Abstract
Background:
Autoimmune thyroid diseases are the most frequent autoimmune disorders, with a global prevalence of about 10%. Several mechanisms have been proposed to induce autoimmune thyroid responses by infectious agents. In this study, we aimed to evaluate the association between parvovirus B19 infection and autoimmune thyroid disorders.Methods:
Adult patients with newly diagnosed Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) and healthy euthyroid controls were recruited. Various clinical and biochemical parameters, including thyroid function tests and serum parvovirus B19 antibody level (IgG), were assessed and compared between the groups.Results:
In this study, data from 404 cases with HT, 248 cases with GD, and 480 healthy individuals as a control group were analyzed. The prevalence of parvovirus B19 infection in patients with HT and GD and controls was 61.1%, 58.9%, and 47.1%, respectively. In the group of patients with HT, there was a significant positive correlation between the B19 IgG and TPOAb (r = 0.764, P < 0.001) and TgAb (r = 0.533, P < 0.001). Also, in patients with GD, the B19 IgG had a significant positive correlation with TPOAb (r = 0.779, P < 0.001) and TgAb (r = 0.467, P < 0.001).Conclusions:
Parvovirus B19 infection is commonly seen in patients with autoimmune thyroid disorders.Keywords
1. Background
Autoimmune thyroid diseases are the most frequent autoimmune disorders, with a global prevalence of about 10% (1). These disorders are caused by immune reactions (either cellular or humoral) to the thyroid gland and include a variety of clinical syndromes with autoimmune hypothyroidism (Hashimoto’s thyroiditis) at one end of the spectrum and autoimmune hyperthyroidism (Graves’ disease) at the other end (2). Hashimoto’s thyroiditis (HT) is the most frequent reason for hypothyroidism in iodine-sufficient areas. Nearly 10% of humans suffer from this disorder, and there is a direct association between its prevalence and age. This disease is characterized by thyroid dysfunction, with or without goiter, and is caused by the destruction of the thyroid gland caused by the apoptosis of thyroid epithelial cells and the existence of antibodies against one or more thyroid antigens in the serum (3). Similar to other autoimmune diseases, a genetic background, along with an environmental factor, is required to initiate HT (4). Graves’ disease (GD) is distinguished by stimulating antibodies against thyroid stimulating hormone (TSH) receptors. This stimulation increases the synthesis of thyroid hormones and leads to the enlargement of the thyroid gland (5, 6).
There have been few studies examining the association between parvovirus B19 infection and autoimmune thyroid disorders (7-9). Generally, parvovirus B19 infection is a health concern on a global scale. The rate of this infection does not vary greatly among different countries, including the United States, Europe, and Asian countries. Almost half of the population with this infection is aged 15 years, and about 60% of the adult population are seropositive for parvovirus B19 infection. This virus causes a diffuse and self-limiting disorder in young and mature individuals, called erythema infectiosum. It may also have manifestations in pregnant women, like arthralgia, arthritis, leukopenia, thrombocytopenia, anemia, vasculitis, miscarriage, and hydrops fetus (10, 11). Parvovirus B19 infection has been reported in several autoimmune diseases involving the connective tissues, joints, and blood vessels. Autoimmune neutropenia, thrombocytopenia, and hemolytic anemia have also been associated with parvovirus B19 infection (12).
The genome (5596 bp) of this single-stranded non-enveloped DNA virus is responsible for encoding non-structural protein 1 (NS1) and two viral capsid proteins, VP1 and VP2 (10). VP1 is similar to VP2 except that it has a unique region (VP1u) of 227 amino acids at its amino-terminal end (13). VP1 and VP2 that shape the icosahedral viral capsid are immune problems related to the immune system (14, 15). Moreover, the B19 protein can activate and upregulate the expression of NF-κB (16). Also, it is reported that B19 NS1 protein is capable of stimulating proinflammatory cytokine interleukin-6 (IL-6) gene production in the NF-kB binding location of the IL - 6 promoter (17). Both NF-κB and IL-6 are involved in the stimulation of different inflammatory and immunological diseases (18). In addition, an expressed phospholipase A2 (PLA2) motif is detected at the VP1u site of B19 (19), and the VP1u-related PLA2 function is required for the stimulation of autoimmune responses (20).
Few studies have examined the association of parvovirus B19 infection with autoimmune thyroid disorders (7-9). Therefore, in this cross-sectional study, we aimed to evaluate the association between parvovirus B19 infection and autoimmune thyroid disorders in three groups of newly diagnosed patients with GD, patients with HT, and euthyroid controls.
2. Methods
The present research was performed among newly diagnosed patients with HT and GD visiting endocrine healthcare centers in Zahedan (Iran) from April 2019 to September 2020. Those with a minimum age of 18 years were continuously enrolled using the consecutive sampling technique. Graves' disease diagnosis was made according to the following laboratory criteria: enhanced free tetraiodothyronine (FT4) and free triiodothyronine (FT3) along with repressed TSH (normal FT4: 0.8-1.8 ng/dL, normal FT3: 2.3-4.2 pg/mL, and normal TSH: 0.4-4.2 mIU/L) and positive TSH-Rec-Ab (normal: up to 1.75 IU/L). Hashimoto's disease was defined according to the following criteria: declined FT4 and FT3 along with increased TSH and positive TPOAb (normal: up to 16 IU/mL) or TgAb (normal: up to 100 IU/mL).
All the participants with a symptom of other health problems, like infectious disorders or malignancies, were excluded from the study. The other exclusion criteria included a history of thyroid medication treatment, tobacco consumption, pregnancy, or lactation. Euthyroid healthy individuals without thyroid disease or any known acute or chronic illness were chosen as the control group from the general population after applying the inclusion and exclusion criteria. The control group was matched according to age and gender with the case groups. The geographical origin and socio-economic status of the control group were similar to those of the case group.
A digital scale was used to measure body weight. In addition, height was measured by a stadiometer in a standing position. Body mass index was calculated by the following formula: weight in kilograms divided by the square of height in meters.
Blood samples were collected from 8 am to 9 am and were stored at -70°C until assay. Thyroid function tests and serum levels of parvovirus B19 IgG were evaluated in patients with GD and HT as well as in the control group. FT4, FT3, and TSH were measured using the immunochemiluminescent assay by an automated analyzer. The intra-assay coefficient of variation for T4 was 4.6%, and its inter-assay coefficient of variation was 3.7%. These values for T3 were 4.5% and 1.9% respectively. TSH assay has intra-assay coefficient of variation of 4.0%, and the inter-assay coefficient of variation of 3.3%. Thyroid peroxidase Ab, thyroglobulin Ab, and TSH-Rec-Ab were measured by the immunochemiluminescent assay employing commercial kits. Inter-assay and intra-assay coefficients of variation were 2% and 0.5% for TPOAb and 1.6% and 0.6% for TgAb, respectively. These values for TSH-Rec-Ab were 4.5% and 1.9%, respectively. IgG anti parvovirus B19 was measured by the enzyme-linked immunoassay method. Values ≥ 5.5 IU/mL were regarded positive. The intra-assay coefficient of variation for IgG was 4.3%, and its inter-assay coefficient of variation was 3.7%.
We conducted the study procedures following obtaining the approval of the research committee (either organizational or national). In addition, we adhered to the principles of the 1964 Helsinki declaration and its amendments. The Ethics Committee of the Zahedan University confirmed the study protocol (ethical code: IR.ZAUMS.REC.1399.326). Informed consent was obtained from all participants.
2.1. Data Analysis
The study variables are described with descriptive statistics, like frequency, percentage, and mean with standard deviation (SD). The normality of the variables was assessed with Shapiro–Wilk test and graphical approaches, like Q - Q plots and histograms. The mean difference of a numerical variable in the three study groups was analyzed with One-way ANOVA test. Also, a post-hoc comparison was conducted based on Bonferroni correction for pairwise comparison. The mean difference of numerical variables in patients with positive and negative B19 IgG was analyzed with independent t-test and Mann-Whitney U test for normally and non-normally distributed variables, respectively. We used Pearson Chi-square or Fisher's exact test for the evaluation of the relationship among categorical parameters with a study group. The correlation between B19 IgG titers and numerical variables was assessed with Pearson correlation coefficient, and important correlations were presented in scatter plots with quadratic fitting and 95% confidence interval. A P-value of less than 0.05 was considered statistically significant. All the analyses were conducted with Stata version 14.
3. Results
Data from 404 patients with HT, 248 patients with GD, and 480 healthy individuals as a control group were analyzed. In all the three groups, about 80% of patients were female, and the mean age was about 35 years. The frequency rates of parvovirus B19 infection in cases with HT, GD, and control group were 61.1%, 58.9%, and 47.1%, respectively (Table 1). The frequency of parvovirus B19 infection in cases with HT and GD was significantly higher than that of the control group, while the prevalence was not significantly different between the two groups of HT and GD (Figure 1).
| Variable | Hashimoto Group (n = 404) | Graves Group (n = 248) | Control Group (n = 480) | P-Value d |
|---|---|---|---|---|
| Sex, female | 327 (80.9) | 199 (80.2) | 376 (78.3) | 0.612 |
| Age (y) | 34.38 ± 10.18 | 34.05 ± 5.97 | 35.25 ± 8.84 | 0.155 |
| BMI (Kg/m2) | 24.19 ± 3.53 A | 23.76 ± 3.69 A | 23.02 ± 3.98 B | < 0.001 |
| FT4 (ng/dL) | 0.45 ± 0.16 A | 3.28 ± 0.91 B | 1.26 ± 0.20 C | < 0.001 |
| FT3 (pg/mL) | 1.63 ± 0.50 A | 6.67 ± 1.54 B | 3.62 ± 0.45 C | < 0.001 |
| TSH (mIU/L) | 79.51 ± 20.40 A | 0.02 ± 0.01 B | 1.55 ± 0.78 B | < 0.001 |
| TPOAb (IU/mL) | 477.47 ± 663.01 A | 343.81 ± 610.14 B | 13.51 ± 19.03 C | < 0.001 |
| Positive TPOAb (≥ 16 IU/mL) | 361 (89.4) A | 202 (81.5) B | 56 (11.7) C | < 0.001 |
| TgAb (IU/mL) | 836.85 ± 1319.05 A | 536.41 ± 1130.52 B | 54.72 ± 49.60 C | < 0.001 |
| Positive TgAb (≥ 100 IU/mL) | 321 (79.5) A | 202 (81.5) A | 56 (11.7) B | < 0.001 |
| B19-Ab (IgG) (IU/mL) | 31.27 ± 34.29 A | 37.47 ± 42.59 A | 22.09 ± 31.27 B | < 0.001 |
| Positive parvovirus B19Ab (≥ 5.5 IU/mL) | 247 (61.1) A | 146 (58.9) A | 226 (47.1) B | < 0.001 |
Distribution of B19 antibody and the prevalence of parvovirus B19 infection by Hashimoto, Graves’ disease and control groups.
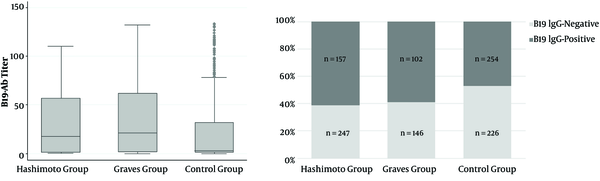
In the HT group, the prevalence of parvovirus B19 infection was 68.4% in TPOAb-positive patients, while none of the TPOAb-negative patients were positive for parvovirus B19 infection. In patients with GD, the prevalence rates of parvovirus B19 infection in TPOAb-positive and negative patients were 71.8% and 2.2%, respectively. Similar to TPOAb results, in the HT group among TgAb-positive patients, the prevalence of parvovirus B19 infection was 71.7%, while this percentage was 20.5% for TgAb-negative patients. In patients with GD, the prevalence of parvovirus B19 infection for TgAb positive and negative patients was 70.8% and 6.5%, respectively.
The clinical and laboratory characteristics of patients with and without parvovirus B19 infection are compared by group in Table 2. In the group of patients with HT and GD, B19 IgG had the highest correlation with TPOAb and TgAb (Figure 2).
Clinical and Biochemical Characteristics in individuals with Positive and Negative Parvovirus B19 Infection by Hashimoto, Graves and Control Groups a
| Hashimoto Group | Graves Group | Control Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative B19 | Positive B19 | P-Value b | Negative B19 | Positive B19 | P-Value b | Negative B19 | Positive B19 | P-Value b | |
| Sex | 0.187 | 0.298 | 0.541 | ||||||
| Female | 131 (40.1) | 196 (59.9) | 84 (42.2) | 115 (57.8) | 199 (52.9) | 177 (47.1) | |||
| Male | 26 (33.9) | 51 (66.2) | 18 (36.7) | 31 (63.3) | 55 (52.9) | 49 (47.1) | |||
| Age (y) | 33.84 ± 10.07 | 34.71 ± 10.25 | 0.402 | 34.41 ± 6.66 | 33.80 ± 5.44 | 0.429 | 35.09 ± 8.90 | 35.42 ± 8.78 | 0.683 |
| BMI (Kg/m2) | 24.61 ± 3.57 | 23.92 ± 3.48 | 0.057 | 24.26 ± 3.41 | 23.41 ± 3.85 | 0.076 | 23.41 ± 4.12 | 22.59 ± 3.77 | 0.025 |
| FT4 (ng/dL) | 0.43 ± 0.15 | 0.45 ± 0.17 | 0.211 | 3.28 ± 0.90 | 3.29 ± 0.92 | 0.901 | 1.28 ± 0.20 | 1.25 ± 0.20 | 0.087 |
| FT3 (pg/mL) | 1.54 ± 0.46 | 1.68 ± 0.51 | 0.005 | 6.82 ± 1.49 | 6.55 ± 1.58 | 0.179 | 3.61 ± 0.46 | 3.63 ± 0.44 | 0.679 |
| TSH (mIU/L) | 83.08 ± 18.81 | 77.23 ± 21.08 | 0.005 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.492 | 1.61 ± 0.80 | 1.48 ± 0.76 | 0.080 |
| TPOAb (IU/mL) | 52.27 ± 72.99 | 747.74 ± 726.66 | < 0.001 | 26.35 ± 25.24 | 565.59 ± 716.43 | < 0.001 | 14.20 ± 20.02 | 12.73 ± 17.87 | 0.399 |
| Positive TPOAb (≥ 16 IU/mL) | < 0.001 | < 0.001 | 0.047 | ||||||
| Yes | 114 (31.6) | 247 (68.4) | 57 (28.2) | 145 (71.8) | 36 (64.3) | 20 (35.7) | |||
| No | 43 (100) | 0 (0.0) | 45 (97.8) | 1 (2.2) | 218 (51.4) | 206 (48.6) | |||
| TgAb (IU/mL) | 282.85 ± 295.23 | 1188.99 ± 1573.10 | < 0.001 | 180.50 ± 192.47 | 785.05 ± 1414.20 | < 0.001 | 57.51 ± 50.01 | 51.59 ± 49.05 | 0.192 |
| Positive TgAb (≥ 100 IU/mL) | < 0.001 | < 0.001 | 0.403 | ||||||
| Yes | 91 (28.3) | 230 (71.7) | 59 (29.2) | 143 (70.8) | 31 (55.4) | 25 (44.6) | |||
| No | 66 (79.5) | 17 (20.5) | 43 (93.5) | 3 (6.5) | 223 (52.6) | 201 (47.4) | |||
The scatter plot with quadratic fitting and 95% confidence interval (CI) of B19 antibody correlation with thyroid antibodies in Hashimoto and Graves’ disease groups.
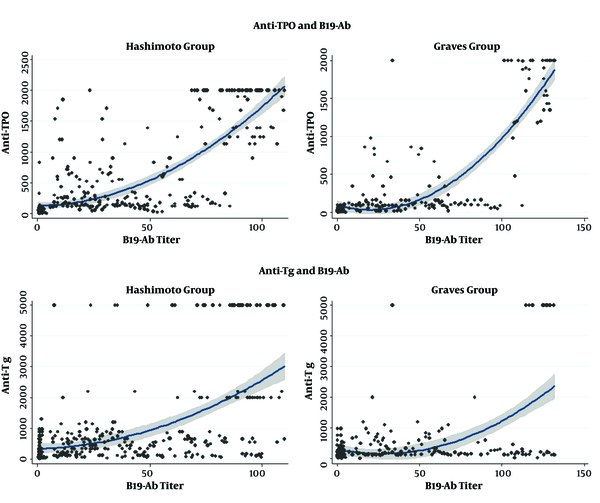
For cases with HT, the level of B19 IgG had a significant negative correlation with BMI and TSH. Also, in this group of patients, there was a significant positive correlation between B19 IgG and FT4, FT3, TPOAb, and TgAb. In patients with GD, B19 IgG had a significant positive correlation with TPOAb and TgAb. No association was found between TRAb and B19 IgG in this group. In the control group, B19 IgG was not significantly correlated with any of the variables (Table 3).
The Pearson Correlation Coefficient Between Serum B19 Ab and Anthropometric and Biochemical Characteristics in the Hashimoto, Graves, and Control Groups
| B19-Ab Titer in | |||
|---|---|---|---|
| Hashimoto Group | Graves Group | Control Group | |
| Age | |||
| r | 0.031 | -0.039 | 0.024 |
| P-value | 0.537 | 0.541 | 0.607 |
| BMI | |||
| r | -0.154 a | -0.118 | -0.078 |
| P-value | 0.002 | 0.065 | 0.086 |
| FT4 | |||
| r | 0.150 a | -0.005 | -0.051 |
| P-value | 0.002 | 0.938 | 0.268 |
| FT3 | |||
| r | 0.166 a | 0.033 | 0.025 |
| P-value | 0.001 | 0.602 | 0.592 |
| TSH | |||
| r | -0.239 a | 0.030 | -0.051 |
| P-value | 0.000 | 0.638 | 0.265 |
| TPOAb | |||
| r | 0.764 a | 0.779 a | 0.012 |
| P-value | 0.000 | 0.000 | 0.800 |
| TgAb | |||
| r | 0.533 a | 0.467 a | -0.010 |
| P-value | 0.000 | 0.000 | 0.822 |
| TRAb | |||
| r | - | 0.023 | - |
| P-value | 0.722 | ||
4. Discussion
Considering the limited information in the literature on the association of parvovirus B19 infection with autoimmune thyroid disorders (7-9, 12, 21, 22), we aimed to provide further information in this area in a study of cases with GD and HT and healthy euthyroid controls (age and gender-matched). In this study, 61% of patients in the HT group, 59% of patients in the GD group, and 47% of subjects in the control group had elevated IgG antibodies against parvovirus B19. These prevalence rates are consistent with those reported by studies on the general population regarding the prevalence of B19 infection based on serological assays (23).
A study of 73 children and adolescents with HT and 73 euthyroid individuals in the age-matched control group showed no significant difference in B19-specific antibodies in the serum of the two groups. However, parvovirus B19 DNA was more abundantly detected in these patients than in the control group. B19 DNA also showed a negative correlation with the course of the disease (8). In another research, the existence of parvovirus B19 DNA in the thyroid tissue of cases with HT was confirmed using PCR (7). Moreover, in a study by Wang et al. in 32 adult patients with HT, the presence of DNA virus and capsid protein in the thyroid tissue was evaluated by nested PCR, in-situ hybridization, and immunohistochemistry (21). Besides, in another study with a small sample size, the thyroid tissue of patients with GD and HT contained capsid B19 proteins, based on immunohistochemistry (22). Also, in a case report, a woman whose child had a skin rash (two weeks before), developed a parvovirus B19 infection, followed by GD, type I diabetes, and rheumatoid arthritis. In this patient, serological tests indicated IgM antibodies against parvovirus B19 and antibodies against TSH receptors (9).
The prevalence of autoimmune thyroid disorders in the general population has been estimated at 1 - 2%. Since studies have shown that the prevalence of parvovirus B19 infection in the general population is much higher than this rate based on serological tests, it is clear that a small proportion of patients with parvovirus B19 infection develop autoimmune thyroid disorders. The B19 virus has a relatively simple pathogenesis in a normal person. Following the acute stage, the virus can be removed from the blood using particular humoral immune responses (24). Nevertheless, the virus genome can be deposited and remain in the tissue in a latent stage following the initial infection (25).
B19 can only infect cells that have the receptors to bind to the virus. Globoside, or a blood group P antigen, is a necessary cellular receptor for the B19 virus (26). Based on the literature, P antigen can be found on erythroid precursors and a number of other cells (27). It has been confirmed that human lymphocytes and thyroid follicular cells contain globosides (blood group P antigens) and can be infected by B19 viruses. They are also considered as target cells in HT (28, 29).
The virus proteins are primarily expressed in thyroid epithelial cells. Autoimmune destruction of the thyroid gland caused by both cellular and humoral immunity is very complex in HT. In the Hashimoto initiation process, active helper T cells play a key role in inducing immune responses to local and foreign antigens (30). B19 virus is capable of stimulating T helper cells by signaling VP1 and VP2 antigens (14, 15, 31). Also, VP1u proteins may be involved in B19-associated inflammatory responses (32). VP1/VP2 antigen expression in the thyroid of patients with HT is significantly higher than that of normal individuals; this suggests the potential role of virus proteins in the pathogenesis of autoimmune thyroid disorders (21). Moreover, previous studies have shown that mononuclear cells infiltrating the thyroid gland increase the NF-κB and IL-6 expression and contribute to inflammatory responses and tissue damage in patients with HT (33). Parvovirus B19 can also increase the expression of NF-κB and IL-6, which are present in thyroid epithelial cells along with viral proteins (21). Hence, their enhanced production can cause inflammatory and autoimmune diseases (18). Also, it has been mentioned that anti-parvovirus antibodies like anti-VP1 IgG can detect and react with human cytokeratin, a protein found extensively in epithelial cells, resulting in an immune response and cell damage (18, 21, 34).
Generally, the identification of infectious agents as etiological factors for human diseases is a very complex process. Also, reporting information about the etiological role of viruses should be done with caution. The existence of antibodies against the virus does not indicate the role of the pathogen in causing the disease, particularly when the infectious pathogen is widespread in the community. However, the lack of viral markers at the initial stage of the disorder does not exclude the role of viruses in the disease, because a triggering primary infection might have occurred years ago. The triggering virus can be completely cleared from the body with only its specific antibodies remaining. Viral agents can remain in the tissue without any signs of systemic inflammation. Evidence of the presence of a virus in the tissue does not necessarily mean that this viral agent is the cause of disease, as it may only be an innocent bystander. Viral diseases are a result of virus-host interactions in which the genetic background plays an essential role. Even when the host shows no clinical signs, the virus can be involved in the pathogenesis of the disease (35).
The present study has several limitations. First, it is an observational study that cannot represent a causal relationship between B19 infection and autoimmune thyroid disorders. Second, in this study, to confirm B19 infection, we used the measurement of serum IgG antibodies in the serum rather than methods such as PCR or in situ hybridization for DNA and immunohistochemistry for capsid proteins in thyroid tissue. Furthermore, the obvious difference in the percentages of positive B19 subjects between the control group and TPO-Ab-negative patients in both HT and GD groups is a questionable finding. Apart from the fact that there was a limited total number of subjects with negative TPO-Ab in the HT (43 subjects) and GD (46 subjects) groups, which makes it difficult to compare them with the 480 participants in the control group, we have no other justification for this finding in this study. On the other hand, having an euthyroid control group and a relatively acceptable sample size are among the strengths of the current research.
In conclusion, parvovirus B19 infection is commonly seen in cases with autoimmune thyroid disorders. Although studying the role of viruses in these disorders is not a new topic, the available results of cross-sectional studies can demonstrate an association between B19 infection and autoimmune thyroid disorders. Further prospective studies are required to confirm the causal role of the B19 virus in the pathogenesis of autoimmune thyroid disorders.
References
-
1.
Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2(3):119-25. https://doi.org/10.1016/s1568-9972(03)00006-5.
-
2.
Ibili ABP, Selver Eklioglu B, Atabek ME. General properties of autoimmune thyroid diseases and associated morbidities. J Pediatr Endocrinol Metab. 2020;33(4):509-15. [PubMed ID: 32126013]. https://doi.org/10.1515/jpem-2019-0331.
-
3.
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-99. [PubMed ID: 11836274]. https://doi.org/10.1210/jcem.87.2.8182.
-
4.
Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-62. https://doi.org/10.1016/s0140-6736(17)30703-1.
-
5.
Latif R, Morshed SA, Zaidi M, Davies TF. The thyroid-stimulating hormone receptor: Impact of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on multimerization, cleavage, and signaling. Endocrinol Metab Clin North Am. 2009;38(2):319-41. viii. [PubMed ID: 19328414]. https://doi.org/10.1016/j.ecl.2009.01.006.
-
6.
Antonelli A, Ferrari SM, Ragusa F, Elia G, Paparo SR, Ruffilli I, et al. Graves' disease: Epidemiology, genetic and environmental risk factors and viruses. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101387. [PubMed ID: 32107168]. https://doi.org/10.1016/j.beem.2020.101387.
-
7.
Mori K, Munakata Y, Saito T, Tani J, Nakagawa Y, Hoshikawa S, et al. Intrathyroidal persistence of human parvovirus B19 DNA in a patient with Hashimoto's thyroiditis. J Infect. 2007;55(2):e29-31. [PubMed ID: 17582502]. https://doi.org/10.1016/j.jinf.2007.05.173.
-
8.
Lehmann HW, Lutterbuse N, Plentz A, Akkurt I, Albers N, Hauffa BP, et al. Association of parvovirus B19 infection and Hashimoto's thyroiditis in children. Viral Immunol. 2008;21(3):379-83. [PubMed ID: 18788945]. https://doi.org/10.1089/vim.2008.0001.
-
9.
Munakata Y, Kodera T, Saito T, Sasaki T. Rheumatoid arthritis, type 1 diabetes, and Graves' disease after acute parvovirus B19 infection. Lancet. 2005;366(9487). https://doi.org/10.1016/s0140-6736(05)67184-x.
-
10.
Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350(6):586-97. [PubMed ID: 14762186]. https://doi.org/10.1056/NEJMra030840.
-
11.
Cohen BJ, Buckley MM. The prevalence of antibody to human parvovirus B19 in England and Wales. J Med Microbiol. 1988;25(2):151-3. [PubMed ID: 3339634]. https://doi.org/10.1099/00222615-25-2-151.
-
12.
Meyer O. Parvovirus B19 and autoimmune diseases. Joint Bone Spine. 2003;70(1):6-11. https://doi.org/10.1016/s1297-319x(02)00004-0.
-
13.
Ozawa K, Ayub J, Kajigaya S, Shimada T, Young N. The gene encoding the nonstructural protein of B19 (human) parvovirus may be lethal in transfected cells. J Virol. 1988;62(8):2884-9. [PubMed ID: 2969055]. [PubMed Central ID: PMC253725]. https://doi.org/10.1128/JVI.62.8.2884-2889.1988.
-
14.
Corcoran A, Doyle S. Advances in the biology, diagnosis and host-pathogen interactions of parvovirus B19. J Med Microbiol. 2004;53(Pt 6):459-75. [PubMed ID: 15150324]. https://doi.org/10.1099/jmm.0.05485-0.
-
15.
von Poblotzki A, Gerdes C, Reischl U, Wolf H, Modrow S. Lymphoproliferative responses after infection with human parvovirus B19. J Virol. 1996;70(10):7327-30. [PubMed ID: 8794392]. [PubMed Central ID: PMC190798]. https://doi.org/10.1128/JVI.70.10.7327-7330.1996.
-
16.
Wang JH, Zhang WP, Liu HX, Wang D, Li YF, Wang WQ, et al. Detection of human parvovirus B19 in papillary thyroid carcinoma. Br J Cancer. 2008;98(3):611-8. [PubMed ID: 18212749]. [PubMed Central ID: PMC2243166]. https://doi.org/10.1038/sj.bjc.6604196.
-
17.
Moffatt S, Tanaka N, Tada K, Nose M, Nakamura M, Muraoka O, et al. A cytotoxic nonstructural protein, NS1, of human parvovirus B19 induces activation of interleukin-6 gene expression. J Virol. 1996;70(12):8485-91. [PubMed ID: 8970971]. [PubMed Central ID: PMC190939]. https://doi.org/10.1128/JVI.70.12.8485-8491.1996.
-
18.
Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7-11. [PubMed ID: 11134171]. [PubMed Central ID: PMC198552]. https://doi.org/10.1172/JCI11830.
-
19.
Zádori Z, Szelei J, Lacoste M, Li Y, Gariépy S, Raymond P, et al. A viral phospholipase A2 is required for parvovirus infectivity. Dev Cell. 2001;1(2):291-302. https://doi.org/10.1016/s1534-5807(01)00031-4.
-
20.
Lehmann HW, von Landenberg P, Modrow S. Parvovirus B19 infection and autoimmune disease. Autoimmun Rev. 2003;2(4):218-23. https://doi.org/10.1016/s1568-9972(03)00014-4.
-
21.
Wang J, Zhang W, Liu H, Wang D, Wang W, Li Y, et al. Parvovirus B19 infection associated with Hashimoto's thyroiditis in adults. J Infect. 2010;60(5):360-70. [PubMed ID: 20153771]. https://doi.org/10.1016/j.jinf.2010.02.006.
-
22.
Adamson LA, Fowler LJ, Clare-Salzler MJ, Hobbs JA. Parvovirus B19 infection in Hashimoto's thyroiditis, papillary thyroid carcinoma, and anaplastic thyroid carcinoma. Thyroid. 2011;21(4):411-7. [PubMed ID: 21190433]. https://doi.org/10.1089/thy.2010.0307.
-
23.
Rohrer C, Gartner B, Sauerbrei A, Bohm S, Hottentrager B, Raab U, et al. Seroprevalence of parvovirus B19 in the German population. Epidemiol Infect. 2008;136(11):1564-75. [PubMed ID: 18198003]. [PubMed Central ID: PMC2870752]. https://doi.org/10.1017/S0950268807009958.
-
24.
Kerr JR. Pathogenesis of human parvovirus B19 in rheumatic disease. Ann Rheum Dis. 2000;59(9):672-83. [PubMed ID: 10976079]. [PubMed Central ID: PMC1753262]. https://doi.org/10.1136/ard.59.9.672.
-
25.
Norja P, Hokynar K, Aaltonen LM, Chen R, Ranki A, Partio EK, et al. Bioportfolio: lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc Natl Acad Sci U S A. 2006;103(19):7450-3. [PubMed ID: 16651522]. [PubMed Central ID: PMC1464359]. https://doi.org/10.1073/pnas.0602259103.
-
26.
Brown KE, Hibbs JR, Gallinella G, Anderson SM, Lehman ED, McCarthy P, et al. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen). N Engl J Med. 1994;330(17):1192-6. [PubMed ID: 8139629]. https://doi.org/10.1056/NEJM199404283301704.
-
27.
Rouger P, Gane P, Salmon C. Tissue distribution of H, Lewis and P antigens as shown by a panel of 18 monoclonal antibodies. Rev Fr Transfus Immunohematol. 1987;30(5):699-708. https://doi.org/10.1016/s0338-4535(87)80138-1.
-
28.
Bouchon B, Portoukalian J, Bornet H. Alterations of the cerebroside fraction of human thyroid glycosphingolipids in Graves' disease. Biochem Int. 1985;11(3):415-24. [PubMed ID: 3840690].
-
29.
Dunstan RA. Status of major red cell blood group antigens on neutrophils, lymphocytes and monocytes. Br J Haematol. 1986;62(2):301-9. [PubMed ID: 3511947]. https://doi.org/10.1111/j.1365-2141.1986.tb02933.x.
-
30.
Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348(26):2646-55. [PubMed ID: 12826640]. https://doi.org/10.1056/NEJMra021194.
-
31.
Dorsch S, Liebisch G, Kaufmann B, von Landenberg P, Hoffmann JH, Drobnik W, et al. The VP1 unique region of parvovirus B19 and its constituent phospholipase A2-like activity. J Virol. 2002;76(4):2014-8. [PubMed ID: 11799199]. [PubMed Central ID: PMC135890]. https://doi.org/10.1128/jvi.76.4.2014-2018.2002.
-
32.
Lu J, Zhi N, Wong S, Brown KE. Activation of synoviocytes by the secreted phospholipase A2 motif in the VP1-unique region of parvovirus B19 minor capsid protein. J Infect Dis. 2006;193(4):582-90. [PubMed ID: 16425138]. https://doi.org/10.1086/499599.
-
33.
Ajjan RA, Watson PF, McIntosh RS, Weetman AP. Intrathyroidal cytokine gene expression in Hashimoto's thyroiditis. Clin Exp Immunol. 1996;105(3):523-8. [PubMed ID: 8809144]. [PubMed Central ID: PMC2200538]. https://doi.org/10.1046/j.1365-2249.1996.d01-784.x.
-
34.
Loizou S, Cazabon JK, Walport MJ, Tait D, So AK. Similarities of specificity and cofactor dependence in serum antiphospholipid antibodies from patients with human parvovirus B19 infection and from those with systemic lupus erythematosus. Arthritis Rheum. 1997;40(1):103-8. [PubMed ID: 9008606]. https://doi.org/10.1002/art.1780400115.
-
35.
Desailloud R, Hober D. Viruses and thyroiditis: An update. Virol J. 2009;6:5. [PubMed ID: 19138419]. [PubMed Central ID: PMC2654877]. https://doi.org/10.1186/1743-422X-6-5.