Abstract
Background:
T helper type 2 (Th2) cells are critical cellular elements in allergic rhinitis. Interleukin-33 (IL-33) produces Th2-related cytokines and binds to the ST2 receptors. This is expressed strongly in mastocytes and discerningly in Th2 cells. Through Th2 cells, IL-33 may also have partly involved in immune responses.Objectives:
This study aimed to measure the IL-33 serum levels in children suffering from allergic rhinitis and investigate its relationship with the disease.Methods:
This case-control study was conducted on a population with the age range of 7-18 years, who referred to the Pediatric Clinic of the Shahid Beheshti Hospital in Kashan, Iran, in 2017. The study sample encompassed 57 patients with allergic rhinitis (case group) and 57 subjects with no allergic rhinitis (control group). The ELISA assay was used to measure the serum level of IL-33 in the case and control groups. Allergic rhinitis was diagnosed by a pediatric immunologist considering the patient’s history and the guidelines set out by the Allergic Rhinitis and its Impact on Asthma (ARIA). All study data were analyzed with SPSS software version 22.Results:
There were significant differences between the two groups in terms of age (P = 0.001), gender (P = 0.0144), family history of atopy (P < 0.001), symptoms duration (P < 0.001), and comorbidities (e.g., atopic dermatitis and asthma) (P < 0.001). Furthermore, compared to the control group, the case group exhibited significantly higher IL-33 serum levels (P < 0.001).Conclusions:
The high serum levels of IL-33 exhibited in patients with allergic rhinitis indicate its involvement in the pathogenesis of the concerned disease.Keywords
1. Background
Allergic Rhinitis or hay fever is a response to specific allergens and is attributed to a set of symptoms caused by nasal congestion such as sneezing, a runny nose, a stuffy nose, an itchy nose, coughing, a sore or scratchy throat, itchy eyes, dark circles under the eyes, and hives. These symptoms appear when allergens such as dust mites or pollens enter the nose via breathing (1) due to increased allergic reactions (2). According to global statistics, above 600 million persons suffer from allergic rhinitis worldwide (3). The increased allergic reactions begin when a person's immune system reacts to allergens such as pollens or dust, generally in the form of a “runny nose” (4, 5). The combination of antibodies with allergens leads to chemical reactions, which release mediators such as histamine and leukotriene, mediating the permeability of the surrounding capillaries and other chemical mediators. These mediators result in nasal congestion, red eyes, runny nose, itching, swollen throat, and other allergic symptoms (6). The symptoms vary from person to person (5).
Allergic rhinitis is a widespread complication, affecting about 10% of individuals (7). This type of inflammation has also been reported in infants due to the consumption of cow milk (8). There are differences between allergic rhinitis and common cold in terms of disease duration (cold lasts less than a week), pathogenic agents, non-contagiousness, and recurrence (9, 10). This disorder can be classified into acute, chronic, seasonal, or permanent types (11, 12). It can also be categorized into intermittent or persistent types concerning the duration of symptoms. If symptoms persist below four days per week or four weeks at a time, they are intermittent. If symptoms persist above four days per week or for four weeks at a time, they are persistent (7).
Interleukin-33 (IL-33) is a newly discovered member of the IL-1 superfamily, found inside the skin, lungs, synovial tissues, and adipose tissues, with similar cellular signals to IL-1ß and IL-18 (13, 14). IL-33 is a 30 kD protein evolutionarily conserved in mammals, with 54% amino acid sequence identity between the human and mouse homologs (15). Unlike IL-1ß and IL-18, maturation processes are not necessary for the IL-33 bioavailability. Similar to IL-1ß and IL-18, IL-33 is first produced as a pre-interleukin in an intracellular form and is then released outside the cell as the main IL-33 after a breakdown (16).
Studies have indicated that IL-33 as a dual-function protein can serve as a pro-inflammatory cytokine or intracellular transcription factor with transcription-regulating functions. Regarding its later role, IL-33 moves toward the cell's nucleus, where it binds to chromatin and regulates gene expression. As presented in Figure 1, the biological effects of IL-33 are mediated by binding to its receptors and activating NF-κB and mitogen-activated protein (MAP) kinases (16). Full-length IL-33 reacts with the NF-κB transcription factor instead of mature IL-33. The receptor of this cytokine, known as ST2, belongs to a large family of IL-1 receptors and plays a critical role in inflammation and immunological responses. Using a heterodimer complex, this receptor can exert its biological impacts. The heterodimer complex consists of IL-1RL1, also called ST2 receptor, and IL-1 receptor accessory protein (IL-1RACP), which is also a part of the IL-1 receptor family (13, 16).
Dendritic cells (DC) are the major antigen-presenting cells and play a pivotal role in immune responses. Moreover, DC directly responds to IL-33 via ST2 (17). DC responds to IL-33 and produces IL-33 under allergic conditions. The interaction between IL-33 and DC may represent a new pathway to initiate Th2-type immune responses (18).
In this regard, IL-33 could induce the production of many cytokines such as IL-5, IL-13, TNF-α, IFN-γ, and IL-2 when binding with ST2 receptors on the surface of Th2 lymphocytes (19, 20). Accordingly, IL33 and ST2 play a critical role in allergic diseases and various mucosal immune responses, suggesting the involvement of the IL-33/ST2 pathway in the pathogenesis of many diseases, especially in the responses based on the Th2 type (21, 22).
Although IL-33 mRNA can be expressed widely in body tissues, its cellular distribution is confined to keratinocytes, fibroblasts, and activated macrophages, including smooth muscles, and epithelial and dendritic cells (23, 24).
IL-33/ST2 signaling pathway.IL-33 is the ligand for ST2. It activates the ST2L/IL-1RAcP dimers or is neutralized by binding to sST2. The interaction of IL-33 with ST2 leads to the recruitment of the myeloid differentiation primary response protein 88 (MyD88), IL-1R-associated kinase 1 (IRAK1), and IRAK4. This would also result in the activation of at least two independent pathways(the transcription factor nuclear factor-κB (NF-κB) and the mitogen-activated protein kinase (MAPK) pathway) and ultimately induce relevant gene expressions. It induces the production of IL-2, IL-5, IL-13, TNF-α, and IFN-γ. IL-33 can also be combined with a single Ig IL-1R-related molecule (SIGIRR), which seems as an inhibitor of the IL-33/ST2 pathway (25).
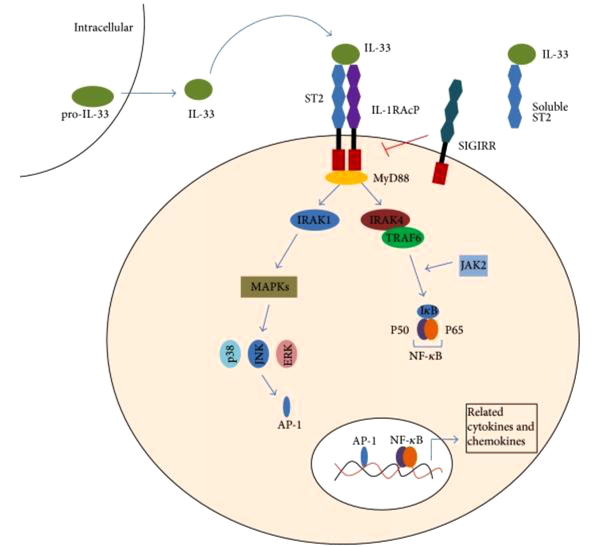
Kakkar’s study has shown that IL-33/ST2 signaling pathway was involved in T cell-mediated immune response and was a potential medium for various inflammatory diseases. IL-33 may function as a modulator of NF-κB and canonical Toll-like receptor/IL-1 receptor signaling (26). Haenuki et al. proposed IL-33 as a therapeutic target in preventing allergic rhinitis, as it disturbs allergic rhinitis responses (27). In another study, Nakanishi et al. found that neutralizing IL-33 can be a potential strategy to treat allergic rhinitis caused by home mites (28). On the other hand, Miller reported that IL-33 could have anti-inflammatory and pro-inflammatory effects, depending on the type of disease and the tested model (23).
2. Objectives
Since IL-33 is one of the recently discovered anti-inflammatory cytokines, there is limited information about changes of this critical cytokine in patients with allergic rhinitis. Accordingly, the present study aimed to measure the serum levels of IL-33 in children suffering from allergic rhinitis and detect its relationship with the disease and its severity.
3. Methods
This case-control study was conducted on a population with the age range of 7-18 years, including healthy individuals and those diagnosed with allergic rhinitis. All study participants had referrals to the pediatric clinic at the Shahid Beheshti Hospital in Kashan, Iran, in 2017. A pediatric immunologist diagnosed allergic rhinitis based on the patient's clinical history and the guidelines set out by the allergic rhinitis and its impacts on asthma (ARIA) (29). A study by Kliegman et al. aimed to determine disease severity (7). According to a study by Gluck et al. (30), the sample size was calculated to be 114 subjects (at least 57 subjects per group). The case and control groups were selected from patients with allergic rhinitis and healthy individuals using the random sampling method.
Inclusion criteria for the case group were being in the age range of 7 - 18 years and being diagnosed with allergic rhinitis. On the other hand, inclusion criteria for the control group were being in the age range of 7 - 18 years and having no symptom of allergic or inflammatory diseases. Exclusion criteria for both groups were as follows: (1) chronic heart, kidney, gastrointestinal, and lung diseases, including bronchopulmonary dysplasia and chronic obstructive pulmonary disease and asthma; (2) infectious diseases such as pneumonia, lung abscess, tuberculosis, and sinusitis; (3) any acute inflammatory disease but allergic rhinitis, such as arthritis, Crohn's disease, and ulcerative colitis; (4) immunodeficiency diseases such as common variable immune deficiency, severe combined immunodeficiency, and hyper-IgM syndrome; (5) congenital diseases such as cystic fibrosis (CF) and malignancies; (6) corticosteroid use in the past three months, and (7) lack of informed consent.
The present study documented demographic information, including age, gender, duration of allergic rhinitis, and family history of allergic diseases, in a prepared checklist after obtaining informed consent from the patients. Intravenous blood (5 cc) was collected from all patients and centrifuged at 10 rpm for 10 minutes. The supernatant was stored in a freezer at -20°C for further analyses. The temperature of the frozen samples was increased by the laboratory temperature after blood sampling, and they were then used for the ELISA assay (ChemWell 2910, Awareness Technology).
The IL-33 kit (7.8 - 500 pg/cc; IBL International GmbH, Germany), with 12 strips and eight wells, was used for experiments. Next, the mean serum level of IL-33 and the severity of the disease were compared between the two groups. According to ethical guidelines, all participants were educated about study methodology and objectives and ensured of data confidentiality. Moreover, they were allowed to quit the study whenever they wished.
The collected data were analyzed with SPSS software version 22. Central tendency and the measures of dispersion were calculated for the quantitative variables, and frequency and percentage were measured for the qualitative variables. The distribution of the quantitative data was assessed using the Kolmogorov-Smirnov test. To investigate the differences in mean serum levels of IL-33 between the two study groups, the independent t-test and Mann-Whitney U tests were performed. Regarding the qualitative demographic characteristics, chi-square and Fisher's exact tests were used to evaluate the group differences.
Moreover, Spearman's test was used to determine the association between the serum levels of IL-33 with different severities of allergic rhinitis. Regression models were used for multivariate analyses. In this study, P < 0.05 was set as the level of significance. The research data are presented as mean values.
4. Results
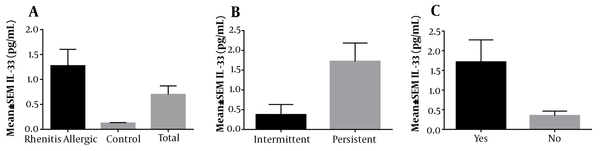
In this study, 114 patients (57 healthy individuals in the control group and 57 patients in the case group) were included. According to the results, the mean age of patients with allergic rhinitis was 9.79 ± 3.57 in the case group and 12.60 ± 3.83 years in the control group, respectively. Among 57 patients with allergic rhinitis, 18 (31.6%) were female, and 39 (68.4%) were male. Moreover, among 57 subjects in the control group, 32 (56.1%) were female, and 25 (43.9%) were male. Data analysis revealed a significant difference between the two study groups in terms of age (P = 0.001) and gender (P = 0.014). As presented in Figure 2A, the mean serum level of IL-33 in patients with allergic rhinitis was 1.27 (ρg/mL), which was significantly higher than that of the control group (0.12 ρg/mL) (P = 0.001). This finding suggested the role of IL-33 in the pathogenesis of the disease.
A, The mean serum levels of IL-33 was 1.27 pg/mL in 57 patients with allergic rhinitis, 0.12 pg/mL in 57 control individuals without allergic rhinitis, and 0.7 pg/mL in all participants. The above result showed a meaningful difference between the studied groups at P = 0.001. B, The mean serum levels of IL-33 were 0.37 pg/mL in patients with intermittent allergic rhinitis and 1.72 pg/mL in patients with persistent allergic rhinitis. There was no relationship between the mean serum level of IL-33 and the duration of symptoms at P = 0.055. C, The mean serum levels of IL-33 were 1.76 pg/mL in patients with comorbidities in the case group and 0.8 pg/mL and 0.46 pg/mL in patients with no comorbidities in both case and control groups. No relationship was noticed between patients with and without comorbidities at P = 0.149.
Moreover, some other variables, including a family history of atopic disease, duration of allergic rhinitis symptoms, and comorbidities (atopic dermatitis and asthma), were examined, and the results are presented in Table 1. As shown in Table 1, there are statistically significant differences between the two groups in terms of a family history of atopic disease, duration of allergic rhinitis symptoms, and comorbidities (atopic dermatitis and asthma).
Family History of Atopic Disease, Duration of Allergic Rhinitis Symptoms, and Comorbidities in Case and Control Groups a
| Variables | Case | Control | P-Value |
|---|---|---|---|
| Family history of atopic disease | < 0.001 | ||
| Positive | 48 (84.2) | 2 (3.5) | |
| Negative | 9 (15.8) | 55 (96.5) | |
| Duration of symptoms | - | ||
| Intermittent | 19 (33.3) | 0 (0) | |
| Continuous | 38 (66.7) | 0 (0) | |
| Comorbidities | < 0.001 | ||
| Yes | 28 (49.1) | 1 (1.8) | |
| No | 29 (50.9) | 56 (98.2) |
Furthermore, the serum levels of IL-33 were investigated in the two groups concerning the duration of symptoms and comorbidities, the results of which are presented in Figures 2B and 2C. As presented Figure 2B, the correlation between the serum levels of IL-33 and duration of symptoms was not statistically significant (P = 0.055). Furthermore, Figure 2C data analysis indicated that the serum levels of IL-33 in patients with comorbidities were not significantly correlated in the case and control groups (P = 0.149).
5. Discussion
Allergic rhinitis is an inflammation of the nasal mucosa. Recent genomic studies have indicated the association between the IL-33 levels with asthma (31) and allergic rhinitis (32). Accordingly, physicians and researchers have paid particular attention to IL-33 due to its effects on allergies. This cytokine also stimulates different types of cells involved in the expression of the membrane ST2 as such it enhances the production of IgE-dependent cytokines and the degranulation of mast cells (33, 34).
Several studies have examined IL-33 and its concentration in patients with allergic rhinitis and some other similar diseases. This study aimed to examine the correlation between the serum levels of IL-33 and allergic rhinitis. The results indicated a significant difference between the control and case groups regarding age, gender, family history of atopy, duration of symptoms, the severity of the disease, and comorbidities. Moreover, there was a statistically significant difference in serum IL-33 levels between the two study groups. More specifically, compared to the control group, patients with allergic rhinitis had higher serum levels of IL-33. Although the serum levels of IL-33 were not significantly correlated with the duration of symptoms or the presence of comorbidities in allergic rhinitis patients. After eliminating the effects of the intervening factors, the difference in the serum level of IL-33 between the case and control groups remained significant.
Moreover, Gluck et al. conducted a study on patients with intermittent allergic rhinitis sensitive to plant pollen, to explore the serum levels of IL-33 and its corresponding ST2 receptor. They found that compared to the control group, the patients in the allergic rhinitis group had significantly higher serum levels of IL-33. The finding was consistent with that of the present study (30). They also reported that IL-33 was associated with disease severity. Accordingly, this cytokine may be effective in the pathogenesis of allergic rhinitis. The serum level of IL-33 seems to be an indicator of Th2-related allergic diseases such as allergic rhinitis. Further, this cytokine is a marker of the severity of allergic rhinitis (30).
Asaka et al. revealed that patients with allergic rhinitis, who are sensitive to Japanese cedar, had higher levels of IL-33 serum in their nasal secretions. Moreover, IL-33 was significantly associated with the total score of nasal symptoms (35). Although this study provided no sufficient evidence, it was suggested that an increase in the level of IL-33 in nasal secretions was associated with the exacerbation of allergic rhinitis (35). The main difference between the method adopted in the aforementioned study and that of the present study was the cytokine measurement method, as they measured nasal secretions while this study measured the serum concentration. Nevertheless, more research is needed to establish the correlation between local eosinophils and IL-33 and determine the mechanisms of pathogenesis.
Recently, IL-33 has been identified as a damage-associated molecular pattern, characterized as a molecule discharged from cells in the necrotic air-filled structure, possibly after an infection or trauma. In this study (35), the level of IL-33 was higher in the common allergy season than the uncommon season, suggesting that the increase in the level of IL-33 is correlated with contact with the allergen and occurs in sensitive individuals.
Haenuki et al. (27) conducted a study in Japan to investigate the function of IL-33 in the development of allergic rhinitis symptoms and found that the primary (sneezing and runny nose) and the final (eosinophil and basophil accumulation) phase symptoms occur after nasal stimulation by the allergen. Subsequently, in the nucleus of the epithelial cells, the IL-33 protein is expressed, resulting in the release of nasal secretions. Basophils and mast cells stimulated by IL-33 intensify the initial and final phases of clinical manifestations caused by the increased histamine release and the production of chemical adsorbents for eosinophils and basophils. Accordingly, the presence of IL-33 is associated with the course of pathogenesis and the symptoms of allergic rhinitis (27).
In a previously mentioned study, the serum levels of IL-33 were measured in a large group of patients suffering from allergic rhinitis, who were also sensitive to Japanese cedar (32). In this group of patients, they found higher serum levels compared to the control group. This is consistent with the results of the present study. Furthermore, this study found that IL-33 and allergic rhinitis were positively correlated. On the whole, in-plant pollen-sensitive animal models, researchers have established the role of IL-33 in the pathogenesis of allergic conjunctivitis (36). In their study, Matsuba-Kitamura et al. provided evidence suggesting that IL-33 could significantly increase T cell capacity to make Th2-type cytokine. This would increase the lymph node secretions in the neck by Th2 cells and eosinophils. The conjunctive tissue then continuously expresses the active form of IL-33; therefore, IL-33 plays a critical role in inducing and increasing allergic conjunctivitis (36).
Moreover, anti-IL-33 antibodies have recently been revealed therapeutic potentials against allergic rhinitis (37). These antibodies clinically decrease the symptoms and reduce the number of eosinophils and Th2-type cytokines in the bronchoalveolar lavage (BAL), leading to a decrease in nasal secretions. This clinical finding can also be extended to the therapeutic potential of IL-33 against human allergies. In general, the result of this study confirms previous research findings in this field, indicating that IL-33 plays a role in Th2-dependent diseases, including allergic rhinitis.
Allergic diseases such as allergic rhinitis and bronchial asthma are activated by Th2-type cytokines, including IL-4, IL-5, and IL-13. Th2 cells secrete these cytokines. Recent research findings suggest that, in addition to acting as a sort of chemical adsorbent for these cells, IL-33 stimulates th2 cells to secrete these cytokines (38). Moreover, the critical role of IL-33 has been confirmed in many inflammatory diseases such as inflammatory bowel diseases, rheumatology, and central nervous system (CNS) inflammatory diseases. Evidence suggests that IL-33 provides a degree of protection against cardiovascular diseases such as type II diabetes and obesity (23, 39). It exerts biological effects by interacting with ST2 and IL-1 sub-protein receptors (40-42). IL-33 also affects some important cells, such as mast cells, in allergic reactions. It degrades mast cells by activating phospholipase D1 and sphingosine kinase 1 (43). Moreover, many studies have determined that IL-33 plays a role in several allergic diseases, including anaphylactic shock, atopic dermatitis, and bronchial asthma (44-46).
5.1. Conclusions
The present study indicated the high serum levels of IL-33 in patients with allergic rhinitis. This finding suggests that IL-33 is involved in the pathogenesis of allergic rhinitis and provides a new perspective on the pathophysiology of this disease and the therapeutic goals. Further studies are recommended to examine the association between the gene expression and the serum level of IL-33 with the severity of allergic rhinitis in children. It is also recommended to compare the serum level of IL-33 between children with allergic rhinitis and those with atopic dermatitis and bronchial asthma.
References
-
1.
Small P, Keith PK, Kim H. Allergic rhinitis. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):51. [PubMed ID: 30263033]. [PubMed Central ID: PMC6156899]. https://doi.org/10.1186/s13223-018-0280-7.
-
2.
Ceylan ME, Cingi C, Özdemir C, Kücüksezer UC, Akdis CA. Pathophysiology of Allergic Rhinitis. All Around the Nose. Springer, Cham; 2020. p. 261-96. https://doi.org/10.1007/978-3-030-21217-9_32.
-
3.
Bousquet J, Dahl R, Khaltaev N. Global alliance against chronic respiratory diseases. Allergy. 2007;62(3):216-23. [PubMed ID: 17298337]. https://doi.org/10.1111/j.1398-9995.2007.01307.x.
-
4.
Tanno LK, Calderon MA, Demoly P, Allergy A. Optimization and simplification of the Allergic and Hypersensitivity conditions classification for the ICD-11. Allergy. 2016;71(5):671-6. [PubMed ID: 26728868]. https://doi.org/10.1111/all.12834.
-
5.
Lei DK, Saltoun C. Allergen immunotherapy: definition, indications, and reactions. Allergy Asthma Proc. 2019;40(6):369-71. [PubMed ID: 31690372]. https://doi.org/10.2500/aap.2019.40.4249.
-
6.
Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012;106(1):9-14. [PubMed ID: 22112783]. https://doi.org/10.1016/j.rmed.2011.09.007.
-
7.
Kliegman RM, Stanton BF, Geme JW, Schor NF. Nelson Textbook of Pediatrics. Allergic Rhinitis 1. 20th ed. Philadelphia: Elsevier; 2016. 1088 p.
-
8.
Gabet S, Ranciere F, Just J, de Blic J, Lezmi G, Amat F, et al. Asthma and allergic rhinitis risk depends on house dust mite specific IgE levels in PARIS birth cohort children. World Allergy Organ J. 2019;12(9):100057. [PubMed ID: 31641405]. [PubMed Central ID: PMC6796773]. https://doi.org/10.1016/j.waojou.2019.100057.
-
9.
Öçal R, Bayar Muluk N, Mullol J. Epidemiology of Allergic Rhinitis. All Around the Nose. Springer, Cham; 2020. p. 297-301.
-
10.
Nathan RA, Meltzer EO, Derebery J, Campbell UB, Stang PE, Corrao MA, et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008;29(6):600-8. [PubMed ID: 19173786]. https://doi.org/10.2500/aap.2008.29.3179.
-
11.
Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med. 2015;372(5):456-63. [PubMed ID: 25629743]. [PubMed Central ID: PMC4324099]. https://doi.org/10.1056/NEJMcp1412282.
-
12.
Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011;378(9809):2112-22. [PubMed ID: 21783242]. https://doi.org/10.1016/S0140-6736(11)60130-X.
-
13.
Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16(11):676-89. [PubMed ID: 27640624]. https://doi.org/10.1038/nri.2016.95.
-
14.
Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89-102. [PubMed ID: 20081871]. https://doi.org/10.1038/nri2691.
-
15.
Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7(6):321-9. [PubMed ID: 21519352]. https://doi.org/10.1038/nrrheum.2011.53.
-
16.
Ali S, Mohs A, Thomas M, Klare J, Ross R, Schmitz ML, et al. The dual function cytokine IL-33 interacts with the transcription factor NF-kappaB to dampen NF-kappaB-stimulated gene transcription. J Immunol. 2011;187(4):1609-16. [PubMed ID: 21734074]. https://doi.org/10.4049/jimmunol.1003080.
-
17.
Kurokawa M, Matsukura S, Kawaguchi M, Ieki K, Suzuki S, Watanabe S, et al. Interleukin-33-activated dendritic cells induce the production of thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Arch Allergy Immunol. 2013;161 Suppl 2:52-7. [PubMed ID: 23711854]. https://doi.org/10.1159/000350363.
-
18.
Su Z, Lin J, Lu F, Zhang X, Zhang L, Gandhi NB, et al. Potential autocrine regulation of interleukin-33/ST2 signaling of dendritic cells in allergic inflammation. Mucosal Immunol. 2013;6(5):921-30. [PubMed ID: 23299617]. [PubMed Central ID: PMC3904307]. https://doi.org/10.1038/mi.2012.130.
-
19.
Blom L, Poulsen BC, Jensen BM, Hansen A, Poulsen LK. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PLoS One. 2011;6(7). e21695. [PubMed ID: 21765905]. [PubMed Central ID: PMC3130774]. https://doi.org/10.1371/journal.pone.0021695.
-
20.
Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, et al. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52(1-2):89-99. [PubMed ID: 22392053]. https://doi.org/10.1007/s12026-012-8283-9.
-
21.
Louten J, Rankin AL, Li Y, Murphy EE, Beaumont M, Moon C, et al. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int Immunol. 2011;23(5):307-15. [PubMed ID: 21422152]. https://doi.org/10.1093/intimm/dxr006.
-
22.
Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20(8):1019-30. [PubMed ID: 18550585]. https://doi.org/10.1093/intimm/dxn060.
-
23.
Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond). 2011;8(1):22. [PubMed ID: 21871091]. [PubMed Central ID: PMC3175149]. https://doi.org/10.1186/1476-9255-8-22.
-
24.
Nile CJ, Barksby E, Jitprasertwong P, Preshaw PM, Taylor JJ. Expression and regulation of interleukin-33 in human monocytes. Immunology. 2010;130(2):172-80. [PubMed ID: 20070408]. [PubMed Central ID: PMC2878462]. https://doi.org/10.1111/j.1365-2567.2009.03221.x.
-
25.
Zhao Q, Chen G. Role of IL-33 and its receptor in T cell-mediated autoimmune diseases. Biomed Res Int. 2014;2014:587376. [PubMed ID: 25032216]. [PubMed Central ID: PMC4084552]. https://doi.org/10.1155/2014/587376.
-
26.
Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7(10):827-40. [PubMed ID: 18827826]. [PubMed Central ID: PMC4277436]. https://doi.org/10.1038/nrd2660.
-
27.
Haenuki Y, Matsushita K, Futatsugi-Yumikura S, Ishii KJ, Kawagoe T, Imoto Y, et al. A critical role of IL-33 in experimental allergic rhinitis. J Allergy Clin Immunol. 2012;130(1):184-94 e11. [PubMed ID: 22460070]. https://doi.org/10.1016/j.jaci.2012.02.013.
-
28.
Nakanishi W, Yamaguchi S, Matsuda A, Suzukawa M, Shibui A, Nambu A, et al. IL-33, but not IL-25, is crucial for the development of house dust mite antigen-induced allergic rhinitis. PLoS One. 2013;8(10). e78099. [PubMed ID: 24205109]. [PubMed Central ID: PMC3808342]. https://doi.org/10.1371/journal.pone.0078099.
-
29.
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63 Suppl 86:8-160. [PubMed ID: 18331513]. https://doi.org/10.1111/j.1398-9995.2007.01620.x.
-
30.
Gluck J, Rymarczyk B, Rogala B. Serum IL-33 but not ST2 level is elevated in intermittent allergic rhinitis and is a marker of the disease severity. Inflamm Res. 2012;61(6):547-50. [PubMed ID: 22349136]. [PubMed Central ID: PMC3345109]. https://doi.org/10.1007/s00011-012-0443-9.
-
31.
Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211-21. [PubMed ID: 20860503]. [PubMed Central ID: PMC4260321]. https://doi.org/10.1056/NEJMoa0906312.
-
32.
Sakashita M, Yoshimoto T, Hirota T, Harada M, Okubo K, Osawa Y, et al. Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin Exp Allergy. 2008;38(12):1875-81. [PubMed ID: 19037964]. https://doi.org/10.1111/j.1365-2222.2008.03114.x.
-
33.
Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179(4):2051-4. [PubMed ID: 17675461]. https://doi.org/10.4049/jimmunol.179.4.2051.
-
34.
Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181(9):5981-9. [PubMed ID: 18941187]. https://doi.org/10.4049/jimmunol.181.9.5981.
-
35.
Asaka D, Yoshikawa M, Nakayama T, Yoshimura T, Moriyama H, Otori N. Elevated levels of interleukin-33 in the nasal secretions of patients with allergic rhinitis. Int Arch Allergy Immunol. 2012;158 Suppl 1:47-50. [PubMed ID: 22627366]. https://doi.org/10.1159/000337764.
-
36.
Matsuba-Kitamura S, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Taki Y, Muto T, et al. Contribution of IL-33 to induction and augmentation of experimental allergic conjunctivitis. Int Immunol. 2010;22(6):479-89. [PubMed ID: 20501612]. https://doi.org/10.1093/intimm/dxq035.
-
37.
Kim YH, Yang TY, Park CS, Ahn SH, Son BK, Kim JH, et al. Anti-IL-33 antibody has a therapeutic effect in a murine model of allergic rhinitis. Allergy. 2012;67(2):183-90. [PubMed ID: 22050307]. https://doi.org/10.1111/j.1398-9995.2011.02735.x.
-
38.
Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37(10):2779-86. [PubMed ID: 17853410]. https://doi.org/10.1002/eji.200737547.
-
39.
Miller AM, Asquith DL, Hueber AJ, Anderson LA, Holmes WM, McKenzie AN, et al. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res. 2010;107(5):650-8. [PubMed ID: 20634488]. [PubMed Central ID: PMC4254700]. https://doi.org/10.1161/CIRCRESAHA.110.218867.
-
40.
Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95(12):6930-5. [PubMed ID: 9618516]. [PubMed Central ID: PMC22690]. https://doi.org/10.1073/pnas.95.12.6930.
-
41.
Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187(5):787-94. [PubMed ID: 9480988]. [PubMed Central ID: PMC2212173]. https://doi.org/10.1084/jem.187.5.787.
-
42.
Lecart S, Lecointe N, Subramaniam A, Alkan S, Ni D, Chen R, et al. Activated, but not resting human Th2 cells, in contrast to Th1 and T regulatory cells, produce soluble ST2 and express low levels of ST2L at the cell surface. Eur J Immunol. 2002;32(10):2979-87. [PubMed ID: 12355452]. https://doi.org/10.1002/1521-4141(2002010)32:10<2979::AID-IMMU2979>3.0.CO;2-5.
-
43.
Pushparaj PN, Tay HK, H'Ng S C, Pitman N, Xu D, McKenzie A, et al. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A. 2009;106(24):9773-8. [PubMed ID: 19506243]. [PubMed Central ID: PMC2700978]. https://doi.org/10.1073/pnas.0901206106.
-
44.
Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164(2):277-81. [PubMed ID: 11463601]. https://doi.org/10.1164/ajrccm.164.2.2008120.
-
45.
Ali M, Zhang G, Thomas WR, McLean CJ, Bizzintino JA, Laing IA, et al. Investigations into the role of ST2 in acute asthma in children. Tissue Antigens. 2009;73(3):206-12. [PubMed ID: 19254249]. https://doi.org/10.1111/j.1399-0039.2008.01185.x.
-
46.
Shimizu M, Matsuda A, Yanagisawa K, Hirota T, Akahoshi M, Inomata N, et al. Functional SNPs in the distal promoter of the ST2 gene are associated with atopic dermatitis. Hum Mol Genet. 2005;14(19):2919-27. [PubMed ID: 16118232]. https://doi.org/10.1093/hmg/ddi323.