Abstract
Background:
Transoral laser microsurgery (TLM) has become the preferred and standardized method for the early treatment of many laryngeal carcinomas, due to the good treatment results, together with the scarcity of local complications.Objectives:
The aim of this study is to investigate the advantages of TLM (diode laser) in the treatment of patients with early-stage (T1-2) glottic laryngeal cancer (GLC).Methods:
In the present study, 228 patients, who were diagnosed with early-stage GLC between 2007 and 2013 and treated with diode TLM in our clinic, were analyzed retrospectively.Results:
During the 5-year follow-up period of the patients, it was found that 5.2% of the patients had therapeutic neck dissection and 2.6% had a tracheostomy. The mean hospitalization period was 24 hours and their speech and swallowing functions were satisfactory. Eight percent of the patients developed local relapse. In addition, the 5-year disease survival 100%, disease-free survival was 92%, and laryngeal preservation was 98.7%.Conclusions:
The TLM method is a minimally invasive treatment for early-stage GLCs and gives treatment results similar to radiotherapy and open surgery methods. However, it is more advantageous than other methods in terms of reducing hospitalization period, low complication rate, preserving speech-swallowing functions, and its re-application in cases of relapse. TLM is a safe and reliable surgical technique with less morbidity that can be repeated in selected tumor recurrence. Anterior commissure monitoring must be important in the treatment of TLM. Especially in anterior commissure, tumors with a tendency to spread in the vertical plane should be monitored for local recurrence. Diode (gallium-arsenide) 980-nm laser has advantages over the standard CO2 laser, and TLM treatment stands out in early-stage GLCs.Keywords
Diode Laser Transoral Endoscopic Microsurgery (TLM) Early-Stage Laryngeal Cancer
1. Background
Of all cancers, the prevalence of laryngeal cancers (LCs) is 2% to 5% in males and 0.4% in females. They are the second most common cancer after skin cancer occurring in the region of the head and neck. Besides, more than 150000 new cases of LG are diagnosed every year worldwide (1). Of LCs, 95% are squamous cell carcinoma. In Turkey, 60% of LCs are glottic, 40% are supraglottic, and 1% are subglottic (2). The aim of surgery for LCs is to remove cancerous tissue and to maintain the laryngeal functions, such as speech, respiration, and swallowing as much as possible (3).
Early-stage glottic laryngeal cancers (GLCs) include T1 and T2 stages. Glottic region T1 tumors are limited to those in the vocal cords. If it affects one cord, it is called as T1a, if it affects both cords, then, it is called T1b. T2 glottic tumors are those that have spread out of the cord but are limited in the larynx or restricted cord movements. Today, radiotherapy (RT) and surgery are accepted as the methods of treatment used in early-stage GLCs. The surgical method is applied as endoscopic (accompanied with or without a transoral laser microscope) or open surgery (3-5).
Recently, new lasers have been developed that use different wavelengths other than the standard CO2 laser. Diode (gallium-arsenide) 980-nm laser is the most important alternative (6). Main advantages of diode laser over CO2 laser include the following: it provides better hemostasis (7), it is cheaper, it is easier to transport and implement (8), cell proliferation and fibroblast activity increase and tissue healing is faster (9), using a thin fiber cable allows the surgeon to hold it like a pencil, as well as angling the flexible feature, and exposing the larynx to areas that are difficult to reach, especially in the CO2 laser. Nevertheless, there are major disadvantages derived from the transoral laser microsurgery (TLM) intrinsically; because of evaporation and carbonation in the tissue, histopathological evaluation is problematic (4, 10).
TLM is still a safe surgical method because of perfect tissue-organ protection rates. In the preoperative evaluation of GLCs, it is important to consider respiratory, voice and swallowing functions, therapy options in case of tumor recurrence, and long-term quality of life (QoL) (4). In recent years, due to the use of robotic surgery in TLM, if needed, a detailed view of the field of enlargement and increased visualization of the surgical field reduce the amount of resected normal tissue and prevented over-treatment. Because of the organ-preserving properties of TLM, postoperative speech and swallowing functions are satisfactorily maintained, and the duration of hospitalization is shortened. In addition, it also enables to be able to avoid tracheostomy, which is among the complications of open surgery, it is high-cost and is difficult to accept for the patient. TLM shows good oncologic and survival results, as well as open surgery and RT. As another important advantage of TLM, it presents the possibility of laser, open partial surgery, or RT in patients with local recurrence or second primary tumor in the head and neck region (6, 8, 11-15).
2. Objectives
Related to all these recent developments in surgery methods and devices, the indications of TLM and the use of diode laser have expanded and their efficacy has increased. This leads to the question of whether the concept of early-stage GLCs should be redefined. The aim of this study is to reveal the oncologic results and to investigate the advantages of diode laser TLM use in the treatment of patients with early-stage GLCs. The detailed comparison with CO2 laser TLM and other conventional (open surgical-RT) treatment methods are planned.
3. Methods
3.1. Data Collection and Ethical Permission
This retrospective study was approved by the Istanbul Yeni Yuzyil University (IYYU), Non-Interventional Clinical Research Ethics Board (24.12.2018/043). The principles of the Declaration of Helsinki and Guidelines for Good Clinical Practices were followed during the study. In this study, retrospective results of 228 patients, who were diagnosed with early-stage GLC (T1a, T1b, and T2,) between 2007 and 2013 and treated with TLM in our clinic (IYYU) and were followed for 5 years, were analyzed.
3.2. The Inclusion and Exclusion Criteria
The exclusion criteria included patients with incomplete information and examination, accompanying other malignancies, incomplete treatment, patient refusal to treatment, and the lack of patient follow-up and data (at least 3 months after tumor resection).
3.3. Working Group
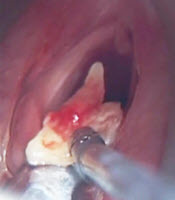
The patients were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) criteria. The diagnosis of laryngeal cancer was made as a result of detailed examination and direct laryngoscopy in the clinic of otolaryngology (Figure 1B) and punch biopsy and histopathological examination. In addition, neck evaluation, palpation, USG, and CT or MRI, when needed in some cases, were performed (Figure 1A). Postoperative, the pathological staging was done according to the 7th edition of the AJCC TNM classification system. Pathological results revealed that all patients had squamous cell carcinoma. One patient had already undergone RT. Before the TLM intervention, physical and endoscopic examinations of the patients were performed. The patients’ oropharynx, hypopharynx, and larynx were examined (16).
A, MR imaging of laryngeal anterior commissure carcinoma; B, pretreatment image of laryngeal carcinoma; C, using a diode laser; D, after 6 months of laser treatment image of the larynx.

Our surgical operations were made under general anesthesia after intubation with Bicakciar’s spiral endotracheal tube. Following adequate larynx exposure with the help of the adjustable laryngoscopes, was utilized for tumor removal (Figure 1C) under microscope diode laser (Lotus 980-England). The modality was continuous-wave radiation delivered to a flexible optical fiber. The output power of the laser was variable, adjusted at 6-12 W, and removal of the tumor was performed until healthy-appearing tissue was evident. The cartilaginous framework of the larynx was always left intact. Following the removal of the tumor, a biopsy of the healthy-appearing mucosa was carried out to rule out margin positivity. Tumor resection was done by one surgeon in the “en-bloc or piece-meal” technique. Endoscopic cordectomy was classified according to the criteria of the European Laryngological Society (ELS). In the cases with anterior commissure invasion, sometimes the perichondrium and partially the thyroid cartilage were resected (or “type VI” cordectomy, according to the ELS, was performed). Border/control biopsies were taken from those, whose tumor sizes were large (17). No tracheostomy was performed for any cases before the intervention. However, following the extubation, respirations of the patients were closely examined. As no lymphadenopathy was detected in the neck examination (all cases were N0), no elective neck dissection was performed. Detailed examination and controls of the patients were carried out for 48 months in 3 to 5 months.
3.4. Analyzed Parameters
Patients’ descriptive information such as age and sex and risk factors such as smoking and alcohol consumption were recorded. Histopathological tumor grade, anterior commissure involvement, performed tracheostomy, positive surgical margins, T stage, the period of hospitalization, post-TLM complication, and laryngeal preservation and after 5-year disease-free survival of patients’ follow-up period were also examined.
The grade is reported in registry systems by the grade value. A 2-grade, 3-grade, or 4-grade system may be used. If a grading system is not specified, generally the following system is used: GX, grade cannot be assessed; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; and G4, undifferentiated (16).
Our follow-up protocol consists of a laryngoscopic examination by microscope under general anesthesia after 6 to 8 weeks in all patients, who were treated by TLM, as well as a clinical follow-up examination with endoscopic laryngoscopy on a regular basis (every 4 - 6 weeks in the 1st, every 2 - 3 months in the 2nd and 3rd, and every 3 - 6 months in the 4th and 5th year, respectively). In our study, the evaluation of voice quality was evaluated by Jacobson et al. developed by. voice handicap index (VHI-10) of Rosen et al. (18), which is a short 10-item version.
Quantitative variables were expressed as mean and minimum-maximum and categorical variables as number and percent.
4. Results
In this study, 228 patients (201 male and 27 female) were followed for 5 years. The mean age of the patients was 61 (42 - 68) years. Among the patients, 212 (93%) were smoking. The tumor stages of patients were T1a (n = 129), T1b (n = 45), and T2 (n = 54). The histological stages were grade 1 (n = 62), grade 2 (n = 163), and grade 3 (n = 3). During diagnosis, vocal cord mobility was limited in 9 patients with the T2 stage. The extension to the anterior commissure (A-com) was detected in 48 (21%) patients (Table 1).
| Patient | Values |
|---|---|
| Sex | |
| Male | 201 (88) |
| Female | 27 (12) |
| Mean age, y [min-max] | 60.82 [42 - 68] |
| Smoking | 212 (93) |
| Tumor stage | |
| T1a | 129 (56.5) |
| T1b | 45 (20) |
| T2 | 54 (23.5) |
| Histological stage | |
| G1 | 62 (27.2) |
| G2 | 163 (71.5) |
| G3 | 3 (1.3) |
| Limited vocal cord mobility | 9 (4) |
| Extension to A-com | 48 (21) |
The GLC stages of the patients and the types of surgical operations performed by TLM are shown in Table 2.
| Cordectomy type | T1a | T1b | T2 | Values |
|---|---|---|---|---|
| II | 31 | - | - | 31 (13.6) |
| III | 33 | - | - | 33 (14.4) |
| IV | 28 | 12 | - | 40 (17.5) |
| Va | 34 | 14 | - | 48 (21) |
| Vb | 3 | 3 | 4 | 10 (4.3) |
| Vc | - | 7 | 29 | 36 (15.8) |
| Vd | - | 5 | 12 | 17 (7.4) |
| VI | - | 4 | 9 | 13 (6) |
| Total | 129 (56.5) | 45 (20) | 54 (23.5) | 228 (100) |
During the follow-up of 228 patients after TLM, granulation tissue was seen in the A-com region of 42 cases, in which the A-com invasive cartilage tissue also emerged. Six of them were regressed, using local corticosteroid treatment and in 36 (16%) patients, these granulation tissues were removed by applying TLM for the second time in the 3rd or 4th month (Table 3). They were examined histopathologically. Among those during the procedure, necrotic cartilage tissue was removed from 11 cases, on whom we applied excessive resection until we exposed the cartilage in the A-com (Figure 2). In 3 of the cases with cartilage necrosis, laryngeal stenosis developed and a tracheostomy was performed. Because 9 cases were + in the biopsies during the follow-up of 12 patients with granulation, this was considered recurrence and 8 patients applied a second TLM operation and the surgical margin biopsy was clear. However, the other patient refused to accept the second TLM initiative; so, the patient was sent to RT. No recurrence was observed in the subsequent follow-up of the 3 patients. In addition, RT was applied to 3 patients after their border biopsy was detected as + TLM.
| Patients | Values |
|---|---|
| Granulation tissue in the A-com region | 42 (18) |
| Local recurrence | 18 (8) |
| Total laryngectomy | 3 (1.5) |
| Cervical lymphadenopathy | 12 (5) |
| Neck dissection | 12 (5) |
| Underwent RT | 4 (1.75) |
| Larynx prevention | 225 (98.5) |
| Surgical margin positivity | 3 (1.3) |
| The second TLM operation | 15 (6) |
| Five-year survival | 228 (100) |
Resection necrotic cartilage

In total, 18 (8%) patients were found local recurrence (3 patients in T1a, 3 patients in T1b, and 12 patients in T2). Of them, 9 resulted from the granular tissue in the A-com region; the other 9 were the recurrences in the regions outside the A-com. All cancer recurrences were seen in the 1st year. The second TLM operation was performed to 15 recurrence cases and RT was applied to one. Total laryngectomy was performed in 3 of the recurrence cases because the lesion would not be completely removed. Local and/or regional recurrences were not observed in the subsequent follow-up of the 18 cases (Table 3).
Because cervical lymphadenopathy was detected in 12 (5%) cases (6 were supraglottic) during the first examination (ipsilateral lymph node measured less than 3 cm = N1), therapeutic neck dissection was performed during TLM intervention (bilaterally for 6). Histopathological examination revealed squamous cell cancer metastasis in lymph nodes. No recurrence developed in the follow-up of these patients (Table 3).
Among our patients, 6 (2.6%) underwent a tracheostomy. Because dyspnea and inspiratory stridor were seen in 3 of the patients with T2 tumors during the first examination, that patient underwent preoperative tracheostomy. The other patients underwent postoperative tracheostomy due to the laryngeal stenosis that developed after the cartilage necrosis. Patients were decannulated within 2 to 3 months.
Of the patients, 8 (3.5%) presented with the postoperative bleeding complaint, and the bleeding stopped without any further intervention as a result of medical treatment applied (Table 4).
| Complication | Values |
|---|---|
| Thyroid cartilage necrosis | 11 (5) |
| Laryngeal stenosis | 3 (1.3) |
| Tracheostomy | 6 (2.6) |
| Bleeding | 8 (3.5) |
| Infection | 21 (9.2) |
| Halitosis | 78 (34) |
| Aspiration | 6 (2.6) |
| Burning of the endotracheal tube | 1 (0.4) |
Because infection developed in 21 cases that extensive resection was applied to in the postoperative period, antibiotic therapy was performed. Because of the aspiration that developed in 6 patients after TLM, the feeding tube was applied for 7 to 10 days (Table 4).
To sum up, 18 (8%) patients were found to have recurrences in this study. Of these cases, 9 (4%) resulted from granulation tissue in the A-com region. Three patients received recovery laryngectomy. Thus, the larynx was preserved in 98.5% of the cases. In total, from the patients, who underwent RT, 3 were due to surgical margin (biopsy was + after TLM) and the others did not accept the second TLM intervention. In addition, because cervical lymphadenopathy (ipsilateral lymph node measured less than 3 cm = N1) was found in 12 (5%) of the cases (6 were supraglottic), therapeutic neck dissection was applied (bilaterally for 6) (Table 3).
5. Discussion
Early-stage GLCs generally have a good prognosis and, therefore, the aims of therapy should be not only removal of the tumor (cure) but also laryngeal protection with optimal voice quality, minimization of complications, and cost reduction. The respiratory, voice and swallowing functions, and long-term QoL are important in the treatment of early-stage GLC. The early-stage GLC without A-com infiltration treated by TLM cordectomy shows desired oncologic and functional results, and effect in equal to better results than the ones treated with radiotherapy (3, 4). TLM (diode laser) microsurgery has an important place in the treatment of GLCs in recent years (19). It is especially superior to open surgeries in early GLCs and is preferential (20). The sound quality is highly maintained in the TLM method and no tracheostomy is performed unless it is compulsory (11, 12, 21). In addition, recent studies have revealed that TLM has yielded good survival results in the treatment of early-stage GLCs at least open surgery and RT do (90% - 95%) (21-23). However, as an advantage of TLM, hospitalization time for patients is short and the complication rate is lower compared to open surgery and RT (13, 24, 25). Short surgery and hospitalization times, low morbidity, high organ preservation, and good functional (respiratory, swallowing, and voice) results make TLM superior to other treatment methods (14, 15, 26). Another study showed that the mean hospitalization period of the patients with early-stage GLC was 14 days after the front lateral laryngectomy (27). In our study, however, the hospitalization period after TLM was 24 hours. This makes TLM treatment advantageous in terms of low labor loss and low cost.
In a study by Pedregal-Mallo et al. (3), a 5-years survival rate without local recurrence was 75% and larynx functions were preserved in 85% of the patients during this period. In a study, Hinni et al. (25) found that larynx has been protected at the rate of 92% as a result of a 5-year follow-up in the treatment of LC to, which they applied TLM. In another study, Peretti et al. (28) reported that specific to 5-year survival rate was 100% in patients with 595 early-stage GLC, whom they treated using TLM. Also, Mendenhall et al. (29) found that a specific to 5-year survival rate was 95% in the early-stage GLCs, and the overall survival rate was 80%. In addition, local control, oncologic outcome, and survival rates showed that TLM, RT, and open surgery were close to each other. In our study, as a result of the TLM method that was applied to 228 patients with early-stage GLC for 5 years, 100% survival, 92% disease-free survival, and 98.5% laryngeal protection were achieved.
In a study, Lucioni et al. (30) found 90% to 98% local control and 98% laryngeal protection in early-stage GLCs with primary laser treatment. In a study of 404 cases in 2015, Canis et al. (31) reported 86% local control, 97% laryngeal protection, and 98% disease-specific-survival in T1a GLCs. In addition, in another study performed by Canis et al. (5), they achieved 93% laryngeal protection in T2a and 83% in T2b GLCs. In our study, after a 5-year follow-up, 98% of T1a, 90% of T1b, 84.5% of T2a, and 66.7% of T2b GLCs local control were observed. Also, 98% of T1 tumors, 93% of T2a, and 83% of T2b laryngeal protection were provided in our study (Figure 1D). At the same time, we achieved a 100% survival and 92% disease-free survival rate in our study series.
Since A-com is a site, where both vocal cords attach to the thyroid cartilage at the front and are, therefore, an area of perichondrium-free, it has an increased risk of tumor spread to the thyroid cartilage (3, 32). But recently, some authors have reported that A-com involvement is independent of local recurrence (33). GLCs with A-com infiltration is often associated with a poor clinical course. This might be due to a higher rate of local recurrence compared to the carcinomas that are solely placed on the vocal cords. In this respect, A-com infiltration of the glottis shows distinct anatomical features compared to vocal cords neoplasms (7). For example, in a study by Pedregal-Mallo et al. (3), the local recurrence rate (50%) was higher and the laryngeal conservation rate (75%) was lower in patients with A-com involvement. In our study, we observed a high local recurrence rate in cases with A-com involvement, T1 or T2, especially those who tend to enlarge above the A-com level of the primary lesion during the first examination. For monitoring relapse and granulation tissue, we recommend that microlaryngoscopic follow-up examination should be performed 6 to 8 weeks after tumor resection and continued with long-term follow-up at short intervals.
In the studies by Peretti et al. (34), the local control rate with TLM was found to be low in lesions with A-com involvement and vertical spread. In early-stage GLCs, especially in lesions with A-com involvement and especially with the tendency to supraglottic extension, it is easier to operate with the advantage of exposure to A-com lesions in TLM procedure by taking advantage of the flexibility of diode laser. The negative aspect of TLM is the development of granulation tissue, especially after intervention in the A-com region. This requires a follow-up of the area in the postoperative period. In our study, A-com granuloma formation was found in 18.4% cases in the 1st year and this rate was consistent with the literature (11). In 4% of these cases, histopathological examination revealed the recurrence of A-com epidermoid cancer. After the second TLM procedure, no recurrence was detected.
Preserving the quality of sound in early-stage GLCs is important. TLM and RT are superior to open surgery in terms of preservation of voice. However, complications such as mucosal damage and xerostomia that can develop in RT are not seen in TLM (3, 34, 35). When the respiratory, swallowing, and voice functions of the larynx are evaluated after TLM treatment, the most affected one is voice function. The effect of voice function is related to the location of the lesion and the quality of the TLM procedure. Especially in type I-III resections, less dysphonia, and satisfactory sound quality were obtained. In publications, the voice handicap index (VHI) is between 19 and 28 over 120 (36). İn our study, when VHI was detected between 16 and 23 and scores below 15 were considered to be normal sound quality, this result was found to be quite satisfactory. Type IV-VI resections showed moderate dysphonia and sound quality. In publications, VHI is between 24 and 39 (36, 37). In our study, VHI was 27 to 41. Obviously, this score cannot be achieved with other conventional treatments considering the size of resection performed.
Quality of life (Qol) is generally good after TLM treatment in early-stage GLC, and there is no significant difference between TLM and control group. In a study by Vilaseca et al. (38), they demonstrated that QoL scores of patients treated with TLM were significantly better than those treated with RT and open surgery.
Dysphagia and aspiration after TLM depend on age, tumor stage, and resection size. In the study performed by Nasef et al. (14), a feeding tube was inserted for a maximum of 5 days due to aspiration in 12.5% of patients after type V resection applied to 40 patients with T2 tumors. In our study, aspiration developed in 11% of 54 T2 tumor patients after TLM and feeding tube was inserted for an average of 8 days.
Preoperative and postoperative complications are very low in TLM procedures compared to other conventional treatments. The complication rate increases proportionally with tumor extension and resection width. The most important complication in TLM is bleeding. Perioperative and postoperative bleeding complications of TLM are less complicated than open surgery (39). In our study, 2% of patients developed bleeding in the postoperative period, but it stopped by using medical treatment. The diode laser we used provides excellent hemostasis compared to the CO2 laser. The reason is that the diode laser is highly absorbed by hemoglobin (7).
Other rare complications of TLM are perichondritis and chondronecrosis. These complications are usually treated with laser ablation and/or antibiotics therapies. A study by Ellies and Steiner (39) reported laryngeal stenosis in 2.3% of the cases after TLM intervention. However, because of the chondronecrosis developed in our 5% cases, necrotic cartilage tissues were removed by applying secondary TLM. A laryngeal stenosis complication developed in 1.3% of the patients and we performed a tracheostomy on these 3 cases.
Despite the rarity of halitosis after TLM (36), 78 (34%) cases (21 of them with postop infection) were found in our study. With symptomatic treatment, he disappeared within an average week.
The most dangerous and fatal complication in TLM operations is the burning of the endotracheal tube (40). Serum physiological fluid lavage and wet pet application were performed in one of our patients, who had this negative complication. In order to avoid this complication, heat resistant and suitable for diode laser tube was used.
5.1. Limitations
Focusing only on early-stage GLCs may be a limitation for our study. It is also considered that patients should be followed-up with more reductions in postoperative complications. Further studies were planned to extend our study to include a larger patient series and supraglottis and subglottis cancers.
5.2. Conclusions
TLM provides similar treatment results in early-stage GLCs as RT and open surgical treatment methods do. Thus, the TLM method has become the first choice in terms of maintaining QoL, sound quality, short hospitalization period, low complication rate, low cost, easy application, and preserving the second treatment chance. A-com monitoring must be important in the treatment of TLM. Especially in A-com, tumors with a tendency to spread in the vertical plane should be monitored for local recurrence. Diode (gallium-arsenide) 980-nm laser has advantages over the standard CO2 laser, and TLM treatment stands out in early-stage GLCs. In conclusion, TLM provides an effective treatment option from the point of oncologic control and function preservation in early-stage GLC with minimal complications.
Acknowledgements
References
-
1.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-86. [PubMed ID: 25220842]. https://doi.org/10.1002/ijc.29210.
-
2.
Koc C. [Otorhinolaryngology and head and neck surgery]. 1st ed. Ankara: Gunes Kitabevi; 2003. p. 1141-54. Turkish.
-
3.
Pedregal-Mallo D, Sanchez Canteli M, Lopez F, Alvarez-Marcos C, Llorente JL, Rodrigo JP. Oncological and functional outcomes of transoral laser surgery for laryngeal carcinoma. Eur Arch Otorhinolaryngol. 2018;275(8):2071-7. [PubMed ID: 29869708]. https://doi.org/10.1007/s00405-018-5027-z.
-
4.
Jacobi C, Freundorfer R, Reiter M. Transoral laser microsurgery in early glottic cancer involving the anterior commissure. Eur Arch Otorhinolaryngol. 2019;276(3):837-45. [PubMed ID: 30604057]. https://doi.org/10.1007/s00405-018-5261-4.
-
5.
Canis M, Martin A, Ihler F, Wolff HA, Kron M, Matthias C, et al. Transoral laser microsurgery in treatment of pT2 and pT3 glottic laryngeal squamous cell carcinoma - results of 391 patients. Head Neck. 2014;36(6):859-66. [PubMed ID: 23720321]. https://doi.org/10.1002/hed.23389.
-
6.
Comert E, Tuncel U, Dizman A, Guney YY. Comparison of early oncological results of diode laser surgery with radiotherapy for early glottic carcinoma. Otolaryngol Head Neck Surg. 2014;150(5):818-23. [PubMed ID: 24486784]. https://doi.org/10.1177/0194599814521775.
-
7.
Saetti R, Silvestrini M, Cutrone C, Narne S. Treatment of congenital subglottic hemangiomas: Our experience compared with reports in the literature. Arch Otolaryngol Head Neck Surg. 2008;134(8):848-51. [PubMed ID: 18711059]. https://doi.org/10.1001/archotol.134.8.848.
-
8.
Bajaj Y, Pegg D, Gunasekaran S, Knight LC. Diode laser for paediatric airway procedures: A useful tool. Int J Clin Pract. 2010;64(1):51-4. [PubMed ID: 18422597]. https://doi.org/10.1111/j.1742-1241.2008.01734.x.
-
9.
Usumez A, Cengiz B, Oztuzcu S, Demir T, Aras MH, Gutknecht N. Effects of laser irradiation at different wavelengths (660, 810, 980, and 1,064 nm) on mucositis in an animal model of wound healing. Lasers Med Sci. 2014;29(6):1807-13. [PubMed ID: 23636299]. https://doi.org/10.1007/s10103-013-1336-z.
-
10.
Tuncel U, Comert E. Preliminary results of diode laser surgery for early glottic cancer. Otolaryngol Head Neck Surg. 2013;149(3):445-50. [PubMed ID: 23629970]. https://doi.org/10.1177/0194599813487684.
-
11.
Ferri E, Armato E. Diode laser microsurgery for treatment of Tis and T1 glottic carcinomas. Am J Otolaryngol. 2008;29(2):101-5. [PubMed ID: 18314020]. https://doi.org/10.1016/j.amjoto.2007.03.004.
-
12.
Ma Y, Green R, Pan S, McCabe D, Goldberg L, Woo P. Long-term voice outcome following radiation versus laser microsurgery in early glottic cancer. J Voice. 2019;33(2):176-82. [PubMed ID: 29229412]. https://doi.org/10.1016/j.jvoice.2017.10.018.
-
13.
Sjogren EV. Transoral laser microsurgery in early glottic lesions. Curr Otorhinolaryngol Rep. 2017;5(1):56-68. [PubMed ID: 28367361]. [PubMed Central ID: PMC5357474]. https://doi.org/10.1007/s40136-017-0148-2.
-
14.
Nasef HO, Thabet H, Piazza C, Del Bon F, Eid M, Banna ME, et al. Prospective analysis of functional swallowing outcome after resection of T2 glottic carcinoma using transoral laser surgery and external vertical hemilaryngectomy. Eur Arch Otorhinolaryngol. 2016;273(8):2133-40. [PubMed ID: 27117690]. https://doi.org/10.1007/s00405-016-4065-7.
-
15.
Diaz-de-Cerio P, Preciado J, Santaolalla F, Sanchez-Del-Rey A. Cost-minimisation and cost-effectiveness analysis comparing transoral CO(2) laser cordectomy, laryngofissure cordectomy and radiotherapy for the treatment of T1-2, N0, M0 glottic carcinoma. Eur Arch Otorhinolaryngol. 2013;270(4):1181-8. [PubMed ID: 22872061]. https://doi.org/10.1007/s00405-012-2139-8.
-
16.
Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz AG, Greene FL. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
-
17.
Remacle M, Van Haverbeke C, Eckel H, Bradley P, Chevalier D, Djukic V, et al. Proposal for revision of the European Laryngological Society classification of endoscopic cordectomies. Eur Arch Otorhinolaryngol. 2007;264(5):499-504. [PubMed ID: 17377801]. https://doi.org/10.1007/s00405-007-0279-z.
-
18.
Rosen CA, Lee AS, Osborne J, Zullo T, Murry T. Development and validation of the voice handicap index-10. Laryngoscope. 2004;114(9):1549-56. [PubMed ID: 15475780]. https://doi.org/10.1097/00005537-200409000-00009.
-
19.
Preuss SF, Cramer K, Klussmann JP, Eckel HE, Guntinas-Lichius O. Transoral laser surgery for laryngeal cancer: Outcome, complications and prognostic factors in 275 patients. Eur J Surg Oncol. 2009;35(3):235-40. [PubMed ID: 18281184]. https://doi.org/10.1016/j.ejso.2008.01.012.
-
20.
Smith JC, Myers EN. Progress in laryngeal surgery. Head Neck. 2002;24(10):955-64. [PubMed ID: 12369075]. https://doi.org/10.1002/hed.10140.
-
21.
Low TH, Yeh D, Zhang T, Araslanova R, Hammond JA, Palma D, et al. Evaluating organ preservation outcome as treatment endpoint for T1aN0 glottic cancer. Laryngoscope. 2017;127(6):1322-7. [PubMed ID: 27778345]. https://doi.org/10.1002/lary.26317.
-
22.
Osguthorpe JD, Putney FJ. Open surgical management of early glottic carcinoma. Otolaryngol Clin North Am. 1997;30(1):87-99. [PubMed ID: 8995138].
-
23.
Abdurehim Y, Hua Z, Yasin Y, Xukurhan A, Imam I, Yuqin F. Transoral laser surgery versus radiotherapy: Systematic review and meta-analysis for treatment options of T1a glottic cancer. Head Neck. 2012;34(1):23-33. [PubMed ID: 21374753]. https://doi.org/10.1002/hed.21686.
-
24.
Kayhan FT, Koc AK, Erdim I. Oncological outcomes of early glottic carcinoma treated with transoral robotic surgery. Auris Nasus Larynx. 2019;46(2):285-93. [PubMed ID: 30217617]. https://doi.org/10.1016/j.anl.2018.08.015.
-
25.
Hinni ML, Salassa JR, Grant DG, Pearson BW, Hayden RE, Martin A, et al. Transoral laser microsurgery for advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2007;133(12):1198-204. [PubMed ID: 18086960]. https://doi.org/10.1001/archotol.133.12.1198.
-
26.
Peretti G, Piazza C, Del Bon F, Mora R, Grazioli P, Barbieri D, et al. Function preservation using transoral laser surgery for T2-T3 glottic cancer: Oncologic, vocal, and swallowing outcomes. Eur Arch Otorhinolaryngol. 2013;270(8):2275-81. [PubMed ID: 23568037]. https://doi.org/10.1007/s00405-013-2461-9.
-
27.
Alkan S, Sozen E, Baylancicek S. Analysis of recurrence after frontolateral laryngectomy. Tukish Arch Otolaryngol. 2008;46(4):298-301.
-
28.
Peretti G, Piazza C, Cocco D, De Benedetto L, Del Bon F, Redaelli De Zinis LO, et al. Transoral CO(2) laser treatment for T(is)-T(3) glottic cancer: The University of Brescia experience on 595 patients. Head Neck. 2010;32(8):977-83. [PubMed ID: 19902535]. https://doi.org/10.1002/hed.21278.
-
29.
Mendenhall WM, Werning JW, Hinerman RW, Amdur RJ, Villaret DB. Management of T1-T2 glottic carcinomas. Cancer. 2004;100(9):1786-92. [PubMed ID: 15112257]. https://doi.org/10.1002/cncr.20181.
-
30.
Lucioni M, Marioni G, Bertolin A, Giacomelli L, Rizzotto G. Glottic laser surgery: Outcomes according to 2007 ELS classification. Eur Arch Otorhinolaryngol. 2011;268(12):1771-8. [PubMed ID: 21769448]. https://doi.org/10.1007/s00405-011-1695-7.
-
31.
Canis M, Ihler F, Martin A, Matthias C, Steiner W. Transoral laser microsurgery for T1a glottic cancer: review of 404 cases. Head Neck. 2015;37(6):889-95. [PubMed ID: 24623709]. https://doi.org/10.1002/hed.23688.
-
32.
Chone CT, Yonehara E, Martins JE, Altemani A, Crespo AN. Importance of anterior commissure in recurrence of early glottic cancer after laser endoscopic resection. Arch Otolaryngol Head Neck Surg. 2007;133(9):882-7. [PubMed ID: 17875854]. https://doi.org/10.1001/archotol.133.9.882.
-
33.
Hoffmann C, Cornu N, Hans S, Sadoughi B, Badoual C, Brasnu D. Early glottic cancer involving the anterior commissure treated by transoral laser cordectomy. Laryngoscope. 2016;126(8):1817-22. [PubMed ID: 26597482]. https://doi.org/10.1002/lary.25757.
-
34.
Peretti G, Piazza C, Mora F, Garofolo S, Guastini L. Reasonable limits for transoral laser microsurgery in laryngeal cancer. Curr Opin Otolaryngol Head Neck Surg. 2016;24(2):135-9. [PubMed ID: 26963672]. https://doi.org/10.1097/MOO.0000000000000240.
-
35.
Bumber Z, Prgomet D, Janjanin S. Endoscopic CO2 laser surgery for supraglottic cancer--ten years of experience. Coll Antropol. 2009;33(1):87-91. [PubMed ID: 19408609].
-
36.
Roh JL, Kim DH, Kim SY, Park CI. Quality of life and voice in patients after laser cordectomy for Tis and T1 glottic carcinomas. Head Neck. 2007;29(11):1010-6. [PubMed ID: 17510971]. https://doi.org/10.1002/hed.20625.
-
37.
Fink DS, Sibley H, Kunduk M, Schexnaildre M, Kakade A, Sutton C, et al. Subjective and objective voice outcomes after transoral laser microsurgery for early glottic cancer. Laryngoscope. 2016;126(2):405-7. [PubMed ID: 26597360]. https://doi.org/10.1002/lary.25442.
-
38.
Vilaseca I, Bernal-Sprekelsen M, Him R, Mandry A, Lehrer E, Blanch JL. Prognostic factors of quality of life after transoral laser microsurgery for laryngeal cancer. Eur Arch Otorhinolaryngol. 2015;272(5):1203-10. [PubMed ID: 24728230]. https://doi.org/10.1007/s00405-014-3030-6.
-
39.
Ellies M, Steiner W. Peri- and postoperative complications after laser surgery of tumors of the upper aerodigestive tract. Am J Otolaryngol. 2007;28(3):168-72. [PubMed ID: 17499132]. https://doi.org/10.1016/j.amjoto.2006.08.008.
-
40.
Dhar P, Malik A. Anesthesia for laser surgery in ENT and the various ventilatory techniques. Trends in Anaesthesia and Critical Care. 2011;1(2):60-6. https://doi.org/10.1016/j.tacc.2011.01.011.