]. https://doi.org/10.1159/000076585.
-
28.
Wu W, Yang D, Tiselius HG, Ou L, Liang Y, Zhu H, et al. The characteristics of the stone and urine composition in Chinese stone formers: primary report of a single-center results. Urology. 2014;83(4):732-7. [PubMed ID: 24485999]. https://doi.org/10.1016/j.urology.2013.11.012.
-
29.
Tsuji H, Umekawa T, Kurita T, Uemura H, Iguchi M, Kin K, et al. Analysis of bone mineral density in urolithiasis patients. Int J Urol. 2005;12(4):335-9. [PubMed ID: 15948718]. https://doi.org/10.1111/j.1442-2042.2005.01049.x.
-
30.
Embon OM, Rose GA, Rosenbaum T. Chronic dehydration stone disease. Br J Urol. 1990;66(4):357-62. [PubMed ID: 2224429]. https://doi.org/10.1111/j.1464-410x.1990.tb14954.x.
-
31.
Asplin JR, Bauer KA, Kinder J, Muller G, Coe BJ, Parks JH, et al. Bone mineral density and urine calcium excretion among subjects with and without nephrolithiasis. Kidney Int. 2003;63(2):662-9. [PubMed ID: 12631132]. https://doi.org/10.1046/j.1523-1755.2003.00763.x.
-
32.
Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8(5):567-73. [PubMed ID: 8511983]. https://doi.org/10.1002/jbmr.5650080507.
-
33.
Ganji Jameshouran MA, Abedi AR, Montazeri S, Tasharrofi MA, Mansouri Tehrani MM. CT-Scan as an Opportunistic Osteoporosis Screening Tool in High-Risk Populations; A Diagnostic Study Conducted on a Sample of Urinary Stone Patients. Iran J Radiol. 2020;17(4). https://doi.org/10.5812/iranjradiol.102177.
-
34.
Arrabal-Polo MA, Arias-Santiago S, de Haro-Munoz T, Lopez-Ruiz A, Orgaz-Molina J, Gonzalez-Torres S, et al. Effects of aminobisphosphonates and thiazides in patients with osteopenia/osteoporosis, hypercalciuria, and recurring renal calcium lithiasis. Urology. 2013;81(4):731-7. [PubMed ID: 23375914]. https://doi.org/10.1016/j.urology.2012.12.013.
-
35.
Krieger NS, Frick KK, Bushinsky DA. Mechanism of acid-induced bone resorption. Curr Opin Nephrol Hypertens. 2004;13(4):423-36. [PubMed ID: 15199293]. https://doi.org/10.1097/01.mnh.0000133975.32559.6b.