Abstract
Background:
Thyroid cancer is one of the most common malignancies related to an endocrine disorder. Due to the widespread increase in thyroid cancer disease all over the world, cancer registries play an important task for improving survival, prevention, and control of cancer plans in developing countries.Objectives:
This study aimed at developing a minimum data set (MDS) for thyroid cancer registry to improve care and treatment core indicators and to revise related guidelines for thyroid cancer in Iran.Methods:
This research was a descriptive and cross sectional study carried out in 2015. Most of the data were collected from the patient's medical records in public hospitals of Ahvaz province, healthcare medicine centers in Ahvaz, in addition to online databases. The data were collected, using a checklist. The decision-making classic Delphi method was used to make a consensus about the data elements. The construct validity and reliability of the questionnaire were approved by the expert’s opinions in the field of endocrinology.Results:
Out of 251 elements of thyroid cancer discussed, 142 and 8 agreed by more than %75 and by 50% to 75% of the experts, respectively. The MDS was divided into 2 categories of identify and clinical data with 1 and 11 sections, respectively.Conclusions:
Comprehensive and uniform data elements about thyroid cancer was not available in Iran. This data set in the field of collecting thyroid cancer information can be useful through facilitating the exchange of health information. The determination of MDS for thyroid cancer will be an effective step to integrate and improve the management of patients’ records.Keywords
1. Background
Thyroid disease is one of the most common endocrine disorders, especially in midlife and elderly individuals. The thyroid gland regulates the body’s metabolism (1). There are 5 general kinds of thyroid disorders such as hypothyroidism, hyperthyroidism, goiter, thyroid nodules, and thyroid cancers (2). Thyroid cancer had an incidence rate generally lower than 3 per 100,000 for men and 5 per 100,000 for women in the world (3). Studies reported an increasing incidence of thyroid cancer in the world during the past several decades (4). The chance of thyroid cancer increased at a rate of 6.5% per year from 1997 to 2006 (5). Based on morphological and clinical features, thyroid cancer is divided into 2 major groups, such as differentiated thyroid tumor, which includes papillary, follicular, medullary, and anaplastic (undifferentiated) thyroid cancer (4, 6). Differentiated thyroid carcinoma accounts approximately for more than 90% of all differentiated thyroid cancer cases. Anaplastic thyroid cancer has an aggressive poor prognosis (5, 7). The prevalence rate of thyroid cancer has been growing significantly and continuously since mid-1990, and it is fastest-growing cancer in both men and women with a growth rate of about 6% per year. Thyroid cancer is mostly diagnosed among people aged 45 to 50 years with the average age diagnosis of 50 years old (8, 9). In a survey conducted by the Iranian Cancer Institute, 1.8% of all cancers and 76.1% of all endocrine cancers constituted by the neoplasm of the thyroid gland (10). Considering the significant widespread growth of the cancer tumors, cancer registries have been created as a basis for a struggling program against the disease. Cancer registries provide information that has great value in conducting research on primary and secondary prevention, health care planning, and cancer management (11). This study aimed at determining a minimum data set (MDS) for thyroid cancer registry to promote information standard for thyroid cancer in Iran. In modern medicine, large amounts of data are produced. Digitizing of data as a part of initiatives has improved the use of the MDS (12). However, there is generally a problem between their collection and their understanding data; in this way, MDSs are prepared. The unified standardization of data can allow the possibility of comparing the collected information from research centers and it gives the credibility (13). The MDS is an important step to further improving provision of services to patients with cancer and this improvement will happen just through the collecting and applying of valid information. The classified data are the most important part of cancer information management; therefore, the MDS is a standard instrumentation for collecting data. Using the MDS, integrated data are used to compare and analyze the activities to access new and valid information on the number of patients, diseases, new therapeutic, and control methods, and their outcomes are collected from all centers (14). The aim of this tool is to determine the data elements that should be considered for each patient and to provide consistent definitions of the necessary information for common terminology (11). The MDS contains much information about the demographic data, health conditions, treatment, sources of payment, and about transfers to other care settings such as hospitals (15). In this research, the minimum set of archival data is defined as a set of data elements including the minimum necessary data, required by physicians for clinical follow-up and medical research. Understanding the problems inherent in traditional archive systems, we have attempted to provide an archive MDS for thyroid cancer based on the opinion of physicians and researchers not only to enable the digitization of medical records but also to access to patient’s follow-up and medical research for two groups. Considering the rising of the thyroid cancer and the lack of a standard tool for collecting the necessary data, there is a need to develop a MDS for thyroid cancer in Iran.
2. Objectives
The aim of this study was to develop an MDS for thyroid cancer registry in order improve care and treatment core indicators and revise related guidelines for thyroid cancer in Iran.
3. Methods
This study was a descriptive and cross-sectional study, which was conducted in 2015. The information was collected from the patient's medical records from the hospitals affiliated with Ahvaz Jundishapur University of Medical Sciences and medical document centers. The list of resources used contains articles, texts, reports, and forms available on the Internet and the patient's medical files. At this stage, an elementary checklist was used for the retrieval of desired information elements. To obtain appropriate resources, studies were identified by searching keywords, including MDS, thyroid cancer data, MDS, and cancer registries in PubMed and Google Scholar. The study is generally limited to studies published from 2005 to 2015. The literature review is limited to Persian and English languages. The articles, whose full texts were unachievable, were excluded from the study. The review and critical analysis continued until the completion of information. Prepared data were divided into clinical and identity groups, using a checklist. Therefore, the data elements extracted from the articles, textbooks, and patient’s medical records were combined and the complete content of the list of MDS was created. The data elements of the mentioned checklist properly prepared the questionnaire. The prepared questionnaire was one of the most important stages in the survey, which contained 2 columns with “Yes” (including required and optional) and “No” in front of each data element. An empty box in front of the end of each element was considered to write necessary data elements according to the expert’s opinions. The validity and reliability of the questionnaire content were evaluated by 41 experts, including 20 endocrinologists, 15 internists, and 6 endocrinology fellowships (with 10 days). The questionnaire design and analyzing the data were performed by SPSS V. 16. The appropriate data elements of thyroid cancer were developed, using a second round of the Delphi technique. The final elements data were chosen by 41 samples of attended experts (demographic characteristics of the samples are described in Table 1). The criteria for the acceptance of data elements in the final MDS were the expert’s agreement or disagreement with the data elements. In this way, data elements with agreement levels below 50% were excluded in the first round, the elements of 50% to 75% agreement were reassessed in the second round, and agreement levels greater than 75% were accepted in the first round of Delphi survey. A score of more than 75% was considered acceptable on each data element in the second round. Therefore, a final list of the data elements of the MDS of thyroid cancer was submitted in two rounds of the Delphi survey.
Demographic Profile of Participants in the Delphi Survey
| Participants | Number | Percentage of Participation | Age Group | Experience |
|---|---|---|---|---|
| Endocrinologist | 20 | 48.78 | 40 - 50.1 | 15 - 20 |
| 50.2 - 60 | 20 - 25 | |||
| Internist | 15 | 36.5 | 30 - 40.1 | 10 - 15 |
| 40.2 - 50 | 15 - 20 | |||
| Endocrinology fellowship | 6 | 14.63 | 35 - 40.1 | 5 - 10 |
| 40.2 - 45 | 10 - 15 |
4. Results
The final data elements of the MDS of the thyroid cancer were designed by 41 samples of participating experts through the Delphi survey in two rounds.
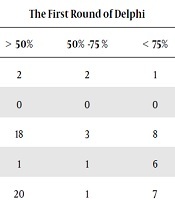
The MDS of thyroid cancer was divided into two main groups. Patient identification data with 1 section and clinical data with 11 sections were classified. A total of 251 final data elements were entered in the Delphi survey. Of these, 151 data elements were accepted in the first round. A total of 150 data elements were confirmed in the second round of the Delphi survey (Tables 2 and 3 show the result of the Delphi technique on the data elements). Patient identifier options include demographic data such as the first and last name, age, gender, occupation, etc.
The first part of the clinical data set was related to clinical history and risk factor elements (such as the personal history of thyroid cancer, family history of thyroid cancer, history of disorders of thyroid dysfunction, etc.).
Administrative and Clinical Data Category for Minimum Data Set for Thyroid Cancer
| Data Sections | Number of Data Elements | The First Round of Delphi | The Second Round of Delphi | The Final Number of Data Elements | ||||
|---|---|---|---|---|---|---|---|---|
| > 50% | 50% -75 % | < 75% | > 50% | 50% - 75% | < 75% | |||
| Demographic | 14 | 2 | 2 | 1 | 0 | 1 | 11 | 12 |
| History and risk factors | 8 | 0 | 0 | 0 | 0 | 0 | 8 | 8 |
| Signs and symptoms | 27 | 18 | 3 | 8 | 1 | 0 | 8 | 8 |
| Examination | 9 | 1 | 1 | 6 | 0 | 0 | 8 | 8 |
| Imaging before and after surgery | 44 | 20 | 1 | 7 | 0 | 0 | 24 | 24 |
| Lab test | 25 | 7 | 9 | 9 | 0 | 7 | 11 | 18 |
| Pathological characteristics | 9 | 1 | 0 | 8 | 0 | 0 | 9 | 9 |
| Surgical Characteristics | 8 | 0 | 0 | 8 | 0 | 0 | 8 | 8 |
| Morphology distribution | 14 | 3 | 7 | 4 | 0 | 0 | 11 | 11 |
| Histopathology | 39 | 16 | 6 | 17 | 0 | 0 | 23 | 23 |
| Tumor staging | 8 | 6 | 0 | 2 | 0 | 0 | 2 | 2 |
| Follow-up | 31 | 19 | 0 | 12 | 0 | 0 | 12 | 12 |
| Procedure | 15 | 8 | 1 | 6 | 0 | 0 | 7 | 7 |
| Total | 251 | 100 | 31 | 120 | 1 | 8 | 142 | 150 |
Examples of Administrative and Clinical Data Elements for a Minimum Data Set for Thyroid Cancer
| Section | Data Elements |
|---|---|
| Lab test data | TSH |
| Total T4&T3 | |
| Free T4&T3 | |
| TG & anti TG Ab | |
| Anti TPO Ab | |
| Fine needle aspiration biopsy findings | |
| Surgery data | Total thyroidectomy |
| Near-total thyroidectomy | |
| Subtotal thyroidectomy | |
| Lobectomy | |
| Lobectomy & Isthmectomy | |
| Morphology data | Tumor focality |
| Tumor margin | |
| Tumor size | |
| Histopathology data | Molecular tumor markers |
| Tumor types | |
| Thyroid capsule invasion | |
| Tumor staging data | TMN staging tumor |
| MACIS staging tumor | |
| Procedure data | Chemotherapy for cancer |
| Radiotherapy for cancer | |
| Radioactive iodine therapy | |
| Demographic data | Patient name |
| Patient family | |
| Gender | |
| Birth data | |
| Medical record number | |
| Race/ethnicity/tribe | |
| level of education | |
| History and risk factors data | Personal history of thyroid cancer |
| Family history of thyroid cancer | |
| Graves’ disease history | |
| Hypothyroidism and hyperthyroidism disease history | |
| Signs and symptoms of data | Hoarseness |
| Dyspnea | |
| Cervical mass | |
| Vital signs and weight | |
| Cough and phlegm | |
| Examination data | Size of the thyroid gland |
| Thyroid consistency | |
| Size of thyroid nodule | |
| Number of thyroid nodule | |
| Radiographic findings before and after surgery | |
| Thyroid scan findings before and after surgery | |
| PET scan findings | |
| Chest and abdomen CT scan findings |
The second section of the clinical data collection included sign and symptom data elements such as vital signs and public data. The next section, the examination data elements included the size of the thyroid gland, size of a thyroid nodule, thyroid consistency, etc. Imaging procedures such as X-rays were categorized as invasive and non-invasive radiological medical tests. Laboratory data included lab tests and pathological reports.
Tumor cell morphology data included tumor size, tumor focality, and tumor margin.
Procedures data encompass surgical procedures, as well as non-surgical and therapeutic procedures. The histopathology of thyroid tumors analysis was divided into cytologic and histogenetic features. Considering that this MDS is used for all patients with thyroid cancer, prognostic scoring of thyroid cancer was categorized based on systems such as TNM and MACIS staging. The follow-up of cancer data including follow-up requests for completing treatments involves regular medical checkups.
5. Discussion
Modern medicine produces a lot of data. But, there is often a problem in their collection, perception, and interpretation of data. In this way, the MDS of information management are developed. According to studies, thyroid cancer is one of the most common endocrine malignancies in Iran similar to developing countries. Studies have an annual percentage growth rate of 2.2 per 100,000 for Iranian males and females (5). The variable content of thyroid cancer information represents the lack of standard tools for data collection and the absence of data set for thyroid cancer in Iran. The lack of standardization of the data of thyroid cancer makes at the regional, national, and international levels a problem. The collection of standard data requires the development of the minimum necessary data set for thyroid cancer in Iran. A research study was conducted by the Royal College of Pathologists of England. The essential data elements proposed in this study include tumor staging and grading, optimal treatment, and prognosis. The data provided in this study, in contrast to the Royal College, contains more information, including identity data, clinical symptoms, laboratory data, and follow-up data. The Royal College dataset is limited to the details of pathological data (16, 17). Dataset for thyroid cancer histopathology developed by the department of pathology of the glands, University of Pennsylvania Medical Center, USA. This is very comprehensive and refers to the detail of the thyroid cancer. This MDS, similar to the MDS presented in our study, histological types of tumor, TNM staging, the size of the tumor, focality of tumor and metastasis are considered as the main element. The data set provided at the University of Pennsylvania does not consider follow-up data and lab tests (18). In a study undertaken in National Cancer Institute of Ireland, it was announced that MDS must be collected for all patients with cancer at the national level, provide comprehensive and accurate information on types of cancer, and also create attitude about similar developments in related areas such as preventive healthcare (14). A study conducted in the Scottish Cancer Therapy Network stated that the MDS played an effective role in collecting necessary cancer data and provided a strong instrument for collecting significant data at the regional and national levels (19). The result of the study by Hawes et al. showed that MDS provides considerable improvement in integrity in nursing home residents, affects the quality of services and quality of life, and reduces the duration of hospitalization (20). Millonig reported that MDSs can provide valuable achievements in clinical, economical, and prevention care systems (21). Reports of a study suggested the documentation of clinical information for the continuity of prevention and care. Also, it is important to develop clinical knowledge, guarantee security, and management of nursing care (22). Creating an MDS is a crucial turning point to help overcome data change between thyroid cancer care centers in the world.
5.1. Conclusions
Due to the lack of classified information elements about thyroid cancer in Iran, an MDS was designed for thyroid cancer in Iran. Creating an MDS will help the standardization and effective management of the data by providing uniform and comprehensive data elements for thyroid cancer. Therefore, an MDS of thyroid cancer was extracted for easy access to information.
Acknowledgements
References
-
1.
Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL. Harrison's principles of internal medicine. 15th ed. McGraw-Hill; 2001.
-
2.
Turanoglu-Bekar E, Ulutagay G, Kantarcı-Savas S. Classification of thyroid disease by using data mining models: A comparison of decision tree algorithms. Oxford J Intell Decis Data Sci. 2016;2016(2):13-28. https://doi.org/10.5899/2016/ojids-00002.
-
3.
Larijani B, Mohagheghi MA, Bastanhagh MH, Mosavi-Jarrahi AR, Haghpanah V, Tavangar SM, et al. Primary thyroid malignancies in Tehran, Iran. Med Princ Pract. 2005;14(6):396-400. [PubMed ID: 16220012]. https://doi.org/10.1159/000088112.
-
4.
Gursoy A. Rising thyroid cancer incidence in the world might be related to insulin resistance. Med Hypotheses. 2010;74(1):35-6. [PubMed ID: 19720470]. https://doi.org/10.1016/j.mehy.2009.08.021.
-
5.
Safavi A, Azizi F, Jafari R, Chaibakhsh S, Safavi AA. Thyroid cancer epidemiology in Iran: A time trend study. Asian Pac J Cancer Prev. 2016;17(1):407-12. [PubMed ID: 26838247]. https://doi.org/10.7314/apjcp.2016.17.1.407.
-
6.
Thoresen SO, Akslen LA, Glattre E, Haldorsen T, Lund EV, Schoultz M. Survival and prognostic factors in differentiated thyroid cancer--a multivariate analysis of 1,055 cases. Br J Cancer. 1989;59(2):231-5. [PubMed ID: 2930688]. [PubMed Central ID: PMC2246999]. https://doi.org/10.1038/bjc.1989.47.
-
7.
Liu AH, Juan LY, Yang AH, Chen HS, Lin HD. Anaplastic thyroid cancer with uncommon long-term survival. J Chin Med Assoc. 2006;69(10):489-91. [PubMed ID: 17098674]. https://doi.org/10.1016/S1726-4901(09)70314-4.
-
8.
Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev. 2009;18(3):784-91. [PubMed ID: 19240234]. [PubMed Central ID: PMC2676561]. https://doi.org/10.1158/1055-9965.EPI-08-0960.
-
9.
Shats O, Goldner W, Feng J, Sherman A, Smith RB, Sherman S. Thyroid cancer and tumor collaborative registry (TCCR). Cancer Inform. 2016;15:73-9. [PubMed ID: 27168721]. [PubMed Central ID: PMC4856228]. https://doi.org/10.4137/CIN.S32470.
-
10.
Larijani B, Aghakhani S, Khajeh-Dini H, Baradar-Jalili R. Clinico-pathological features of thyroid cancer as observed in five referral hospitals in Iran--a review of 1177 cases. Acta Oncol. 2003;42(4):334-7. [PubMed ID: 12899505]. https://doi.org/10.1080/02841860310001547.
-
11.
Ahmadi M, Alipour J, Mohammadi A, Khorami F. Development a minimum data set of the information management system for burns. Burns. 2015;41(5):1092-9. [PubMed ID: 25561018]. https://doi.org/10.1016/j.burns.2014.12.009.
-
12.
Mohammadi A, Ahmadi M, Gharagozlu A. Developing a minimum data set for an information management system to study traffic accidents in Iran. Iran Red Crescent Med J. 2016;18(3). e23677. en. https://doi.org/10.5812/ircmj.23677.
-
13.
Lee AS, Baskerville RL. Generalizing generalizability in information systems research. Inform Syst Res. 2003;14(3):221-43. https://doi.org/10.1287/isre.14.3.221.16560.
-
14.
Ghaneie M, Rezaie A, Ghorbani NR, Heidari R, Arjomandi M, Zare M. Designing a minimum data set for breast cancer: A starting point for breast cancer registration in iran. Iran J Public Health. 2013;42(Supple1):66-73. [PubMed ID: 23865019]. [PubMed Central ID: PMC3712595].
-
15.
Kowal PR, Wolfson LJ, Dowd JE. Creating a minimum data set on ageing in sub-Saharan Africa. South Afr J Gerontol. 2000;9(2):18-23. https://doi.org/10.21504/sajg.v9i2.203.
-
16.
Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014;81 Suppl 1:1-122. [PubMed ID: 24989897]. https://doi.org/10.1111/cen.12515.
-
17.
Paterson IC, Greenlee R, Adams Jones D. Thyroid cancer in Wales 1985-1996: A cancer registry-based study. Clin Oncol (R Coll Radiol). 1999;11(4):245-51. [PubMed ID: 10473721]. https://doi.org/10.1053/clon.1999.9057.
-
18.
Baloch ZW, Cibas ES, Clark DP, Layfield LJ, Ljung BM, Pitman MB, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: A summation. Cytojournal. 2008;5:6. [PubMed ID: 18394201]. [PubMed Central ID: PMC2365970]. https://doi.org/10.1186/1742-6413-5-6.
-
19.
Parkin DM. The evolution of the population-based cancer registry. Nat Rev Cancer. 2006;6(8):603-12. [PubMed ID: 16862191]. https://doi.org/10.1038/nrc1948.
-
20.
Hawes C, Morris JN, Phillips CD, Fries BE, Murphy K, Mor V. Development of the nursing home Resident Assessment Instrument in the USA. Age Ageing. 1997;26 Suppl 2:19-25. [PubMed ID: 9464550]. https://doi.org/10.1093/ageing/26.suppl_2.19.
-
21.
Millonig MK. Mapping the route to medication therapy management documentation and billing standardization and interoperabilility within the health care system: Meeting proceedings. J Am Pharm Assoc (2003). 2009;49(3):372-82. [PubMed ID: 19357067]. https://doi.org/10.1331/JAPhA.2008.09518.
-
22.
Nunes SR, Rego G, Nunes R. The experience of an information system for nursing practice: The importance of nursing records in the management of a care plan. Comput Inform Nurs. 2014;32(7):322-32. [PubMed ID: 24781812]. https://doi.org/10.1097/CIN.0000000000000060.