Abstract
Background:
Recent studies have suggested a significant role for Caveolin-1 (CAV1) gene in the pathogenesis of breast carcinomas; however, current evidence is conflicting as some report a tumor suppression effect while others implicate this gene as a promoter of cancer development.Objectives:
The present study aimed to provide further evidence on this subject by comparing Caveolin-1 expression between specimens obtained from normal breast tissues, benign lesions and malignant tumors.Methods:
In this cross-sectional study, subjects with specimens resected from their breast tissues for various reasons in Shohadaye-Tajrish Hospital, Tehran, Iran during 2013 were included as the sample population. A total of 100 subjects participated in this survey (53 invasive breast cancer, 26 ductal carcinoma in situ (DCIS), 13 ductal hyperplasia, and 8 normal). Their tissue specimens were prepared and immunohistochemical studies were performed to assess the expression of Caveolin-1, human epidermal growth factor receptor 2 (HER2), estrogen receptor (ER), and progesterone receptor (PR). Correlations between these factors and the survival of patients were assessed using SPSS software.Results:
Epithelial Caveolin-1 expression was found to have a positive significant correlation with pathology group of the patients (P = 0.039), grade of the tumor (P = 0.032), and lymph node positivity (P = 0.046). A negative correlation was also observed between epithelial Caveolin-1 positivity with HER2 score (P = 0.036) and HER2 positivity (P = 0.012). The overall survival of the patients was negatively affected by epithelial Caveolin-1 expression (28.3 vs. 38.9 months; P = 0.001), grade of the tumor (P < 0.001), and lymph node involvement (34.1 vs. 40.5; P = 0.051), while ER (39.7 vs. 28.9; P = 0.001) and PR (39.6 vs. 31.0; P = 0.010) positivity were associated with improved survival.Conclusions:
According to the findings, it seems that Caveolin-1 expression might be associated with worse outcomes in patients with breast cancer.Keywords
Breast Cancer Caveolin-1 Human Epidermal Growth Factor Receptor 2 Estrogen Receptor Progesterone Receptor
1. Background
Breast cancer is the most common cancer among women, comprising 29% of all new cases diagnosed with cancers in this population. It is also the second most common cause of cancer-related deaths in women, accounting for 14% of these deaths annually (1). Accordingly, extensive research aimed at revealing the complex interactions between growth factors, steroids, oncogenes, and tumor suppressor genes that might be involved in breast carcinogenesis has been conducted.
In this regard, recent studies have suggested a significant role for Caveolin-1 (CAV1) gene in the pathogenesis of breast carcinomas (2, 3). This gene is located at 7q31.1, encodes an integral membrane protein (4, 5), and has been reported to be frequently deleted in breast cancers (5). Caveolin proteins participate in the formation of caveolae, omega-shaped invaginations of the plasma membrane that is involved in various biological functions including endocytosis, transcytosis, cell adhesion and migration, molecular transport, and signal transduction (6).
Caveolin-1 mediates the transport of cholesterol, fatty acid, low-density lipoprotein and, albumin and facilitates insulin secretion through interacting with G-protein coupled receptor, via ATP-dependent potassium channels (2). It has also been reported to play an important role in cell proliferation by binding to several proteins such as epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGF), endothelial nitric oxide synthase, H-ras, sex combs reduced (Scr) proteins, human epidermal growth factor receptor 2 (HER2) and protein kinase C (7-9).
Current evidence on the role of CAV1 in human cancers is conflicting. Dysregulations of CAV1 expression have been reported in breast cancer cells (10-12). Its forced expression in these cell lines has been found to exert inhibitory effects on cancer progression and metastasis (13). An inactivating substitution at codon 132 (proline to leucine) within the CAV1 gene was first reported in 2001 to be present in 16% of primary breast cancer specimens. This mutation was hypothesized to induce premalignant changes in mammary epithelial (14, 15). However, this association was later found to be restricted to estrogen receptor (ER)-positive breast cancers (16). On the other hand, CAV1 has also been reported to have oncogenic properties as well. Its transfection into breast cancer cell lines was found to induce proliferation and formation of colonies (17). Studies have also shown the overexpression of CAV1 in primary breast cancers (18). Gupta et al. suggested that whether Caveolin-1 exerts tumor suppressor effects or oncogenic properties depends on the stage of carcinogenesis (3). It seems that at the initial stages, this CAV1 expression is associated with tumor suppression.
There is also evidence on the relation between CAV1 expression and treatment response in patients with breast cancer. Increased expression of this gene was reported to be associated with resistance against trastuzumab (19), while in another study conducted on breast cancer cells, the loss of CAV1 expression was linked to resistance against tamoxifen (20). Wang et al. also reported upregulation in CAV1 expression in breast cancer stem cells, particularly post-chemotherapy, and suggested the expression of this gene to be responsible for resistance to chemotherapy (21).
2. Objectives
Considering the disagreements between studies on the role of Caveolin-1 in breast carcinomas, the present study aimed to provide further evidence on this subject by comparing CAV1 expression between specimens obtained from normal breast tissues, benign lesions, and malignant tumors.
3. Methods
3.1. Study Design and Sample Population
In this cross-sectional study, the target population was determined as the patients who had specimens resected from their breast tissues for various reasons in Shohadaye Tajrish Hospital, Tehran, Iran during 2013. Eventually, through convenience sampling method, a total of 100 participants were recruited for the study, of which 53 were diagnosed with invasive breast cancer, 26 had ductal carcinoma in situ (DCIS), 13 had ductal hyperplasia and 8 individuals had undergone breast reduction mammoplasty and were included as normal subjects. Resected specimens obtained from the participants who underwent immunohistochemical evaluations to determine positivity of ER, progesterone receptor (PR), HER2, and Caveolin-1 in components of stromal and epithelial. Patients with invasive breast cancer were contacted to acquire information about their final outcome, and if expired, their time of death was recorded for survival analysis.
3.2. Immunohistochemistry
Paraffin-embedded tissue blocks fixed via the routine procedure were prepared and sectioned with a thickness of 3 µm. Sections were transferred to micro slides coated with polylysine, were dewaxed and hydrated again. Immersed in Reveal Emulgator (Biocarta, Hamburg, Germany), the slides were boiled for 5 min in a pressure cooker at a pressure of 103 kPa for antigen retrieval. In order to block the binding of unspecific agents, after being washed in distilled water and phosphate buffered saline, the slides were exposed to Aurion-BSA-c10% (Aurion, Wageningen, Netherlands). Subsequently, the sections were subjected to the mice-derived primary monoclonal IgG1-anti-Caveolin-1 antibody (Biosciences Pharmingen, Heidelberg, Germany) for at least 8 hours at a temperature of 4ºC. After being washed in PBS, to inhibit endogenous peroxidase activity, hydrogen peroxidase-containing methanol was applied to the slides and then they were incubated for one hour with goat-derived anti-mouse immunoglobulins conjugated with dextran polymer that was labeled with horseradish peroxidase (HRP). The sections were washed again in PBS and then using Vector SG Substrate Kit for HRP (Vector Laboratories, Burlingame, CA, USA), peroxidase enzyme was visualized and nuclear counterstaining was performed with hematoxylin. Smooth muscle cells and endothelial cells were considered as positive controls, as they contain high amounts of Caveolin-1, while slides that were not exposed to the primary antibody were considered as negative controls.
Expression of estrogen receptor (ER) and progesterone receptor (PR) was evaluated using mouse-derived anti-ER monoclonal antibody (DAKO, Denmark) and mouse-derived anti-PR monoclonal antibody (DAKO, Carpinteria, CA, USA). Immunostaining was performed as described for immunohistochemistry of Caveolin-1 with an incubation period of 25 min.
In immunohistochemistry assessment for HER2, the steamer was used for antigen retrieval before treatment. Sections were incubated for 25 min with diluted (1:4000) polyclonal anti-c-ErbB2 antibody (DAKO, oncoprotein) and LSAB-kit was used for detection.
3.3. Microscopic Assessments
Regardless of cytoplasmic staining, staining of the cell membranes determined Caveolin-1 positivity, Entrapped vessels within the sections were considered as internal positive controls for the evaluation of Caveolin-1 expression. Assessments were performed for two distinct epithelial and stromal components of the breast tissue and the specimens were classified as Caveolin-1 negative and positive, accordingly. Caveolin-1 positive sections were further scored semi-quantitatively based on the pattern of staining as focal, patchy, diffuse (weak), diffuse (moderate), and diffuse (strong) (Figure 1).
Caveolin-1 staining in the specimens: (A) epithelial component; (B1 and B2) myoepithelial component as the positive control; (C1) weak staining of stromal component; (C2) moderate staining of stromal component; (C1) strong staining of stromal component

Staining for ER and PR were also scored semi-quantitatively in accordance to the Quick Score method (22) on a total scale of 0 to 7 which is determined by adding the two scores of staining intensity (1: weak, 2: moderate, 3: strong) and the percentage of nuclei-positive cells (1: less than 25%, 2: 25 to 50%, 3: 50 to 75%, 4: greater than 75%). A score of 0 - 3 was considered as negative and a score of 4 - 7 was recorded as positive.
HER2 expression was scored on a semi-quantitative scale of 0: membrane staining in less than 10% of tumor cells, 1+: faint partial membrane staining in more than 10% of tumor cells, 2+: weak to moderate membrane staining in more than 10% of tumor cells, and 3+: strong staining of the entire membrane in more than 10% of tumor cells, as described by Wulfing et al. (23). Tissue specimens that scored as 0 and 1+ were classified as HER2-negative, 2+ was considered borderline, and 3+ was recorded as HER2-positive. In order to separate the highest intensity of HER2 staining from lower levels, a dichotomous variable was also defined with 2+ specimens considered as HER2-negative as well.
3.4. Ethical Considerations
The aims and methods of the present study were thoroughly explained to the patients and they were reassured that their inclusion in this survey will not affect their treatment, their data will be considered confidential, used anonymously and will only be accessible by the main researchers of the study and they can withdraw from the study at their will. Witten informed consent was obtained from the all participants. The Ethics Committee of Shahid Beheshti University of Medical Sciences reviewed and approved the study protocol. The survey was conducted in accordance with the guidelines of the Helsinki’s Declaration.
3.5. Statistical Analysis
Data were entered into SPSS software for windows version 22.0 (IBM corp., Armonk, NY, USA) for analysis (24). Descriptive statistics of the results were presented as frequency and percentage since all the evaluated variables were qualitative. To assess the correlation between these factors chi-squared test and Fisher’s exact test were used as needed. Kaplan-Meier method was used to assess the survival of the patients with invasive breast cancer and determine its correlation with evaluated markers. Statistical significance was defined as P Value less than 0.05 in all analysis.
4. Results
4.1. Descriptive Statistics
As mentioned, a total of 100 subjects participated in this survey, including 53 (53.0%) patients with invasive breast cancer, 26 (26.0%) with DCIS, 13 (13.0%) with ductal hyperplasia, and 8 (8.0%) normal subjects. Table 1 presents the descriptive statistics of the sample population.
Descriptive Statistics of Evaluated Variables
| Variables | Count | Percent % |
|---|---|---|
| Group | ||
| Normal | 8 | 8.0 |
| Ductal hyperplasia | 13 | 13.0 |
| DCIS | 26 | 26.0 |
| Invasive | 53 | 53.0 |
| Epithelial Caveolin-1 | ||
| Negative | 82 | 82.0 |
| Positive | 18 | 18.0 |
| Epithelial staining pattern | ||
| Focal | 2 | 11.1 |
| Patchy | 4 | 22.2 |
| Diffuse (weak) | 2 | 11.1 |
| Diffuse (moderate) | 7 | 38.9 |
| Diffuse (strong) | 3 | 16.7 |
| Stromal Caveolin-1 | ||
| Negative | 6 | 6.0 |
| Positive | 94 | 94.0 |
| Stromal staining pattern | ||
| Focal | 0 | 0.0 |
| Patchy | 10 | 10.6 |
| Diffuse (weak) | 25 | 26.6 |
| Diffuse (moderate) | 46 | 48.9 |
| Diffuse (strong) | 13 | 13.8 |
| Grade | ||
| 1 | 4 | 9.5 |
| 2 | 18 | 42.9 |
| 3 | 20 | 47.6 |
| Lymph node | ||
| Negative | 13 | 25.5 |
| Positive | 38 | 74.5 |
| ER | ||
| Negative | 18 | 43.9 |
| Positive | 23 | 56.1 |
| PR | ||
| Negative | 20 | 48.8 |
| Positive | 21 | 51.2 |
| HER2 score | ||
| 0-1+ (negative) | 24 | 53.3 |
| 2+ (borderline) | 6 | 13.3 |
| 3+ (positive) | 15 | 33.3 |
| HER2 | ||
| Negative | 30 | 66.7 |
| Positive | 15 | 33.3 |
In all included subjects, Caveolin-1 was expressed in the myoepithelial component of the specimens, which was evaluated as the positive control in the study. Epithelial Caveolin-1 staining was reported to be negative in the majority of included subjects (82.0%). Among the 18 participants with positive epithelial Caveolin-1, staining was found to be focal in 2 subjects, patchy in 4, diffuse (weak) in 2, diffuse (moderate) in 7 and diffuse (strong) in 3 individuals. On the other hand, stromal Caveolin-1 staining was positive in 94.0% of the sample population with the staining pattern found to be patchy in 10 subjects, diffuse (weak) in 25, diffuse (moderate) in 46 and diffuse (strong) in 13 participants.
The grade of the tumor was determined in 42 patients with invasive breast cancer among which, 4 patients (9.5%) were reported as grade 1, 18 (42.9%) were reported as grade 2, and 20 (47.6%) were reported to have grade 3 breast tumors. Lymph node involvement was positive in 38 (74.5%) of these patients. As for the hormone receptors, 23 patients (56.1%) were ER-positive and 21 (51.2%) were PR-positive. HER2 expression was classified as 0-1+ or negative in 24 patients (53.3%), 2+ or borderline in 6 (13.3%), and 3+ or positive in 15 (33.3%) subjects. Considering borderlines as negative, 15 patients (33.3%) were HER2-positive and 30 (66.7%) were HER2-negative.
The patients with invasive breast cancer were followed for an average of 32.3 ± 8.9 months with a minimum of 10 and a maximum of 42 months. During this period, 16 patients (31.4%) expired and the overall survival was calculated to be 35.7 months.
4.2. Analytical Statistics
As presented in Table 2, epithelial Caveolin-1 positivity was found to have a positive significant correlation with the group of patients (P = 0.039), grade of the tumor in patients with invasive breast cancer (P = 0.032), and lymph node positivity (P = 0.046). Accordingly, the highest rates of positive epithelial Caveolin-1 were found in patients with invasive breast cancer (28.3%), subjects with grade 3 tumors (40.0%), and lymph node positive patients (36.8%). On the other hand, a negative correlation was observed between epithelial Caveolin-1 positivity with HER2 score (P = 0.036) and HER2 positivity (P = 0.012) in subjects who were diagnosed with invasive breast cancer. As for the staining pattern, none of the evaluated factors showed a significant association with the epithelial staining pattern.
Correlation Between Epithelial Caveolin-1 Staining and Its Patterns with Evaluated Variables in the Studya
| Variables | Epithelial Caveolin-1 | P Value | Epithelial Staining Pattern | P Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Negative (N = 82) | Positive (N = 18) | Focal (N = 2) | Patchy (N = 4) | Diffuse (Weak) (N = 2) | Diffuse (Moderate) (N = 7) | Diffuse (Strong) (N = 3) | |||
| Group | 0.039 | 0.062 | |||||||
| Normal | 8 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Ductal hyperplasia | 12 (92.3) | 1 (7.7) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| DCIS | 24 (92.3) | 2 (7.7) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Invasive | 38 (71.7) | 15 (28.3) | 0 (0.0) | 3 (20.0) | 2 (13.3) | 7 (46.7) | 3 (20.0) | ||
| Grade | 0.032 | 0.630 | |||||||
| 1 | 4 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 2 | 15 (83.3) | 3 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 1 (33.3) | ||
| 3 | 10 (50.0) | 10 (50.0) | 0 (0.0) | 3 (30.0) | 1 (10.0) | 4 (40.0) | 2 (20.0) | ||
| Lymph node | 0.046 | 0.232 | |||||||
| Negative | 12 (92.3) | 1 (7.7) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Positive | 24 (63.2) | 14 (36.8) | 0 (0.0) | 2 (14.3) | 2 (14.3) | 7 (50.0) | 3 (21.4) | ||
| ER | 0.923 | 0.120 | |||||||
| Negative | 12 (66.7) | 6 (33.3) | 0 (0.0) | 3 (50.0) | 1 (16.7) | 1 (16.7) | 1 (16.7) | ||
| Positive | 15 (65.2) | 8 (34.8) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 5 (62.5) | 2 (25.0) | ||
| PR | 0.368 | 0.061 | |||||||
| Negative | 15 (75.0) | 5 (25.0) | 0 (0.0) | 3 (60.0) | 1 (20.0) | 1 (20.0) | 0 (0.0) | ||
| Positive | 13 (61.9) | 8 (38.1) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 5 (62.5) | 2 (25.0) | ||
| HER2 score | 0.036 | 0.587 | |||||||
| 0-1+ (negative) | 13 (54.2) | 11 (45.8) | 0 (0.0) | 3 (27.3) | 1 (9.1) | 4 (36.4) | 3 (27.3) | ||
| 2+ (borderline) | 4 (66.7) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | ||
| 3+ (positive) | 14 (93.3) | 1 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | ||
| HER2 | 0.012 | 0.697 | |||||||
| Negative | 17 (56.7) | 13 (43.3) | 0 (0.0) | 3 (23.1) | 2 (15.4) | 5 (38.5) | 3 (23.1) | ||
| Positive | 14 (93.3) | 1 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | ||
Table 3 presents the correlations between stromal Caveolin-1 staining and its pattern with an evaluated factor in the study. Based on the findings, none of the evaluated variables were found to have a significant correlation with neither stromal Caveolin-1 positivity nor its observed pattern in the slides.
Correlation Between Stromal Caveolin-1 Staining and Its Patterns with Evaluated Variables in the Studya
| Variables | Stromal Caveolin-1 | P Value | Stromal Staining Pattern | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Negative (N = 6) | Positive (N = 94) | Patchy (N = 10) | Diffuse (Weak) (N = 25) | Diffuse (Moderate) (N = 46) | Diffuse (Strong) (N = 13) | |||
| Group | ||||||||
| Normal | 1 (12.5) | 7 (87.5) | 0.241 | 2 (28.6) | 2 (28.6) | 2 (28.6) | 1 (14.3) | 0.225 |
| Ductal hyperplasia | 0 (0.0) | 13 (100.0) | 2 (15.4) | 5 (38.5) | 4 (30.8) | 2 (15.4) | ||
| DCIS | 0 (0.0) | 26 (100.0) | 1 (3.8) | 3 (11.5) | 19 (73.1) | 3 (11.5) | ||
| Invasive | 5 (9.4) | 48 (90.6) | 5 (10.4) | 15 (31.3) | 21 (43.8) | 7 (14.6) | ||
| Grade | ||||||||
| 1 | 0 (0.0) | 4 (100.0) | 0.693 | 0 (0.0) | 0 (0.0) | 3 (75.0) | 1 (25.0) | 0.521 |
| 2 | 2 (11.1) | 16 (88.9) | 3 (18.8) | 6 (37.5) | 5 (31.3) | 2 (12.5) | ||
| 3 | 3 (15.0) | 17 (85.0) | 1 (5.9) | 6 (35.3) | 8 (47.1) | 2 (11.8) | ||
| Lymph node | ||||||||
| Negative | 2 (15.4) | 11 (84.6) | 0.433 | 1 (9.1) | 4 (36.4) | 3 (27.3) | 3 (27.3) | 0.482 |
| Positive | 3 (7.9) | 35 (92.1) | 4 (11.4) | 10 (28.6) | 17 (48.6) | 4 (11.4) | ||
| ER | ||||||||
| Negative | 3 (16.7) | 15 (83.3) | 0.439 | 1 (6.7) | 6 (40.0) | 6 (40.0) | 2 (13.3) | 0.724 |
| Positive | 2 (8.7) | 21 (91.3) | 4 (19.0) | 6 (28.6) | 8 (38.1) | 3 (14.3) | ||
| PR | ||||||||
| Negative | 3 (15.0) | 17 (85.0) | 0.592 | 2 (11.8) | 6 (35.3) | 8 (47.1) | 1 (5.9) | 0.793 |
| Positive | 2 (9.5) | 19 (90.5) | 2 (10.5) | 7 (21.1) | 7 (36.8) | 3 (15.8) | ||
| HER2 score | ||||||||
| 0-1+ (negative) | 4 (16.7) | 20 (83.3) | 0.407 | 2 (10.0) | 7 (35.0) | 8 (40.0) | 3 (15.0) | 0.603 |
| 2+ (borderline) | 0 (0.0) | 6 (100.0) | 2 (33.3) | 1 (16.7) | 3 (50.0) | 0 (0.0) | ||
| 3+ (positive) | 1 (6.7) | 14 (93.3) | 1 (7.1) | 5 (35.7) | 5 (35.7) | 3 (21.4) | ||
| HER2 | ||||||||
| Negative | 4 (13.3) | 26 (86.7) | 0.502 | 4 (15.4) | 8 (30.8) | 11 (42.3) | 3 (11.5) | 0.740 |
| Positive | 1 (6.7) | 14 (93.3) | 1 (7.1) | 5 (35.7) | 5 (35.7) | 3 (21.4) | ||
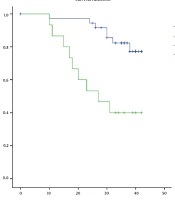
The overall survival of patients with invasive breast cancer according to the evaluated variables is presented in Table 4. Results showed that the overall survival of the patients with epithelial Caveolin-1 expression was significantly lower than subjects with negative expression of this protein (28.3 vs. 38.9 months; P = 0.001) (Figure 2), however, the positivity of stromal Caveolin-1 did not significantly affect the overall survival of patients. A grade of the tumor (P < 0.001) and lymph node involvement (34.1 vs. 40.5; P = 0.051) negatively affected the overall survival, while ER (39.7 vs. 28.9; P = 0.001) and PR (39.6 vs. 31.0; P = 0.010) positivity was found to significantly improve survival in these patients. HER2 score and HER2 positivity showed no significant effects on the overall survival of patients with invasive breast cancer.
Overall survival of patients with invasive breast cancer according to their epithelial Caveolin-1 expression status

Correlation Between Stromal Caveolin-1 Staining and Its Patterns with Evaluated Variables in the Study
| N (Column %) | Events (Row %) | Overall Survival, Mean ± SE | P Value (Log-Rank test) | |
|---|---|---|---|---|
| Group | - | |||
| Invasive | 53 (100) | 16 (30.2) | 35.7 ± 1.4 | |
| Epithelial Caveolin-1 | 0.001 | |||
| Negative | 38 (71.7) | 7 (18.4) | 38.9 ± 1.2 | |
| Positive | 15 (28.3) | 6 (60.0) | 28.3 ± 3.2 | |
| Stromal Caveolin-1 | 0.657 | |||
| Negative | 5 (9.4) | 2 (40.0) | 32.6 ± 4.0 | |
| Positive | 48 (90.6) | 14 (29.2) | 35.9 ± 1.5 | |
| Grade | < 0.001 | |||
| 1 | 4 (9.5) | 0 (0.0) | - | |
| 2 | 18 (42.9) | 2 (11.9) | - | |
| 3 | 20 (47.6) | 14 (60.0) | - | |
| Lymph node | 0.051 | |||
| Negative | 13 (25.5) | 1 (7.7) | 40.5 ± 1.4 | |
| Positive | 38 (74.5) | 15 (39.5) | 34.1 ± 1.8 | |
| ER | 0.001 | |||
| Negative | 18 (43.9) | 11 (61.1) | 28.9 ± 2.4 | |
| Positive | 23 (56.1) | 3 (13.0) | 39.7 ± 1.3 | |
| PR | 0.010 | |||
| Negative | 20 (48.8) | 10 (50.0) | 31.0 ± 2.3 | |
| Positive | 21 (51.2) | 18 (14.3) | 39.6 ± 1.4 | |
| HER2 score | 0.891 | |||
| 0-1+ (negative) | 24 (53.3) | 8 (33.3) | 35.3 ± 2.1 | |
| 2+ (borderline) | 6 (13.3) | 1 (16.7) | 38.2 ± 2.6 | |
| 3+ (positive) | 15 (33.3) | 5 (33.3) | 34.3 ± 2.4 | |
| HER2 | 0.878 | |||
| Negative | 30 (66.7) | 9 (30.0) | 36.0 ± 1.8 | |
| Positive | 15 (33.3) | 5 (33.3) | 34.3 ± 2.4 |
5. Discussion
The present study, using immunohistochemical studies, assessed Caveolin-1 expression in a sample of Iranian patients diagnosed with invasive breast cancer, DCIS, and ductal hyperplasia along with 8 normal subjects. Findings showed a positive significant correlation between epithelial Caveolin-1 positivity with the diagnosis of the patients, grade of the tumor in patients with invasive breast cancer, and the involvement of lymph nodes. In addition, a negative correlation was also observed between epithelial Caveolin-1 positivity with HER2 score and HER2 positivity in subjects diagnosed with invasive breast cancers. On the other hand, the expression of Caveolin-1 in the stromal component of the specimens had no significant association with any of the evaluated factors.
Survival analysis also showed a significantly lower overall survival in patients with epithelial Caveolin-1 expression compared to those subjects with negative expression of this protein. The grade of the tumor and lymph node involvement negatively affected the overall survival, while ER and PR positivity were associated with improved survival in these patients. Overall, it seems that Caveolin-1 expression might be associated with worse outcomes in patients with breast cancer.
Multiple studies have performed immunohistochemical analyses to evaluate Caveolin-1 expression in human breast cancer and its clinical relevance. In the survey conducted by Yang et al. Caveolin-1 expression was assessed in a sample of patients with invasive breast cancer (n = 15), intraductal breast cancer (n = 15), and lymph node metastasis (n = 9). They reported Caveolin-1 expression to be significantly higher in all these three groups of patients, compared to normal breast epithelium, which was also found to be minimally stained (25). In another study, Hurlstone et al. reported no epithelial expression of Caveolin-1 in specimens obtained from 10 normal subjects who had undergone reduction mammoplasties. It was found to be highly expressed in breast epithelial cells of these subjects and subsequently, tumors derived from breast myoepithelium had high levels of Caveolin-1 expression (26). The findings of this survey was quite compatible with our results, as we also found all of our normal subjects to be epithelial Caveolin-1 negative, while specimens obtained from invasive breast cancers had higher rates of Caveolin-1 expression.
Savage et al. also assessed Caveolin-1 expression and its distribution in benign and malignant breast lesions, breast cancer precursors, and normal breast tissue. They reported no expression of Caveolin-1 in the epithelial component of normal breast tissues or the luminal epithelial cells of benign lesions. However, in 13.4% of the cases with DCIS and 9.4% of the invasive breast cancer patients, Caveolin-1 expression was observed in their luminal epithelial cells. They found Caveolin-1 expression to be negatively correlated with expression of hormonal markers (ER and PR), HER2, and cyclin D1. In addition, higher rates of Caveolin-1 positivity were reported in higher grade tumors which were also found to be a significant correlation. Disease-free survival and overall survival were also found to be significantly lower in patients with Caveolin-1 expression (18). The results of their study were comparable to our findings. Similarly, none of the specimens obtained from our normal participants expressed epithelial Caveolin-1 in their immunohistochemical evaluations, while expression of this protein was observed in 7.7% of subjects with ductal hyperplasia, 7.7% of patients with DCIS, and 28.3% of cases diagnosed with invasive breast cancer. Although we found no significant correlation between Caveolin-1 expression in the epithelium with ER and PR status of our subjects, congruent with Savage et al.’s study, we reported a negative significant association between expression of this protein and HER2 positivity. Caveolin-1 expression negatively affected overall survival of our patients as well.
In another study conducted by Zhang et al. the relationship between expression of Caveolin-1 with clinical findings and expression of EGFR, HER2, and Ki-67 was evaluated using immunohistochemical techniques in 50 patients diagnosed with invasive breast cancer. Their results showed Caveolin-1 expression to be significantly correlated with lymph node metastasis and grade of the tumor. Although they found the rate of HER2 positivity was lower in specimens where Caveolin-1 was highly expressed, the differences were not statistically significant with a borderline P value of 0.067 (27). Hence, it could be said that Zhang et al.’s findings were compatible with our findings. However, in their survival analysis, patients with low Caveolin-1 levels were found to have lower survival compared to subjects with high Caveolin-1 expression. In this regard, the findings of the two studies are incompatible.
In the study conducted by Liedtke et al., they reported no Caveolin-1 expression in the epithelial component of specimens obtained from 5 normal breast tissues, 295 benign lesions, and 108 DCIS cases, while 29.4% of 109 invasive breast cancer specimens were found to be positive for Caveolin-1 expression. These researchers found no significant correlation between epithelial Caveolin-1 expression neither with evaluated clinical and pathological markers, nor with overall survival or disease-free survival (28). Hence, the higher rate of Caveolin-1 expression they reported in patients with invasive breast cancer was compatible with our findings. Given the effects of Caveolin-1 expression on patients’ survival, the results of the two surveys were incompatible.
As can be seen, there are still many disagreements between the studies that have evaluated the role of Caveolin-1 in human breast cancer. Although some evidence suggests a tumor suppressive role for Caveolin-1 in development of breast cancer (29), others have implicated overexpression of this protein as a promoting factor for certain steps of carcinogenesis such as inhibiting anoikis in MCF7 breast cancer cells (30). Breast cancer cell growth that is induced by medroxyprogesterone acetate has also been shown to be mediated by Caveolin-1 (31). Non-inflammatory carcinomas have been reported to present with lower Caveolin-1 expression compared to inflammatory breast cancer, which is a very aggressive form of invasive breast cancer (32). These discrepancies could be justified to some extent by the hypothesis that Gupta et al. proposed stating that whether Caveolin-1 exerts tumor suppressor effects or oncogenic properties, depends on the stage of carcinogenesis (3). It seems that at the initial stages of breast cancer development, Caveolin-1 expression is associated with tumor suppression.
Caveolin-1 protein has been reported to be involved in regulating the function of caveolae by altering the assembly of cell membrane components that are responsible for signal transduction and interactions between distinct pathways (2). The role of this membrane protein in gathering different protein components near each other in the caveolae could be the key to its dual effects reported by current evidence. Also, the combination of proteins present in the cell determines the overall effect rather than the expression of Caveolin-1 alone. Nevertheless, further investigations are required to determine the exact mechanism through which this protein affects breast cancer pathogenesis at different stages and to establish its role as a prognostic marker for patients’ treatment response and survival.
One of the limitations of the present study was the small sample population included, which might have affected the results. In addition, considering the methods of this study, survival analysis was performed based on the retrospectively obtained information through phone calls with the patients or their families, which subjects the results of this study to recall bias. Future studies on this topic are recommended to include larger sample populations with a wider variety of breast lesions and follow patients prospectively to minimize the effects of recall bias in determining the role of Caveolin-1 as a prognostic marker.
5.1. Conclusions
The findings of the present study showed a positive significant correlation between epithelial Caveolin-1 positivity with the diagnosis of the patients, grade of the tumor in subjects with invasive breast cancer, and involvement of lymph nodes. Moreover, a negative correlation was observed between epithelial Caveolin-1 positivity with HER2 score and HER2 positivity in subjects diagnosed with invasive breast cancers. Caveolin-1 expression was also found to be negatively correlated with the survival of the patients and overall it seems to be associated with worse outcomes in breast cancer patients.
Acknowledgements
References
-
1.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. [PubMed ID: 26742998]. https://doi.org/10.3322/caac.21332.
-
2.
Mercier I, Lisanti MP. Caveolin-1 and breast cancer: A new clinical perspective. Adv Exp Med Biol. 2012;729:83-94. [PubMed ID: 22411315]. https://doi.org/10.1007/978-1-4614-1222-9_6.
-
3.
Gupta R, Toufaily C, Annabi B. Caveolin and cavin family members: Dual roles in cancer. Biochimie. 2014;107 Pt B:188-202. [PubMed ID: 25241255]. https://doi.org/10.1016/j.biochi.2014.09.010.
-
4.
Hnasko R, Lisanti MP. The biology of caveolae: Lessons from caveolin knockout mice and implications for human disease. Mol Interv. 2003;3(8):445-64. [PubMed ID: 14993453]. https://doi.org/10.1124/mi.3.8.445.
-
5.
Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288(3):C494-506. [PubMed ID: 15692148]. https://doi.org/10.1152/ajpcell.00458.2004.
-
6.
Kiss AL. Caveolae and the regulation of endocytosis. Adv Exp Med Biol. 2012;729:14-28. [PubMed ID: 22411311]. https://doi.org/10.1007/978-1-4614-1222-9_2.
-
7.
Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271(16):9690-7. [PubMed ID: 8621645]. https://doi.org/10.1074/jbc.271.16.9690.
-
8.
Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272(41):25437-40. [PubMed ID: 9325253]. https://doi.org/10.1074/jbc.272.41.25437.
-
9.
Zhang EY, Cristofanilli M, Robertson F, Reuben JM, Mu Z, Beavis RC, et al. Genome wide proteomics of ERBB2 and EGFR and other oncogenic pathways in inflammatory breast cancer. J Proteome Res. 2013;12(6):2805-17. [PubMed ID: 23647160]. [PubMed Central ID: PMC4142215]. https://doi.org/10.1021/pr4001527.
-
10.
Qian N, Ueno T, Kawaguchi-Sakita N, Kawashima M, Yoshida N, Mikami Y, et al. Prognostic significance of tumor/stromal caveolin-1 expression in breast cancer patients. Cancer Sci. 2011;102(8):1590-6. [PubMed ID: 21585620]. https://doi.org/10.1111/j.1349-7006.2011.01985.x.
-
11.
El-Gendi SM, Mostafa MF, El-Gendi AM. Stromal caveolin-1 expression in breast carcinoma. Correlation with early tumor recurrence and clinical outcome. Pathol Oncol Res. 2012;18(2):459-69. [PubMed ID: 22057638]. https://doi.org/10.1007/s12253-011-9469-5.
-
12.
Simpkins SA, Hanby AM, Holliday DL, Speirs V. Clinical and functional significance of loss of caveolin-1 expression in breast cancer-associated fibroblasts. J Pathol. 2012;227(4):490-8. [PubMed ID: 22488553]. https://doi.org/10.1002/path.4034.
-
13.
Sloan EK, Stanley KL, Anderson RL. Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene. 2004;23(47):7893-7. [PubMed ID: 15334058]. https://doi.org/10.1038/sj.onc.1208062.
-
14.
Hayashi K, Matsuda S, Machida K, Yamamoto T, Fukuda Y, Nimura Y, et al. Invasion activating caveolin-1 mutation in human scirrhous breast cancers. Cancer Res. 2001;61(6):2361-4. [PubMed ID: 11289096].
-
15.
Sotgia F, Williams TM, Schubert W, Medina F, Minetti C, Pestell RG, et al. Caveolin-1 deficiency (-/-) conveys premalignant alterations in mammary epithelia, with abnormal lumen formation, growth factor independence, and cell invasiveness. Am J Pathol. 2006;168(1):292-309. [PubMed ID: 16400031]. [PubMed Central ID: PMC1592656]. https://doi.org/10.2353/ajpath.2006.050429.
-
16.
Li T, Sotgia F, Vuolo MA, Li M, Yang WC, Pestell RG, et al. Caveolin-1 mutations in human breast cancer: Functional association with estrogen receptor alpha-positive status. Am J Pathol. 2006;168(6):1998-2013. [PubMed ID: 16723714]. [PubMed Central ID: PMC1606619]. https://doi.org/10.2353/ajpath.2006.051089.
-
17.
Wu P, Qi B, Zhu H, Zheng Y, Li F, Chen J. Suppression of staurosporine-mediated apoptosis in Hs578T breast cells through inhibition of neutral-sphingomyelinase by caveolin-1. Cancer Lett. 2007;256(1):64-72. [PubMed ID: 17618736]. https://doi.org/10.1016/j.canlet.2007.05.007.
-
18.
Savage K, Lambros MB, Robertson D, Jones RL, Jones C, Mackay A, et al. Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin Cancer Res. 2007;13(1):90-101. [PubMed ID: 17200343]. https://doi.org/10.1158/1078-0432.CCR-06-1371.
-
19.
Sekhar SC, Kasai T, Satoh A, Shigehiro T, Mizutani A, Murakami H, et al. Identification of caveolin-1 as a potential causative factor in the generation of trastuzumab resistance in breast cancer cells. J Cancer. 2013;4(5):391-401. [PubMed ID: 23833684]. [PubMed Central ID: PMC3701809]. https://doi.org/10.7150/jca.6470.
-
20.
Thomas NB, Hutcheson IR, Campbell L, Gee J, Taylor KM, Nicholson RI, et al. Growth of hormone-dependent MCF-7 breast cancer cells is promoted by constitutive caveolin-1 whose expression is lost in an EGF-R-mediated manner during development of tamoxifen resistance. Breast Cancer Res Treat. 2010;119(3):575-91. [PubMed ID: 19288272]. https://doi.org/10.1007/s10549-009-0355-8.
-
21.
Wang Z, Wang N, Li W, Liu P, Chen Q, Situ H, et al. Caveolin-1 mediates chemoresistance in breast cancer stem cells via beta-catenin/ABCG2 signaling pathway. Carcinogenesis. 2014;35(10):2346-56. [PubMed ID: 25085904]. https://doi.org/10.1093/carcin/bgu155.
-
22.
Reiner A, Neumeister B, Spona J, Reiner G, Schemper M, Jakesz R. Immunocytochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res. 1990;50(21):7057-61. [PubMed ID: 2208173].
-
23.
Wulfing P, Diallo R, Muller C, Wulfing C, Poremba C, Heinecke A, et al. Analysis of cyclooxygenase-2 expression in human breast cancer: High throughput tissue microarray analysis. J Cancer Res Clin Oncol. 2003;129(7):375-82. [PubMed ID: 12884024]. https://doi.org/10.1007/s00432-003-0459-1.
-
24.
IBM Corp. IBM SPSS statistics for windows, version 22.0. Armonk, NY: IBM Corp; 2013.
-
25.
Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, et al. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998;4(8):1873-80. [PubMed ID: 9717814].
-
26.
Hurlstone AF, Reid G, Reeves JR, Fraser J, Strathdee G, Rahilly M, et al. Analysis of the CAVEOLIN-1 gene at human chromosome 7q31.1 in primary tumours and tumour-derived cell lines. Oncogene. 1999;18(10):1881-90. [PubMed ID: 10086342]. https://doi.org/10.1038/sj.onc.1202491.
-
27.
Zhang G, Zhu T, Cui Y, Mishra R, Luan T, Xie P, et al. Caveolin-1 regulates proliferation and metastasis of human breast cancer cells by activating Her-2. Int J Clin Exp Med. 2016;9(5):7700-9.
-
28.
Liedtke C, Kersting C, Burger H, Kiesel L, Wulfing P. Caveolin-1 expression in benign and malignant lesions of the breast. World J Surg Oncol. 2007;5:110. [PubMed ID: 17915016]. [PubMed Central ID: PMC2082377]. https://doi.org/10.1186/1477-7819-5-110.
-
29.
Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, et al. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279(49):51630-46. [PubMed ID: 15355971]. https://doi.org/10.1074/jbc.M409214200.
-
30.
Fiucci G, Ravid D, Reich R, Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002;21(15):2365-75. [PubMed ID: 11948420]. https://doi.org/10.1038/sj.onc.1205300.
-
31.
Salatino M, Beguelin W, Peters MG, Carnevale R, Proietti CJ, Galigniana MD, et al. Progestin-induced caveolin-1 expression mediates breast cancer cell proliferation. Oncogene. 2006;25(59):7723-39. [PubMed ID: 16799639]. https://doi.org/10.1038/sj.onc.1209757.
-
32.
Van den Eynden GG, Van Laere SJ, Van der Auwera I, Merajver SD, Van Marck EA, van Dam P, et al. Overexpression of caveolin-1 and -2 in cell lines and in human samples of inflammatory breast cancer. Breast Cancer Res Treat. 2006;95(3):219-28. [PubMed ID: 16244790]. https://doi.org/10.1007/s10549-005-9002-1.