Abstract
Keywords
1. Introduction
Tuberculosis is the second leading cause of death from infectious diseases globally, especially in developing countries, accounting for around 1.5 million people each year. Some deaths are also due to the increasing rate of HIV infection (1, 2). One of the tuberculosis manifestations is tuberculous meningoencephalitis, accounting for as much as 5% of extrapulmonary tuberculosis cases. Tuberculous meningoencephalitis often presents with atypical symptoms, sometimes leading to diagnosis and therapy delays (3-5).
In Indonesia, the number of tuberculosis cases found and treated in 2020 was 344,992, with 11% being extrapulmonary tuberculosis (6). Emerging at the end of 2019, COVID-19 has infected 60 million people and caused 1.5 million deaths worldwide. In Indonesia, COVID-19 has infected 600,000 people, leading to 19,000 deaths. Besides, COVID-19 has been shown to affect various organs (7). An exaggerated inflammatory response causes damage to these organs. Although most symptoms are in the respiratory system, neurological manifestations are often found, proving that COVID-19 may act as an opportunistic pathogen in the nervous system, especially the brain (6, 8, 9). Several neurological manifestations were found, including stroke, polyneuropathy, myopathy, and seizures, but there are still limited data about COVID-19 in tuberculous meningoencephalitis (4, 9).
Several case reports have shown a close link between tuberculosis infection and COVID-19. Risk factors are old age and several comorbidities, such as diabetes mellitus and chronic respiratory disease, closely associated with poor outcomes in tuberculosis and COVID-19 (10, 11). In a cohort study conducted by Tadolini et al., 38.8% of tuberculosis patients receiving anti-tuberculosis therapy were still at risk of COVID-19 infection, and higher mortality rate, especially elderly patients (2). This case report discusses the presence of COVID-19 infection in a young tuberculous meningoencephalitis patient already receiving anti-tuberculosis drugs with poor treatment response and outcome.
2. Case Presentation
A 21-year-old woman diagnosed with tuberculous meningoencephalitis in the intensive phase of tuberculous therapy presented with a complaint of dysphagia from one day before admission. The patient complained of difficulty swallowing drinks and soft foods. The patient also complained of dysphonia that appeared simultaneously. There were cough and fever for two days. There were other complaints such as double vision, improving at the beginning of tuberculosis therapy but worsening a few days before admission. Headache and neck stiffness are still present.
The patient had been diagnosed with tuberculous meningoencephalitis for one month since August 2020. Due to hydrocephalus complications, a ventriculoperitoneal (VP) shunting was performed on the patient at the first hospitalization. The patient received oral anti-tuberculosis (streptomycin, rifampin, isoniazid, and pyrazinamide). When first diagnosed, the patient also had a focal to bilateral motor onset tonic-clonic seizure and was routinely receiving the oral antiepilepsy drug (phenytoin). The patient was previously diagnosed with tuberculous lymphadenitis and was finished its treatment.
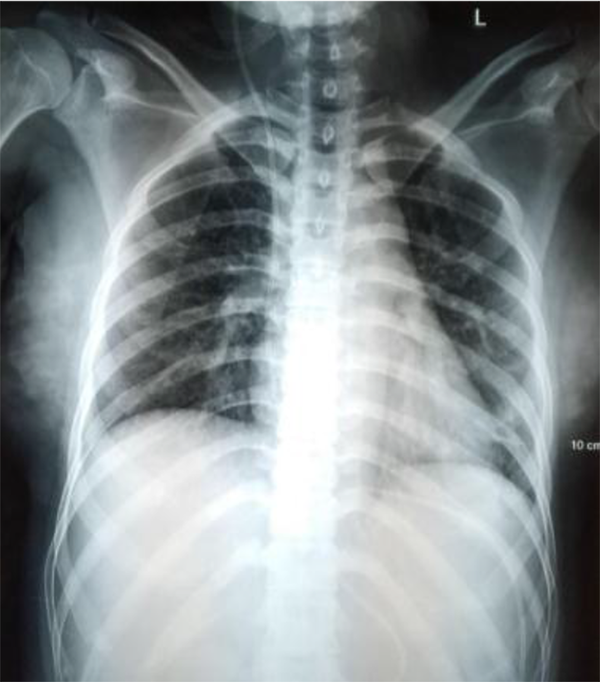
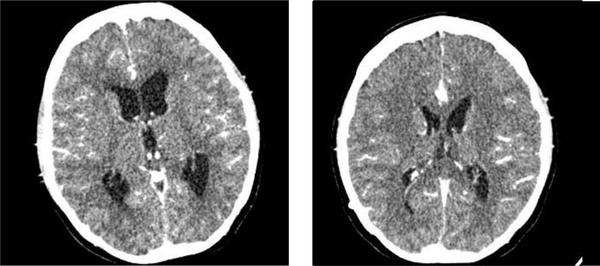
We found neck stiffness as a meningeal sign. There were also left abducens nerve, right glossopharyngeal nerve, and right vagus nerve palsy. Besides, there were bidirectional nystagmus and dysmetria on the right side. On laboratory examination, euvolemic hypoosmolar hyponatremia was found, and the presence of reactive COVID-19 rapid test results was confirmed by a positive PCR test from the second day after readmission. On CSF examination, we found an increase in protein by 731 mg/dL, low glucose 14 mg/dL, and a predominance of MN of 90% with positive Nonne's and Pandy's tests, which was typical for tuberculous meningoencephalitis infection. The patient was tested with Gene X pert for tuberculosis in the CSF and sputum, without resistance to anti-tuberculosis drugs. The CXR results showed pneumonia and minimal effusion in the left lung field (Figure 1). Head CT scan showed increased leptomeningeal enhancement compared to the CT scan one month earlier. There was a hypodense appearance in the left temporal lobe and head CT, probably due to vasculitis. The VP shunt was still in place and did not show any signs of hydrocephalus (Figure 2).
Chest X-ray AP projection. Pneumonia and minimal left pleural effusion are seen.
Head CT of patients with and without contrast in August 2020 (right) and September 2020 (left). Appropriate with tuberculous meningoencephalitis with increased leptomeningeal enhancement. Subacute infarction of the left temporal lobe is possibly due to vasculitis. There are no visible features of hydrocephalus and VP shunt on site.
Later, the patient was diagnosed with tuberculous meningoencephalitis with vasculitis and confirmed COVID-19 infection. The patient was treated with intravenous dexamethasone, anti-tuberculosis drugs, phenytoin, aspirin, N-acetylcysteine, oseltamivir, and hydroxychloroquine. We give oseltamivir and hydroxychloroquine to patients because these therapies are used in the early phase of the COVID-19 pandemic in Indonesia, where there is no available therapy according to the recommendations of the FDA. The patient was treated with high flow oxygen at the beginning of her treatment. The patient was intubated on the third day of hospitalization because oxygen saturation dropped to 78%. The patient died four days after hospitalization due to respiratory failure.
3. Discussion
Tuberculous meningoencephalitis is the most common manifestation of TB in the central nervous system associated with high sequelae and mortality if not treated properly (12). In this study, we discussed a 21-year-old woman diagnosed with tuberculous meningoencephalitis and was still in the intensive phase of therapy, presenting with new complaints of dysphagia and dysphonia. The patient also had worsening complaints of diplopia, headache, and fever. Tuberculous meningoencephalitis often occurs in patients with immunodeficiency due to malnutrition, aging, malignancy, and HIV infection. Patients may also have another type of tuberculosis manifestation, such as pulmonary tuberculosis, occurring in 50% of the patients (12, 13). In this patient, there was a history of tuberculous lymphadenitis with completed previous therapy.
Tuberculous meningoencephalitis is characterized by a subacute onset with prodromal symptoms such as fever, headache, and vomiting a few weeks earlier, showing severe headache symptoms, altered consciousness, stroke, hydrocephalus symptoms, and neuropathy of cranial nerves (12). In this patient, there were symptoms characteristic of TB meningoencephalitis, such as headache, fever, neck stiffness, left abducens, right glossopharyngeal, and right vagus nerve palsy. In addition, one could find bidirectional nystagmus and dysmetria on the right side. The complaint of tuberculous meningoencephalitis is dominated by the rupture of the subependymal or subpial tubercles into the subarachnoid space. Tuberculous meningoencephalitis primarily affects the cerebellum, brain stem, and basal cisterns by forming a gelatinous exudate around the area, inducing edema and perivascular infiltration. In addition, there is also an inflammatory mechanism in the walls of blood vessels that can cause infarction and bleed due to damage to these vessels (12, 13).
This patient also had a focal to bilateral motor onset tonic-clonic seizure when first diagnosed and was routinely receiving phenytoin. Seizures are one of the symptoms of tuberculous meningoencephalitis, which is quite frequent in 17 - 93% of cases. The seizures can be either acute symptomatic or unprovoked seizures. The cause of these seizures is mainly the inflammation of the brain, nerve damage, and activation of glial cells (13, 14). This patient also showed a subacute infarction in the temporal lobe, probably due to vasculitis and appropriated with the semiology of the patient's focal to bilateral motor onset tonic clonic seizures.
In the clinical picture of the patient described previously, there was a worsening of the symptoms, especially cranial nerve disorders with previously existing deficits. In the chest CXR, there was a picture of pneumonia and minimal effusion in the left lung field with a positive rapid test and confirmed COVID-19 infection, which was not found in the previous admission. In the head CT scan, the meningoencephalitis process worsened, marked by an increase in the leptomeningeal enhancement compared with the previous head CT.
The poor clinical outcome of tuberculous meningoencephalitis may be correlated with COVID-19 infection. Several studies have suggested that tuberculosis and viral respiratory infections affect the immune response, where both synergisms can worsen the patient's clinical condition (1, 15). In a meta-analysis, patients with tuberculosis showed 2.1 times more risk of developing severe COVID-19 infection. Tuberculosis infection in people with systemic lupus erythematosus (SLE) shows a longer duration of illness with high CRP levels and low CD4+ counts (11). This suggests that tuberculosis infection can suppress the immune system, increasing the risk of infection with other pathogens and worsening outcomes. Besides, despite being very important, steroid therapy in tuberculous meningoencephalitis patients can also aggravate the immunosuppressive condition in these patients (4, 16).
Some evidence suggests that COVID-19 can initiate excessive inflammation by increasing the secretion of IL-1b, IFN-y, TNF-a, IL-2, IL-4, and IL-10, which, in turn, will cause a cytokine storm. This cytokine storm can correlate with the ability of TB to invade the brain by decreasing the blood-brain barrier ability. In a mouse model study, COVID-19 was able to reactivate dormant Mycobacterium tuberculosis from mesenchymal stem cells CD271+3. Several studies found an increased secretion of type I interferon (such as IFN-1 and IFN-b) occurring in the early stages of viral infection. However, IFN type I can decrease the ability of macrophages to respond to IFN-y, which is essential for controlling the intracellular growth of M. tuberculosis (11, 16).
Apart from increasing the risk of reactivation and increasing the pathogenicity of M. tuberculosis, COVID-19 itself can be neuroinvasive or induce immune reactions, damaging the nervous system. Besides, COVID-19 can invade the CNS via the hematogenous route or by retrograde synaptic transmission through the olfactory nerves route. The COVID-19 receptor, ACE-2, is highly expressed in the CNS. Meningoencephalitis was associated with COVID-19 infection in several cases, but PCR results from the CSF were negative. Another mechanism can explain the negative PCR result. COVID-19-related encephalitis is probably caused by immune mediation after infection or para-infection. The presence of respiratory failure can also explain the poor prognosis of COVID-19 in CNS infection due to the involvement of medullary respiratory centers (4, 8). Coinfection with M. tuberculosis as tuberculous meningoencephalitis and COVID-19 can result in poor outcomes in cases of tuberculous meningoencephalitis receiving adequate anti-tuberculous therapy.
References
-
1.
Crisan-Dabija R, Grigorescu C, Pavel CA, Artene B, Popa IV, Cernomaz A, et al. Tuberculosis and COVID-19: Lessons from the Past Viral Outbreaks and Possible Future Outcomes. Can Respir J. 2020;2020:1401053. [PubMed ID: 32934758]. [PubMed Central ID: PMC7479474]. https://doi.org/10.1155/2020/1401053.
-
2.
Tadolini M, Garcia-Garcia JM, Blanc FX, Borisov S, Goletti D, Motta I, et al. On tuberculosis and COVID-19 co-infection. Eur Respir J. 2020;56(2). [PubMed ID: 32586888]. [PubMed Central ID: PMC7315815]. https://doi.org/10.1183/13993003.02328-2020.
-
3.
Freij BJ, Gebara BM, Tariq R, Wang AM, Gibson J, El-Wiher N, et al. Fatal central nervous system co-infection with SARS-CoV-2 and tuberculosis in a healthy child. BMC Pediatr. 2020;20(1):429. [PubMed ID: 32907595]. [PubMed Central ID: PMC7479402]. https://doi.org/10.1186/s12887-020-02308-1.
-
4.
Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135-53. [PubMed ID: 32561990]. [PubMed Central ID: PMC7304377]. https://doi.org/10.1007/s00415-020-09990-2.
-
5.
Munir B, Prayudi F, Setianto CA, S S. Factors Affecting Prognosis of Tuberculous Meningitis in Saiful Anwar General Hospital Malang. Malang Neurol J. 2020;6(1):1-4. https://doi.org/10.21776/ub.mnj.2020.006.01.1.
-
6.
Munir B, Rianawati SB, Kurniawan SN, Santoso WM, Arisetijono E, Candradikusuma D, et al. Neurological Manifestation on Hospitalized Patient with Probable Covid-19 in Saiful Anwar Hospital Indonesia (Serial Cases). Malang Neurol J. 2020;6(2):51-5. https://doi.org/10.21776/ub.mnj.2020.006.02.1.
-
7.
World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Geneva, Switerland: World Health Organization; 2021, [cited 2021].
-
8.
Yachou Y, El Idrissi A, Belapasov V, Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci. 2020;41(10):2657-69. [PubMed ID: 32725449]. [PubMed Central ID: PMC7385206]. https://doi.org/10.1007/s10072-020-04575-3.
-
9.
Assari S. COVID-19 Pandemic and Neurological Disease: A Critical Review of the Existing Literature. Hosp Pract Res. 2020;5(3):81-6. [PubMed ID: 33094214]. [PubMed Central ID: PMC7577209].
-
10.
Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione MC. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J. 2020;56(1). [PubMed ID: 32482787]. [PubMed Central ID: PMC7263070]. https://doi.org/10.1183/13993003.01708-2020.
-
11.
Mandal N, De N, Jana P, Sannigrahi A, Chattopadhyay K. Correlation between CNS Tuberculosis and the COVID-19 Pandemic: The Neurological and Therapeutic Insights. ACS Chem Neurosci. 2020;11(18):2789-92. [PubMed ID: 32880441]. https://doi.org/10.1021/acschemneuro.0c00546.
-
12.
Marx GE, Chan ED. Tuberculous meningitis: diagnosis and treatment overview. Tuberc Res Treat. 2011;2011:798764. [PubMed ID: 22567269]. [PubMed Central ID: PMC3335590]. https://doi.org/10.1155/2011/798764.
-
13.
Mucaj S, Dreshaj S, Kabashi S, Hundozi H, Gashi S, Zhjeqi V, et al. Tuberculous meningoencephalitis. Med Arh. 2010;64(3):189-90. [PubMed ID: 20645518].
-
14.
Tan JL, Nordin S, Besari AM. Rare Clinical Presentation of Tuberculous Meningitis: A Case Report. Malays J Med Sci. 2017;24(5):119-23. [PubMed ID: 29386980]. [PubMed Central ID: PMC5772823]. https://doi.org/10.21315/mjms2017.24.5.14.
-
15.
Zandifar S, Zandifar Z. Acute viral encephalitis associated with SARS-CoV-2. Ann Clin Case Rep. 2020;5:1845.
-
16.
Ata F, Yousaf Q, Veliyankodan Parambil J, Parengal J, Mohamedali MG, Yousaf Z. A 28-Year-Old Man from India with SARS-Cov-2 and Pulmonary Tuberculosis Co-Infection with Central Nervous System Involvement. Am J Case Rep. 2020;21. e926034. [PubMed ID: 32813683]. [PubMed Central ID: PMC7458692]. https://doi.org/10.12659/AJCR.926034.