Abstract
Background:
Genital tract infection is one of the main causes of male infertility. The increasing frequency of drug resistance and side effects of antibiotics have urged researchers to seek alternative sources of antimicrobial agents, such as medicinal herbs, for the treatment of antibiotic-resistant infections.Objectives:
The present study aimed to determine the frequency of potentially pathogenic microorganisms in the semen of infertile men and investigate the antimicrobial activity of Silybum marianum extract against the isolates.Methods:
Semen samples were collected from 96 infertile men who referred to the Pasteur Laboratory in Gorgan, Iran. Semen samples were first analyzed according to the World Health Organization guidelines and then underwent microbiological tests to identify pathogens. Antimicrobial susceptibility to S. marianum extract was evaluated using the disk diffusion (Kirby-Bauer) method. Moreover, the active components of the extract were identified by the gas chromatography-mass spectrometry technique.Results:
In the semen analysis, 64% of the samples had problems in the parameters of sperm count, motility, and morphology. In addition, the bacterial contamination was observed in 36% of semen samples. The most and the least common isolates were Staphylococcus aureus (45%) and Klebsiella pneumoniae (11%) and Staphylococcus epidermidis (11%), respectively. In Gram-positive isolates, S. aureus was mostly resistant to azithromycin (57%). In Gram-negative isolates, Escherichia coli was mostly resistant to gentamicin (37%). The inhibitory activity of S. marianum flower extract was significantly higher than that of S. marianum leaf extract (P < 0.01). Silybin (2.64%) and silychristin (3.07%) were the most abundant constituents of S. marianum flower extract.Conclusions:
Bacterial infections play an important role in male infertility and S. marianum extract after purification can be potentially used for the treatment of antibiotic-resistant genital tract infections.Keywords
Semen Infection Infertility Antibiotic Resistance Silybum marianum
1. Background
Infertility can increase sexual dysfunction and reduce sexual satisfaction and the frequency of sexual activity. For many people, infertility is a major crisis and source of psychological stress that can cause emotional stress and a range of negative psychological reactions, including depression, anxiety, worry, anger, shame, jealousy, loneliness, despair, low self-esteem, and imbalance. Emotionally, it can lead to feelings of inadequacy and sexual dysfunction. Infertility is a complex life crisis that is psychologically threatening and emotionally stressful. Due to the impact of infertility on human life, it has become an important part of medicine and research in the medical community (1).
It has been reported that one in every four couples is affected by infertility. About 40% of these cases are related to “male factor” infertility. One of the most important causes of male infertility is the genital tract infection, which can directly affect the sperm count, motility, and morphological normality (2). These infections can also damage the testicles and stimulate inflammatory and autoimmune reactions, all of which can lead to male infertility. A wide range of microorganisms is involved in the development of male infertility (3). Enterococci and E. coli are often found in the semen and usually lower sperm parameters compared to normal. Staphylococci are bacteria often found in the urethra of men and can contaminate the semen (4). Generally, bacterial infections and subsequent sperm agglutination can result in reduced sperm motility, epididymitis, prostatitis, and impaired spermatogenesis. Despite the favorable effects of antibiotic therapy on sperm parameters, the increasing rate of antibiotic resistance has made the treatment of such infections extremely difficult (5, 6).
Nowadays, the use of medicinal herbs and traditional medicine is recognized as an important aspect of global health promotion. Medicinal plants comprise a variety of biocompatible and nontoxic compounds that can exert therapeutic effects without unwanted side effects (7). Silybum marianum is a commonly used medicinal plant from the family Compositae that grows wild in various parts of the world, including the northern, western, and southern areas of Iran. Silymarin is the active compound of S. marianum seeds that contain flavonolignans, including silybin, isosilybin, silychristin, and silydianin. These compounds possess antioxidant, anti-inflammatory, anti-arthritic, and antimicrobial properties (8).
2. Objectives
This study aimed to investigate the in vitro effects of S. marianum extract on microbial agents isolated from the semen of infertile men.
3. Methods
3.1. Isolation of Microorganisms
In this study, 96 semen samples were collected from men aged 20 - 35 years who were referred to the Pasteur Laboratory in Gorgan, Iran, between February 2018 to May 2019. The study procedures were approved by the Ethics Committee of Islamic Azad University, AliAbad branch (code No. 1398.008), Golestan, Iran, and were performed according to medical ethics standards. The semen analysis and culture were performed to identify possible genital bacterial pathogens in men with no sex in the past 3 - 5 days. The study also included a control group consisting of 50 semen samples taken from men with proven fertility and no history of infection. The samples were incubated for 30 minutes at 37ºC before Papanicolaou staining. The semen volume, time of fluidization, pH, concentration, morphology, motility, and white blood cell count were determined according to the World Health Organization guidelines (9).
To identify microorganisms, 10 µL of each semen sample was transferred onto blood agar and eosin methylene blue agar (Merck, Germany) using sterile pipette tips. After incubation at 37ºC for 24 hours, bacterial strains were identified using catalase, coagulase, oxidase, and differential tests. The samples were also cultured on Sabrodextrose agar (Biolife, Italy) and incubated at 32ºC for 2 - 5 days to isolate possible fungal agents.
The antibiotic resistance pattern of Gram-positive and Gram-negative bacterial isolates was determined by the disk diffusion (Kirby-Bauer) test using the following antibiotic disks: vancomycin (30 µg), cefazolin (30 µg), doxycycline (30 µg), azithromycin (15 µg), ceftriaxone (30 µg), gentamicin (10 µg), levofloxacin (5 µg), and cefixime (5 µg). All antibiotic disks were purchased from PadTanTeb Co., Iran. The results were analyzed according to the standards described by the Clinical and Laboratory Standards Institute (M100-S25) in 2015 (10).
3.2. Preparation of Silybum marianum Extract
The plant was collected from Ziarat village in Gorgan, northern Iran, and confirmed in the herbarium of the Islamic Azad University of Gorgan. Then, the leaves and flowers of the plant were separated, dried, and powdered completely. The ethanolic extract of the plant was obtained by maceration. For this purpose, the obtained powder was mixed with a 1:10 volume of 70% ethanol in an aluminum-coated Erlenmeyer flask at room temperature. The mixture was placed on a shaker at 130ºC for 48 hours and then passed through a Whatman No. 4 filter paper (USA). The solvent was separated from the obtained extract using a vacuum rotary evaporator at 60ºC. Next, the extract was evaporated to be dried under vacuum at 45ºC and 25 mmHg. Finally, the extract was passed through 0.45-micron filters and stored in a sterile dark glass container in a refrigerator.
3.3. Identification of Silybum marianum Extract Components
First, the extract was incubated at -20ºC for 48 hours to remove wax and fat. After filtration, the mixture was mixed with an equal volume of dichloromethane for 2 hours at 286 rpm. The obtained uniformed mixture was left for 15 minutes until separation into two phases. Finally, the dichloromethane phase was injected into a Gas Chromatography-Mass Spectrometry (GC-MS) instrument (Agilent, USA) to identify compounds.
3.4. Determination of Antibacterial Effect of Silybum marianum Extract by Agar Well Diffusion Method
To prepare the initial concentrations of flower (F1) and leaf (L1), the dried leaf and flower extract was inoculated with 1% dimethyl sulfoxide and distilled water to obtain a final concentration of 500 mg/mL. The resulting mixtures were then diluted to 250 mg/mL and labeled as F2 and L2. A bacterial suspension with a density equal to 0.5 McFarland standard was cultured on Mueller-Hinton agar (Merck, Germany). Five wells with a diameter of 5 mm were created on the agar plate and were inoculated with 50 µL of the initial and diluted concentrations of the leaf and flower extracts (B1, B2, G1, G2). The plates were incubated at 37ºC for 24 hours, and the diameters of growth inhibition zones were measured. A well containing only distilled water was considered as the negative control. An inhibition zone diameter of < 12 mm and < 10 mm indicated sensitivity and resistance, respectively. Data were analyzed by SPSS-16 using the Kruskal-Wallis nonparametric test. In addition, the Microsoft Excel (2010) software was used to calculate the difference of means and to draw tables and charts. A p-value of less than 0.01 was considered statistically significant.
4. Results
Of 96 semen samples collected from infertile men, 35 samples (36%) were culture-positive. Of these samples, 16 (45%) were positive for Staphylococcus aureus, 11 (33%) for Escherichia coli, four (11%) for Klebsiella, and four (11%) for Staphylococcus epidermidis. No fungi were isolated from the samples. Most positive cultures (38.8%) were related to men aged 27 - 30 years, while the frequency of positive cultures was lowest among samples collected from those aged 20 - 25 years.
4.1. Results of Semen Analysis
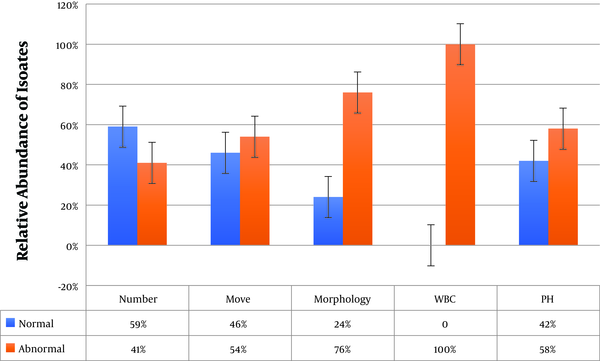
The frequency of sperm count, motility, and morphology problems was 41%, 54%, and 76% among culture-positive semen samples, respectively. In addition, 64% of the samples had problems in all three parameters. The WBC count was between 6 and 18 in culture-positive samples, which was higher than the normal range (0 - 1). Moreover, the pH of 42% of the samples was higher than the normal range (7.1 - 8.0) (Figure 1).
Results of semen analysis of infertile men
4.2. Results of Antibiotic Susceptibility Testing
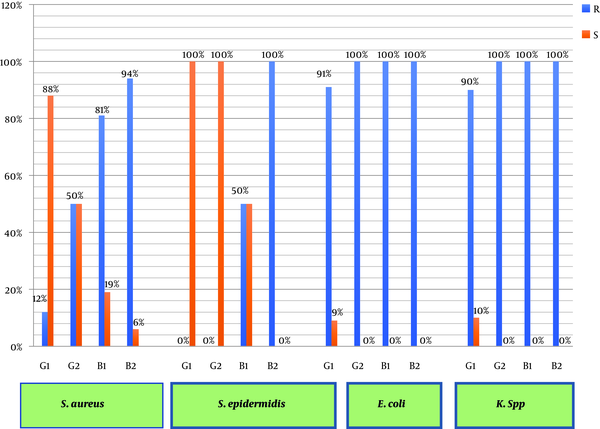
In Gram-positive bacteria, the highest rates of resistance and susceptibility among S. aureus isolates were observed against erythromycin (57%) and vancomycin (100%), respectively. In Gram-negative bacteria, the highest rates of resistance and susceptibility among E. coli isolates were related to gentamicin (37%) and ceftriaxone (100%), respectively (Table 1).
| Bacteria | Cefazolin | Doxycycline | Azithromycin | Vancomycin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | I | S | R | I | S | R | I | S | R | I | S | |
| S. epidermidis | 3 (75) | - | 1 (25) | 1 (25) | - | 3 (75) | 2 (50) | - | 2 (100) | - | - | 4 (100) |
| S. aureus | 2 (12) | 3 (19) | 11 (69) | 7 (44) | 2 (12) | 7 (44) | 9 (57) | 1 (4) | 6 (39) | - | - | 16 (100) |
| Ceftriaxone | Levofloxacin | Cefixime | Gentamicin | |||||||||
| R | I | S | R | I | S | R | I | S | R | I | S | |
| E. coli | 1 (5) | 1 (5) | 9 (90) | 3 (27) | - | 8 (73) | 2 (13) | 2 (13) | 7 (64) | 4 (37) | 1 (8) | 6 (55) |
| Klebsiella spp. | - | - | 4 (100) | 1 (25) | - | 3 (75) | 1 (25) | 1 (25) | 2 (50) | 1 (25) | - | 3 (75) |
4.3. Antibacterial Effect of S. marianum Extract
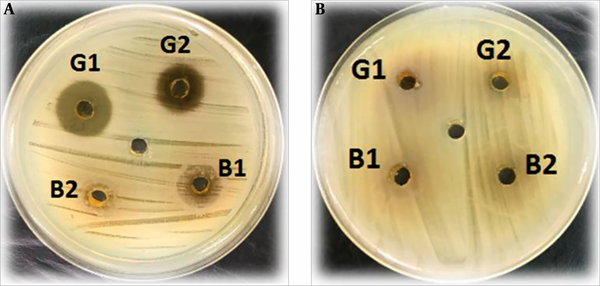
Based on the results, the inhibitory properties of S. marianum was more significant against Gram-positive bacteria than against Gram-negative bacteria. In addition, the mean diameter of the inhibition zone generated by the extract of S. marianum flower (13 ± 3 mm) was larger than the mean diameter of the inhibition zone generated by the extract of S. marianum leaf (7 ± 2 mm). The results of the analysis of variance indicated a significant relationship between the antibacterial effect of the S. marianum flower extract and the diameter of the inhibition zone (P < 0.01) (Figure 2). Moreover, 80% and 60% of Gram-positive bacteria were susceptible to the extracts of S. marianum flower and S. marianum leaf, respectively. However, all Gram-negative bacteria were resistant to the S. marianum leaf extract and only were 13% resistant to the S. marianum flower extract. In addition, the inhibitory activity of the extracts was concentration-dependent (Figure 3).
Comparison of growth inhibition zones generated by S. marianum flower and leaf extracts against Gram-positive (A) and Gram-negative (B) isolates.
Relative frequency of the antibacterial effect of S. marianum flower and leaf extracts on bacterial isolates from the semen of infertile men
4.4. Constituents of S. marianum Extract
Given its stronger antibacterial activity, we performed the GC-MS analysis on the S. marianum flower extract. Based on the results, the extract contained various compounds, including silybin (2.64%) and silychristin (3.07%). The peaks related to silybin and silychristin appeared at the retention times of 35,747 and 36,447, respectively. These compounds were identified with a probability of > 99%.
5. Discussion
Infertility is a fairly common problem worldwide. The main causes of male infertility include genital injuries, semen contamination, epididymitis, and ejaculatory duct obstruction. Male infertility is initially investigated by the semen analysis. Abnormal spermiogram findings simply raise the possibility of reduced fertility. In addition, sperm motility is the most important determinant factor of infertility (11). In our study, 64% of the semen samples from infertile men had problems in parameters including sperm count, motility, morphology, and pH, which is almost identical to the rates in previous studies in Iran (11, 12). Nevertheless, some researchers believe that infertility is possible even in asymptomatic men, often due to infections that decrease semen quality by affecting sperm motility and morphology (13, 14).
A wide range of bacteria can play a role in male infertility (15). In the present study, 36% of samples from infertile men were positive for bacterial infection, which is similar to the rate reported in two previous studies in Iran (16, 17) and a study in Tunisia (18). However, it should be noted that the abundance of bacterial infections varies depending on the causative bacterium. For instance, in a study in 2018, the frequency of S. aureus infection was 16% among semen samples of infertile men (19), which is notably lower than the rate observed in our study (45%). In another study in 2018 (20), the frequency of S. aureus isolates in semen samples of infertile men was reported to be 65%, which is higher than the rate found in our study. In the mentioned study, the frequency of S. epidermidis (24.5%) and Klebsiella (4.8%) isolates was also higher than the frequency found in our study (11%). Studies in Nigeria (21) and Iraq (22) also reported the frequency of staphylococcal infection to be 68% and 65%, respectively. These inconsistencies could be related to differences in age, physiology, anatomy, and diet of study subjects.
Although antibiotic therapy is the most commonly used method of controlling genital infections, the increasing rate of drug resistance has reduced the effectiveness of treatment (23, 24). Therefore, researchers have investigated alternative therapies, the use of medicinal plants, or natural antimicrobial agents for the treatment of such infections.
We demonstrated the inhibitory activity of S. marianum extract against bacteria isolated from the semen of infertile men, especially Gram-positive bacteria. This finding is consistent with the results of some studies in Iran (25, 26) and South Korea (27). However, our findings were inconsistent with the findings of a study by de Oliveira et al. (28), which can be due to the difference in the bacterial species, subjects, time of plant harvest, and the active components of the plant. Silybin and silychristin were among the most abundant active components of S. marianum extract. Previous studies revealed the antibacterial (27) and DNA-dependent RNA polymerase I stimulating activities (29) of these compounds.
5.1. Conclusion
The results of our study showed that bacterial semen infection could play a significant role in male infertility. The S. marianum flower extract has good antibacterial properties, particularly against Gram-positive bacteria such as S. aureus and S. epidermidis. Therefore, this extract after purification can be potentially used as a suitable alternative for the treatment of antibiotic-resistant infections of the male reproductive tract.
Acknowledgements
References
-
1.
Kamel RM. Management of the infertile couple: an evidence-based protocol. Reprod Biol Endocrinol. 2010;8:21. [PubMed ID: 20205744]. [PubMed Central ID: PMC2844387]. https://doi.org/10.1186/1477-7827-8-21.
-
2.
Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl. 2005;26(4):459-69. [PubMed ID: 15955884]. https://doi.org/10.2164/jandrol.04104.
-
3.
Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, et al. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch Androl. 2003;49(2):83-94. [PubMed ID: 12623744]. https://doi.org/10.1080/01485010390129269.
-
4.
Sanocka-Maciejewska D, Ciupinska M, Kurpisz M. Bacterial infection and semen quality. J Reprod Immunol. 2005;67(1-2):51-6. [PubMed ID: 16112738]. https://doi.org/10.1016/j.jri.2005.06.003.
-
5.
Shang XJ, Li K, Ye ZQ, Chen YG, Yu X, Huang YF. Analysis of lipid peroxidative levels in seminal plasma of infertile men by high-performance liquid chromatography. Arch Androl. 2004;50(6):411-6. [PubMed ID: 15669606]. https://doi.org/10.1080/01485010490484138.
-
6.
Ahmad MK, Mahdi AA, Shukla KK, Islam N, Jaiswar SP, Ahmad S. Effect of Mucuna pruriens on semen profile and biochemical parameters in seminal plasma of infertile men. Fertil Steril. 2008;90(3):627-35. [PubMed ID: 18001713]. https://doi.org/10.1016/j.fertnstert.2007.07.1314.
-
7.
Bibi Y, Nisa S, Chaudhary FM, Zia M. Antibacterial activity of some selected medicinal plants of Pakistan. BMC Complement Altern Med. 2011;11:52. [PubMed ID: 21718504]. [PubMed Central ID: PMC3141602]. https://doi.org/10.1186/1472-6882-11-52.
-
8.
Evren E, Yurtcu E. In vitro effects on biofilm viability and antibacterial and antiadherent activities of silymarin. Folia Microbiol (Praha). 2015;60(4):351-6. [PubMed ID: 25937395]. https://doi.org/10.1007/s12223-015-0399-6.
-
9.
Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231-45. [PubMed ID: 19934213]. https://doi.org/10.1093/humupd/dmp048.
-
10.
Patel JB, Cockerill FR, Bradford PA. Performance standards for antimicrobial susceptibility testing: twenty-fifth informational supplement. CLINICAL AND LABORATORY STANDARDS INSTITUTE. 2015;35(3):29-50.
-
11.
Tavalaee M, Azadi L, Nasr-Esfahani MH; Barekat. Comparison of sperm parameters and its functional characteristics between fertile. Cell Tissue Res. 2016;6(4):523-32.
-
12.
Tafvizi F, Baghdadi K, Roodbari NH. Lack of relatedness between Human cytomegalovirus in semen and male infertility. Iranian Journal of Medical Microbiology. 2016;10(3):39-46.
-
13.
Tafvizi F, Tahmasebi Fard Z. Lack of association between HPV and sperm parameters as a risk factor in infertile men admitted to infertility clinic. Pars of Jahrom University of Medical Sciences. 2015;13(3):37-45. https://doi.org/10.29252/jmj.13.3.37.
-
14.
Sharma A. Male Infertility; Evidences, Risk Factors, Causes, Diagnosis and Management in Human. Annals of Clinical and Laboratory Research. 2017;5(3). https://doi.org/10.21767/2386-5180.1000188.
-
15.
Gdoura R, Kchaou W, Znazen A, Chakroun N, Fourati M, Ammar-Keskes L, et al. Screening for bacterial pathogens in semen samples from infertile men with and without leukocytospermia. Andrologia. 2008;40(4):209-18. [PubMed ID: 18727730]. https://doi.org/10.1111/j.1439-0272.2008.00845.x.
-
16.
Khalili MB, Khalili MA. Correlation between asymptomatic urethritis with bacteriospermia in seminal plasma of fertile and infertile men. Journal of Reproduction & Infertility. 2002;3(4).
-
17.
Nabi A, Khalili MA, Halvaei I. An Investigation of Bacterial Infection of Seminal Fluid in Men with Infertility with Unknown Etiology. Qom University of Medical Sciences Journal. 2014;8(5):48-53.
-
18.
Gdoura R, Kchaou W, Ammar-Keskes L, Chakroun N, Sellemi A, Znazen A, et al. Assessment of Chlamydia trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, and Mycoplasma genitalium in semen and first void urine specimens of asymptomatic male partners of infertile couples. J Androl. 2008;29(2):198-206. [PubMed ID: 18077823]. https://doi.org/10.2164/jandrol.107.003566.
-
19.
Esmailkhani A, Akhi MT, Sadeghi J, Niknafs B, Zahedi Bialvaei A, Farzadi L, et al. Assessing the prevalence of Staphylococcus aureus in infertile male patients in Tabriz, northwest Iran. Int J Reprod Biomed (Yazd). 2018;16(7):469-74. [PubMed ID: 30234189]. [PubMed Central ID: PMC6129373].
-
20.
Malekshahi M, Razavian MH, Aghaee S. Evaluation of malone-di-aldehide as an indicatorfor semen bacterial contamination. JSSU. 2018;26(5):426-38.
-
21.
Emokpae MA, Uadia PO, Sadiq NM. Contribution of bacterial infection to male infertility in Nigerians. Online Journal of Health and Allied Sciences. 2009;8(1).
-
22.
AlJanabi A, Jubair A, Pemmaraju S, Pruthi P, Pruthi V. The role of bacterial infections on male infertility in Al-Anbar province of Iraq. International Journal of Medical Science and Public Health. 2014;3(2):177. https://doi.org/10.5455/ijmsph.2013.191120131.
-
23.
Nourbakhsh F, Momtaz H. Detection of antibiotic resistance patterns in Staphylococcus aureus strains isolated from patients admitted to Isfahan hospitals during 2014-2015. KAUMS Journal (FEYZ). 2015;19(4):356-63.
-
24.
Amiri E, Anvari M. Surveying drug resistance of Staphylococcus aureus strains resistant to the antibiotic vancomycin in some hospitals in Rasht. Medical Sciences Journal. 2018;28(1):74-80. https://doi.org/10.29252/iau.28.1.74.
-
25.
Hasanloo T, Sepehrifar R, Farahmand S. The effect of different doses of silymarin, extracted from the seeds of the Silybum marianum L. Gaertn on the growth of 6 bacterial species. Research in Medicine. 2015;38(4):193-9.
-
26.
Lahlah ZF, Meziani M, Maza A. Silymarin natural antimicrobial agent extracted from Silybum marianum. Journal Academia. 2012;2:164-9.
-
27.
Lee DG, Kim HK, Park Y, Park SC, Woo ER, Jeong HG, et al. Gram-positive bacteria specific properties of silybin derived from Silybum marianum. Arch Pharm Res. 2003;26(8):597-600. [PubMed ID: 12967193]. https://doi.org/10.1007/BF02976707.
-
28.
de Oliveira DR, Tintino SR, Braga MF, Boligon AA, Athayde ML, Coutinho HD, et al. In vitro antimicrobial and modulatory activity of the natural products silymarin and silibinin. Biomed Res Int. 2015;2015:292797. [PubMed ID: 25866771]. [PubMed Central ID: PMC4377387]. https://doi.org/10.1155/2015/292797.
-
29.
Machicao F, Sonnenbichler J. Mechanism of the stimulation of RNA synthesis in rat liver nuclei by silybin. Hoppe Seylers Z Physiol Chem. 1977;358(2):141-7. [PubMed ID: 844797]. https://doi.org/10.1515/bchm2.1977.358.1.141.